Biocontrol Potential of the New Codling Moth Granulovirus (CpGV) Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Insects
2.2. Preparation Procedure
2.3. Molecular Genetic Identification of CpGV Strains
2.4. Comparative Genomic Analysis
2.5. Phylogenetic Analysis
2.6. Entomopathogenic Activity
2.7. Data Analysis
3. Results
3.1. Molecular Genetic Identification of CpGV Strains
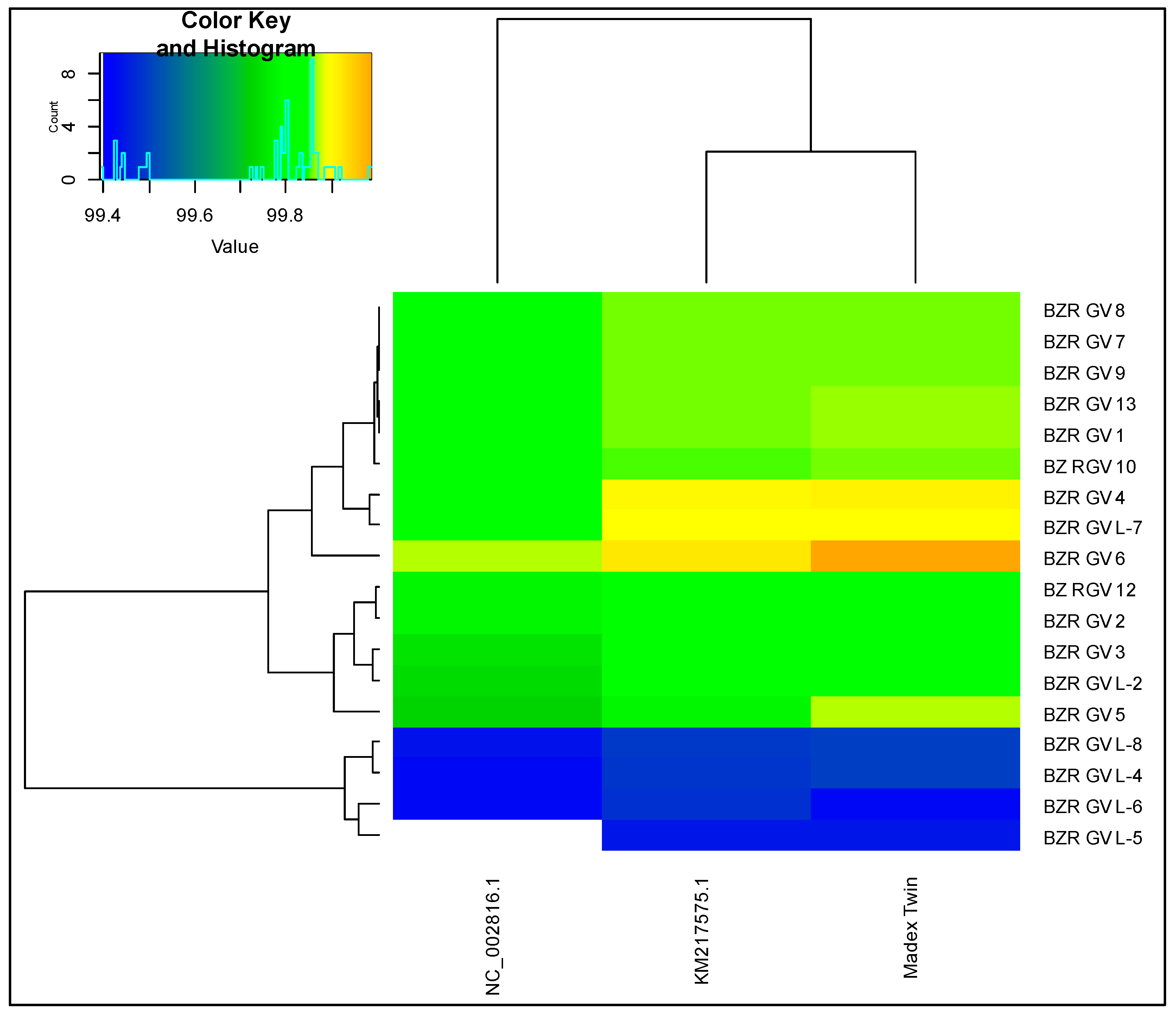
3.2. Genomic Analysis Based on Average Nucleotide Identity
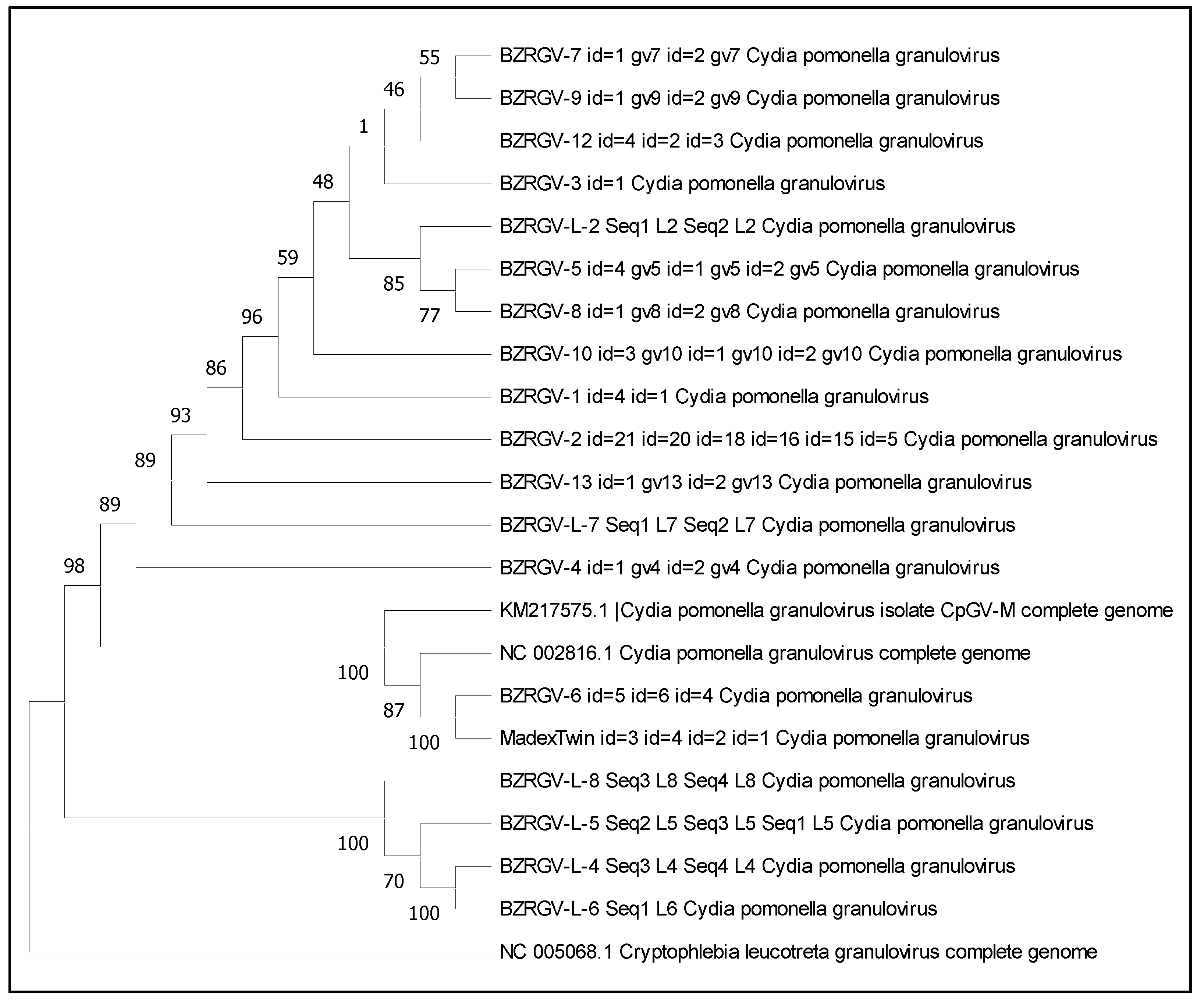
3.3. Phylogenetic Tree
3.4. Entomopathogenic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Black, B.C.; Brennan, L.A.; Dierks, P.M.; Gard, I.E. Commercialization of Baculoviral Insecticides. In The Baculoviruses. The Viruses; Miller, L.K., Ed.; Springer: Boston, MA, USA, 1997; pp. 341–387. [Google Scholar]
- Beas-Catena, A.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. Baculovirus biopesticides: An overview. J. Anim. Plant Sci. 2014, 24, 362–373. [Google Scholar]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, Z.; Gencer, D.; Demir, I. Development of novel betabaculovirus (HycuGV-Hc1) as a biopesticide (HycuGV-TR61) and its efficacy on the fall webworm, Hyphantria cunea Drury (Lepidoptera: Erebidae) larvae. Egypt. J. Biol. Pest Control. 2023, 33, 21. [Google Scholar] [CrossRef]
- Ismailov, V.; Agasjeva, I.; Ananko, G.; Kolosov, A. Virin HSK and Helicovex SK—Efficacy and safety of baculovirus-based bioinsecticides. BIO Web Conf. 2020, 21, 00022. [Google Scholar] [CrossRef]
- Andermatt Biocontrol Suisse AG. [Electronic Resource]. Available online: https://www.biocontrol.ch/de-ch/produkte-und-webshop--c22466?page=1&itemsPerPage=12&sortBy=relevance&categories=22466&facets=22592%253A15930 (accessed on 30 January 2024).
- Certis Biological. CYD-X. [Electronic Resource]. Available online: https://www.certisbio.com/products/insecticidal-viruses/cyd-x-hp (accessed on 30 January 2024).
- Sauer, A.J.; Fritsch, E.; Undorf-Spahn, K.; Nguyen, P.; Marec, F.; Heckel, D.G.; Jehle, J.A. Novel resistance to Cydia pomonella granulovirus (CpGV) in codling moth shows autosomal and dominant inheritance and confers cross-resistance to different CpGV genome groups. PLoS ONE 2017, 12, e0179157. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Sciocco-Cap, A.; Romanowski, V. Baculovirus Insecticides in Latin America: Historical Overview, Status and Future Perspectives. Viruses 2015, 7, 2230–2267. [Google Scholar] [CrossRef] [PubMed]
- Eberle, K.E.; Asser-Kaiser, S.; Sayed, S.M.; Nguyen, H.T.; Jehle, J.A. Overcoming the resistance of codling moth against conventional Cydia pomonella granulovirus (CpGV-M) by a new isolate CpGV-I12. J. Invertebr. Pathol. 2008, 98, 293–298. [Google Scholar] [CrossRef]
- Siegwart, M.; Graillot, B.; Blachere Lopez, C.; Besse, S.; Bardin, M.; Nicot, P.C.; Lopez-Ferber, M. Resistance to bio-insecticides or how to enhance their sustainability: A review. Front Plant Sci. 2015, 19, 381. [Google Scholar] [CrossRef]
- Explained. Eurostat Statistics: Agricultural Production—Orchards. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_orchards#Apple_trees (accessed on 30 April 2024).
- Time for Apples from Europe: Sector. Available online: https://applesfromeurope.eu/sector/ (accessed on 30 April 2024).
- ProBusiness 72. The Apple Market in Russia and the World. [Electronic Resource]. Available online: https://www.pbs72.ru/articles/mneniya/rynok-yablok-v-rossii-i-mire/ (accessed on 27 March 2022). (In Russian).
- Akroute, D.; Douaik, A.; Habbadi, K.; ElBakkali, A.; BenBouazza, A.; Benkirane, R.; El Iraqui El Houssaini, S. Influence of Some Fruit Traits on Codling Moth (Cydia pomonella L.) Preference among Apple Varieties in Two Contrasted Climatic Conditions. Horticulturae 2023, 9, 788. [Google Scholar] [CrossRef]
- Hussain, B.; Masoodi, K.Z.; War, A.R.; Hakak, A.S.; Ahmad, N.; Masoodi, T. Occurrence of granulovirus infecting Cydia pomonella in high altitude cold arid region of India. VirusDisease 2020, 31, 517–525. [Google Scholar] [CrossRef]
- Cherkezova, S.R. Development of technology for protecting an apple orchard against a complex of lepidopteran pests under weather stress. Fruit Grow. Vitic. South Russ. 2019, 55, 107–119. (In Russian) [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef] [PubMed]
- The State Catalog of Pesticides and Agrochemicals Approved for Use in the Territory of the Russian Federation. Part I. Pesticides; Ministry of Agriculture of the Russian Federation: Moscow, Russia, 2024; pp. 55–56. (In Russian)
- Jorio, H.; Tran, R.; Kamen, A. Stability of Serum-Free and Purified Baculovirus Stocks under Various Storage Conditions. Biotechnol. Prog. 2006, 22, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Avilés, N.; Martínez, A.M.; Pineda, S.; Bravo-Patiño, A.; Figueroa, I.; Lasa, R. Cool-textured diets for use in baculovirus production. Biocontrol Sci. Technol. 2017, 27, 1327–1338. [Google Scholar] [CrossRef]
- Mitrofanov, V.B. Granulosa of the apple moth Laspeyresia pomonella L. Dissertation of the candidate of biological sciences. Leningrad, 1976, 182. (In Russian). Available online: https://search.rsl.ru/ru/record/01009531473 (accessed on 31 July 2024).
- FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 10 April 2024).
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve Years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Guyomar, C.; Delage, W.; Legeai, F.; Mougel, C.; Simon, J.-C.; Lemaitre, C. MinYS: Mine Your Symbiont by Targeted Genome Assembly in Symbiotic Communities. NAR Genom. Bioinform. 2020, 2, lqaa047. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Guizelini, D.; Raittz, R.T.; Cruz, L.M.; Souza, E.M.; Steffens, M.B.R.; Pedrosa, F.O. GFinisher: A New Strategy to Refine and Finish Bacterial Genome Assemblies. Sci. Rep. 2016, 6, 34963. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Graillot, B.; Berling, M.; Blachere-López, C.; Siegwart, M.; Besse, S.; López-Ferber, M. Progressive Adaptation of a CpGV Isolate to Codling Moth Populations Resistant to CpGV-M. Viruses 2014, 6, 5135–5144. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput ANI Analysis of 90K Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv 2013, arXiv:2004.01574. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- The State Catalog of Pesticides and Agrochemicals Approved for Use in the Territory of the Russian Federation. Part I. Pesticides; Ministry of Agriculture of the Russian Federation: Moscow, Russia, 2023; pp. 53–54. (In Russian)
- Graillot, B.; Blachere-López, C.; Besse, S.; Siegwart, M.; López-Ferber, M. Importance of the Host Phenotype on the Preservation of the Genetic Diversity in Codling Moth Granulovirus. Viruses 2019, 11, 621. [Google Scholar] [CrossRef]
- Liao, Z.H.; Kuo, T.C.; Shih, C.W.; Tuan, S.J.; Kao, Y.H.; Huang, R.N. Effect of juvenile hormone and pyriproxyfen treatments on the production of Spodoptera lituranuclear polyhedrosis virus. Entomol. Exp. Appl. 2016, 161, 112–120. [Google Scholar] [CrossRef]
- Berling, M.; Blachere-Lopez, C.; Soubabere, O.; Lery, X.; Bonhomme, A.; Sauphanor, B.; Lopez-Ferber, M. Cydia pomonella granulovirus Genotypes Overcome Virus Resistance in the Codling Moth and Improve Virus Efficiency by Selection against Resistant Hosts. Appl. Environ. Microbiol. 2008, 75, 925–930. [Google Scholar] [CrossRef]
- Henderson, C.F.; Tilton, E.W. Tests with acaricides against brown wheat mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Kishore, J.; Goel, M.; Khanna, P. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wennmann, J.T.; Wang, D.; Jehle, J.A. Novel diversity and virulence patterns found in new isolates of Cydia pomonella granulovirus from China. Appl. Environ. Microbiol. 2020, 86, e02000-19. [Google Scholar] [CrossRef]
- Lynn, D.E.; Harrison, R.L. Routine Maintenance and Storage of Lepidopteran Insect Cell Lines and Baculoviruses. In Baculovirus and Insect Cell Expression Protocols; Springer: Berlin/Heidelberg, Germany, 2016; pp. 197–221. [Google Scholar] [CrossRef]
- Fraser, M.J.; Smith, G.E.; Summers, M.D. Acquisition of Host Cell DNA Sequences by Baculoviruses: Relationship between Host DNA Insertions and FP Mutants of Autographa californica and Galleria mellonella Nuclear Polyhedrosis Viruses. J. Virol. 1983, 47, 287–300. [Google Scholar] [CrossRef]
- Ishimwe, E.; Hodgson, J.J.; Passarelli, A.L. Expression of the Cydia pomonella granulovirus matrix metalloprotease enhances Autographa californica multiple nucleopolyhedrovirus virulence and can partially substitute for viral Cathepsin. Virology 2015, 481, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Tristem, M.; O’Reilly, D.R.; Crook, N.E.; Maeda, S. Identification and characterization of the Cydia pomonella granulovirus cathepsin and chitinase genes. J. Gen. Virol. 1998, 79, 2283–2292. [Google Scholar] [CrossRef]
- Tsygan, V.N. The role of apoptosis in the regulation of the immune response. Rev. Clin. Pharmacol. Drug Ther. 2004, 3, 62–77. (In Russian) [Google Scholar]
- Mascarin, G.M.; Delalibera, I. Insecticidal Activity of the Granulosis Virus in Combination with Neem Products and Talc Powder Against the Potato Tuberworm Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2012, 41, 223–231. [Google Scholar] [CrossRef]
- Apaire-Marchais, V.; Ogliastro, M.; Chandre, F.; Pennetier, C.; Raymond, V.; Lapied, B. Virus and calcium: An unexpected tandem to optimize insecticide efficacy. Environ. Microbiol. Rep. 2016, 8, 168–178. [Google Scholar] [CrossRef]
- Ditta Abid, A.; Saeed, S.; Muhammad Zaka, S.; Ali, M.; Sohail Shahzad, M.; Iqbal, M.; Shahzad, U.; Iqbal, N.; Alghanem, S.M. Interaction of HaNPVs with two novel insecticides against Helicoverpa armigera Hubner (Noctuidae: Lepidoptera). Saudi J. Biol. Sci. 2020, 27, 2124–2128. [Google Scholar] [CrossRef] [PubMed]
- Slinin, A.S.; Bydanov, O.I.; Karachunskiy, A.I. Analysis of survival and possibility of certain events in patients with acute leucosis. Pediatr. Haematol./Oncol. Immunopathol. 2016, 15, 34–39. [Google Scholar] [CrossRef]
- Black, J.L.; Lorenz, G.M.; Cato, A.J.; Bateman, N.R.; Seiter, N.J. Efficacy of Helicoverpa Armigera Nucleopolyhedrovirus on Soybean for Control of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in Arkansas Agriculture. Insects 2022, 13, 91. [Google Scholar] [CrossRef]
- Goto, C.; Mukawa, S.; Mitsunaga, T. Two Year Field Study to Evaluate the Efficacy of Mamestra brassicae Nucleopolyhedrovirus Combined with Proteins Derived from Xestia c-nigrum Granulovirus. Viruses 2015, 7, 1062–1078. [Google Scholar] [CrossRef]
- Kalha, C.S.; Singh, P.P.; Kang, S.S.; Hunjan, M.S.; Gupta, V.; Sharma, R. Entomopathogenic Viruses and Bacteria for Insect-Pest Control. In Integrated Pest Management; Elsevier: Amsterdam, The Netherlands, 2014; pp. 225–244. [Google Scholar]
| Ingredient | g or mL |
|---|---|
| Agar-agar | 12 |
| Corn flour | 45 |
| Wheat bran | 45 |
| Malt sprouts | 54 |
| Soy flour | 40 |
| Ascorbic acid | 5.4 |
| Citric acid | 4.5 |
| Sorbic acid | 0.81 |
| Methylparaben | 2.25 |
| Water | 800 |
| No. | Strain/Code | Titer, Granules/mL | Biological Efficacy, %, Days | Live Larvae, Ind., Days | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 7 | 10 | 15 | 1 | 2 | 3 | 5 | 7 | 10 | 15 | |||
| 1 | BZR GV 1 | (8.00 ± 3.00) × 106 | 25.15 | 32.81 | 31.94 | 36.81 | 73.05 | 100.00 | 100.00 | 3.5 a,b | 3.0 b | 2.8 a,b,c,d | 2.5 b,c,d,e | 1.0 a,b | 0.0 a | 0.0 a |
| 2 | BZR GV 2 | (9.00 ± 2.00) × 106 | 6.43 | 7.93 | 5.76 | 6.59 | 46.11 | 77.42 | 79.17 | 4.4 c,d,e | 4.1 d,e,f,g | 4.0 e,f,g,h | 3.7 f,g,h,i | 2.0 b,c,d,e,f | 0.7 a,b,c,d | 0.6 a,b,c,d |
| 3 | BZR GV 3 | (1.00 ± 0.40) × 107 | 0 | 12.91 | 10.99 | 17.58 | 55.09 | 87.10 | 100.00 | 5.0 e | 3.8 b,c,d,e,f | 3.7 d,e,f,g,h | 3.3 c,d,e,f,g,h | 1.6 a,b,c,d,e | 0.4 a,b,c | 0.0 a,b |
| 4 | BZR GV 4 | (2.13 ± 0.32) × 107 | 0 | 7.93 | 5.76 | 9.34 | 46.11 | 83.87 | 89.58 | 5.0 e | 4.1 d,e,f,g | 4.0 f,g,h | 3.6 e,f,g,h,i | 2.0 b,c,d,e,f | 0.5 a,b,c | 0.3 a,b,c,d |
| 5 | BZR GV 5 | (3.40 ± 0.79) × 107 | 11.11 | 20.37 | 18.85 | 31.32 | 55.09 | 64.52 | 86.11 | 4.2 c,d | 3.5 b,c,d,e | 3.4 c,d,e,f,g | 2.7 b,c,d,e,f | 1.6 a,b,c,d,e | 1.2 b,c,d | 0.4 a,b,c,d |
| 6 | BZR GV 6 | (2.97 ± 2.02) × 107 | 1.75 | 7.93 | 5.76 | 14.84 | 52.10 | 70.97 | 93.06 | 4.6 d,e | 4.1 d,e,f,g | 4.0 e,f,g,h | 3.4 d,e,f,g,h,i | 1.7 b,c,d,e,f | 1.0 a,b,c,d | 0.2 a,b |
| 7 | BZR GV 7 | (1.50 ± 0.20) × 107 | 4.09 | 5.44 | 8.38 | 20.33 | 76.05 | 83.87 | 100.00 | 4.5 c,d,e | 4.2 d,e,f,g | 3.8 e,f,g,h | 3.2 c,d,e,f,g,h | 0.8 a,b | 0.5 a,b,c | 0.0 a,b |
| 8 | BZR GV 8 | (1.47 ± 0.41) × 107 | 4.09 | 7.93 | 18.85 | 31.32 | 61.08 | 70.97 | 89.58 | 4.5 c,d,e | 4.1 d,e,f,g | 3.4 b,c,d,e,f | 2.7 b,c,d,e,f | 1.4 a,b,c | 1.0 a,b,c,d | 0.3 a,b,c,d |
| 9 | BZR GV 9 | (1.03 ± 0.25) × 107 | 0 | 17.88 | 26.70 | 45.05 | 73.05 | 77.42 | 86.11 | 5.0 e | 3.6 b,c,d,e,f | 3.1 b,c,d,e,f | 2.2 a,b,c | 1.0 a,b | 0.7 a,b,c,d | 0.4 a,b,c,d |
| 10 | BZR GV 10 | (1.93 ± 0.05) × 107 | 8.77 | 12.91 | 29.32 | 39.56 | 61.08 | 74.19 | 100.00 | 4.3 c,d | 3.8 c,d,e,f | 3.0 a,b,c,d,e | 2.4 a,b,c,d | 1.4 a,b,c | 0.8 a,b,c,d | 0.0 a,b |
| 11 | BZR GV 12 | (2.40 ± 0.52) × 107 | 0 | 0 | 0 | 0 | 13.17 | 51.61 | 72.22 | 5.0 e | 5.0 g | 4.6 h,i | 4.5 i | 3.2 g,h | 1.6 d | 0.8 b,c,d |
| 12 | BZR GV 13 | (1.37 ± 0.32) × 107 | 0 | 0 | 0 | 0 | 52.10 | 70.97 | 89.58 | 5.0 e | 5.0 g | 5.0 i | 4.5 i | 1.7 b,c,d,e,f | 1.0 a,b,c,d | 0.3 a,b,c,d |
| 13 | BZR GV L-2 | (2.70 ± 0.36) × 107 | 0 | 0 | 0.52 | 12.09 | 67.07 | 74.19 | 79.17 | 5.0 e | 4.5 f,g | 4.2 g,h,i | 3.5 d,e,f,g,h,i | 1.2 a,b,c | 0.8 a,b,c,d | 0.6 a,b,c,d |
| 14 | BZR GV L-4 | (4.53 ± 2.91) × 107 | 0 | 10.42 | 18.85 | 28.57 | 49.10 | 70.97 | 75.69 | 5.0 e | 4.0 c,d,e,f | 3.4 c,d,e,f,g | 2.8 b,c,d,e,f,g | 1.8 b,c,d,e,f | 1.0 a,b,c,d | 0.7 a,b,c,d |
| 15 | BZR GV L-5 | (6.77 ± 4.60) × 107 | 1.75 | 10.42 | 5.76 | 28.57 | 58.08 | 77.42 | 82.64 | 4.6 d,e | 4.0 c,d,e,f | 4.0 f,g,h | 2.8 b,c,d,e,f,g | 1.5 a,b,c,d | 0.7 a,b,c,d | 0.5 a,b,c,d |
| 16 | BZR GV L-6 | (1.39 ± 0.19) × 108 | 6.43 | 22.86 | 37.17 | 50.55 | 70.06 | 77.42 | 82.64 | 4.4 c,d,e | 3.4 b,c,d | 2.6 a,b,c | 2.0 a,b | 1.1 a,b,c | 0.7 a,b,c,d | 0.5 a,b,c,d |
| 17 | BZR GV L-7 | (9.00 ± 3.61) × 106 | 15.79 | 15.39 | 13.61 | 12.09 | 28.14 | 58.06 | 65.28 | 4.0 b,c | 3.7 b,c,d,e,f | 3.6 d,e,f,g,h | 3.5 d,e,f,g,h,i | 2.6 d,e,f,g,h | 1.4 c,d | 1.1 c |
| 18 | BZR GV L-8 | (9.57 ± 1.71) × 107 | 11.11 | 30.32 | 42.41 | 64.29 | 85.03 | 90.32 | 100.00 | 4.2 c,d | 3.1 b,c | 2.4 a,b | 1.4 a | 0.5 a | 0.3 a,b | 0.0 a,b |
| 19 | «Madex Tween» | (3.08 ± 0.23) × 106 | 27.49 | 50.23 | 50.26 | 53.30 | 64.07 | 67.74 | 89.58 | 3.4 a | 2.2 a | 2.1 a | 1.8 a,b | 1.3 a,b,c | 1.1 b,c,d | 0.3 a,b,c,d |
| 20 | control | - | - | - | - | - | - | - | - | 4.7 d,e | 4.4 e,f,g | 4.2 g,h,i | 4.0 g,h,i | 3.7 h | 3.4 e | 3.2 e |
| No. | Strain/Code | p-Value (Error Level) | Average Time of Death, Days | Median Time of Death, Days |
|---|---|---|---|---|
| 1 | BZR GV 1 | <0.001 | 5.2 | 7.0 |
| 2 | BZR GV 2 | <0.001 | 7.7 | 7.0 |
| 3 | BZR GV 3 | <0.001 | 7.0 | 7.0 |
| 4 | BZR GV 4 | <0.001 | 7.6 | 7.0 |
| 5 | BZR GV 5 | <0.001 | 7.2 | 7.0 |
| 6 | BZR GV 6 | <0.001 | 7.8 | 7.0 |
| 7 | BZR GV 7 | <0.001 | 6.6 | 7.0 |
| 8 | BZR GV 8 | <0.001 | 7.0 | 7.0 |
| 9 | BZR GV 9 | <0.001 | 6.1 | 5.0 |
| 10 | BZR GV 10 | <0.001 | 6.5 | 5.0 |
| 11 | BZR GV 12 | <0.001 | 10.0 | 10.0 |
| 12 | BZR GV 13 | <0.001 | 8.7 | 7.0 |
| 13 | BZR GV L-2 | <0.001 | 7.6 | 7.0 |
| 14 | BZR GV L-4 | <0.001 | 7.4 | 7.0 |
| 15 | BZR GV L-5 | <0.001 | 7.2 | 7.0 |
| 16 | BZR GV L-6 | <0.001 | 5.8 | 5.0 |
| 17 | BZR GV L-7 | <0.001 | 8.5 | 10.0 |
| 18 | BZR GV L-8 | <0.001 | 4.6 | 3.0 |
| 19 | «Madex Tween» | <0.001 | 5.6 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsygichko, A.A.; Asaturova, A.M.; Lakhova, T.N.; Klimenko, A.I.; Lashin, S.A.; Vasiliev, G.V. Biocontrol Potential of the New Codling Moth Granulovirus (CpGV) Strains. Microorganisms 2024, 12, 1991. https://doi.org/10.3390/microorganisms12101991
Tsygichko AA, Asaturova AM, Lakhova TN, Klimenko AI, Lashin SA, Vasiliev GV. Biocontrol Potential of the New Codling Moth Granulovirus (CpGV) Strains. Microorganisms. 2024; 12(10):1991. https://doi.org/10.3390/microorganisms12101991
Chicago/Turabian StyleTsygichko, Aleksandra A., Anzhela M. Asaturova, Tatiana N. Lakhova, Alexandra I. Klimenko, Sergey A. Lashin, and Gennady V. Vasiliev. 2024. "Biocontrol Potential of the New Codling Moth Granulovirus (CpGV) Strains" Microorganisms 12, no. 10: 1991. https://doi.org/10.3390/microorganisms12101991
APA StyleTsygichko, A. A., Asaturova, A. M., Lakhova, T. N., Klimenko, A. I., Lashin, S. A., & Vasiliev, G. V. (2024). Biocontrol Potential of the New Codling Moth Granulovirus (CpGV) Strains. Microorganisms, 12(10), 1991. https://doi.org/10.3390/microorganisms12101991







