1. Introduction
Two flagship missions are under development to explore the surfaces of Mars and Europa and eventually return samples from both planetary bodies. Both missions have the potential to detect extant or extinct life elsewhere in the solar system. The Perseverance rover is the first of three spacecraft within the Mars Sample Return (MSR) mission architecture [
1,
2,
3] and is currently collecting and caching rock, regolith, and aeolian dust samples in the Jezero Crater. Cached samples will be collected by a future Sample Return Lander (SRL) and launched into low Mars orbit (LMO) for pick-up by an Earth Return Orbiter (ERO). Earth-to-Mars transits for all three spacecraft will take between 6 and 8 months, and the first two vehicles will operate on the Martian terrain for many months. In contrast, the Europa Clipper (EC) mission is the first of multiple missions to Jovian space to map, land, and potentially return Europan samples to the Earth [
4,
5,
6]. The EC spacecraft will follow a 6.5-year Earth-to-Jupiter cruise phase that will include one flyby of Venus and two flybys of the Earth for gravity assists (i.e., called the VEEGA trajectory).
Both missions will be exposed to five dominant biocidal space conditions during transit including high vacuum, extreme desiccation, solar heating, solar UV irradiation on external surfaces, and ionizing radiation [
7]. The MSR Perseverance rover and the SRL spacecraft will be further exposed to up to 20 biocidal conditions present on the Martian surface including solar UV irradiation, desiccation, volatile oxidants, low pressure, high salt concentrations, extremes in dust pH, an anoxic CO
2-dominated atmosphere, and periodic solar particle events (to mention a few) [
8,
9,
10,
11]. The Mars landers during transit will be encased within cruise-phase systems with heat shields and back shells protecting most surfaces from direct solar heating and solar UV and thus partially shield the spacecraft from the space biocidal conditions listed above. In contrast, the EC and ERO spacecraft will be partially or fully deployed during their interplanetary cruise phases and will receive significantly greater amounts of solar heating and solar UV irradiation during transit.
In order to protect the scientific integrity of both mission architectures, planetary protection protocols (PPs) have been (Perseverance and EC spacecraft; e.g., [
12,
13,
14,
15]), and will be, developed to (i) minimize pre-launch bioburdens on critical subsystems that will directly handle samples; (ii) sterilize and place, behind biobarriers, criticality-1 subsystems that must be free of terrestrial contamination prior to deployment on surface terrains; (iii) streamline collection and caching activities to prevent the recontamination of pre-sterilized components; (iv) select unique materials for specific surfaces to compliment the mitigation of recontamination; and (v) develop quantitative models to predict the inactivation of adhered bioburdens during interplanetary flight. Regarding the latter PP component, three quantitative models for microbial survival in interplanetary space have been developed [
16,
17,
18]. The study by Dillon et al. [
16] focused on thermal issues related to landed spacecraft on the Moon. The Lunar Microbial Survival (LMS) model [
17] included the interactive effects of solar heating, solar UVC irradiation, vacuum, and ionizing radiation on microbial survival on the Moon. And the Cruise-Phase Microbial Survival (CPMS) model [
18] extended the LMS model to predict bioburden inactivation rates for interplanetary spacecraft traversing out to >10 astronomical units (AU). The CPMS model predicts that synergism between solar heating and vacuum for internal surfaces will dominate as biocidal factors out to approx. 10 AU, after which vacuum alone will dominate because of the extremely low cryogenic temperatures beyond the orbit of Saturn [
18]. Solar UV irradiation contributes to the sterilization of external spacecraft surfaces out to 100 AU and beyond [
18].
The primary objectives of the current research were to (i) characterize the biocidal effects of three interactive factors under simulated space conditions applied concurrently to spores of three Bacillus spp.; (ii) determine whether the concomitant effects of vacuum (VAC), simulated solar heating (HEAT), and simulated solar ultraviolet irradiation (UV) exhibited additive or synergistic relationships for killing Bacillus spp. spores; and (iii) develop datasets that can be used to validate the LMS and CPMS models and initiate the development of a Mars Microbial Survival (MMS) model. The results are expected to directly impact predictions on how microbial bioburdens can be inactivated on multiple spacecraft within the MSR and EC mission architectures.
Three
Bacillus spp. were selected for this research because their dormant spores are highly resistant to the biocidal conditions in interplanetary space [
7,
8,
15] and they are used as proxies for monitoring the total bioburdens on spacecraft surfaces [
19].
Bacillus subtilis was selected because it is a lab standard for planetary protection research,
B. pumilus was selected as a UV-resistant species, and
B. atrophaeus was selected as a heat-tolerant species [
19].
3. Results
Four experiments were conducted to characterize the synergistic interactions among three space biocidal factors (i.e., VAC, HEAT, and UV) encountered during the interplanetary travel of spacecraft. The research was based on several key microbial assumptions for interplanetary spacecraft, including the following: (i) spores on spacecraft surfaces occurred as individual propagules adhered to surfaces as monolayers [
13,
23]; (ii) the potential effects of multi-layered aggregates [
23] of spores were ignored (iii) because
Bacillus spp. endospores are significantly more resistant to space biocidal conditions than non-spore-forming species [
7], the sterilization of vegetative cells was assumed to occur at faster rates for non-spore-forming species, and (iv) spores on external spacecraft surfaces are not shielded from UV exposures during interplanetary travel.
The results across all four experiments were similar. First, two controls were included in each experiment and were composed of (i) a lab control maintained at 1013 mbar (i.e., average sea level pressure) and room temperature (between 22–25 °C) and (ii) a low-pressure control within the PAC that was maintained at 10
−2 mbar (Exp-1) or 10
−6 mbar (Exp-2, -3, and -4) and room temperature. In all four experiments, these two controls exhibited similar and not significantly different survival plots (
Figure 3,
Figure 4 and
Figure 5 and
Figure 7). The results indicated that there was no apparent biocidal effect of VAC alone on spores of all three
Bacillus spp. (
p > 0.05). Second, the biocidal effects of HEAT or UV irradiation were significantly enhanced when VAC was included in the experimental conditions.
Based on comparing the slopes of the inactivation plots for the HEAT-only assays in Exp-1,
B. atrophaeus 9372 was the most resistant to high temperature alone (
Figure 3A) followed by
B. subtilis 168 (
Figure 3C) and
B. pumilus SAFR-032 (
Figure 3B). In contrast, when considering the synergistic effects of adding high vacuum to the assays, the order of resistance to VAC + HEAT was observed as
B. atrophaeus ≈
B. subtilis >
B. pumilus.
Vacuum-plus-UV-irradiation assays were split into two different experiments in which a low UVC flux of 0.672 W m
−2 was tested in Exp-2 (
Figure 4; similar to 3.16 AUs in the asteroid belt) and a high UVC flux of 3.6 W m
−2 was tested in Exp-3 (
Figure 5 and
Figure 6; similar to Mars perihelion in LMO). The two UVC fluence rates were tested because previous work [
26] had established extremely rapid inactivation rates for seven
Bacillus spp. when exposed to LMO-like UVC rates, and thus, synergism between VAC and UV would have been much more difficult to examine if only the LMO rate of 3.6 W m
−2 was used. Furthermore, the low UV flux would yield data applicable to interplanetary spacecraft traveling through the asteroid belt [
18].
Results for the VAC + low UVC flux demonstrated only moderate inactivation kinetics for UVC exposures when conducted at lab-normal pressures of 1013 mbar for all three
Bacillus spp. tested (
Figure 4). In contrast, when vacuum levels close to 10
−6 mbar were concurrently used in the VAC + low UVC assays, synergistic interactions were observed that increased the lethalities at 30 min of UV exposure to −5.5 logs for
B. pumilus and −4.5 logs for
B. subtilis and
B. atrophaeus. Thus, the order of resistance to VAC + low UVC was observed to be
B. atrophaeus ≈
B. subtilis >
B. pumilus.
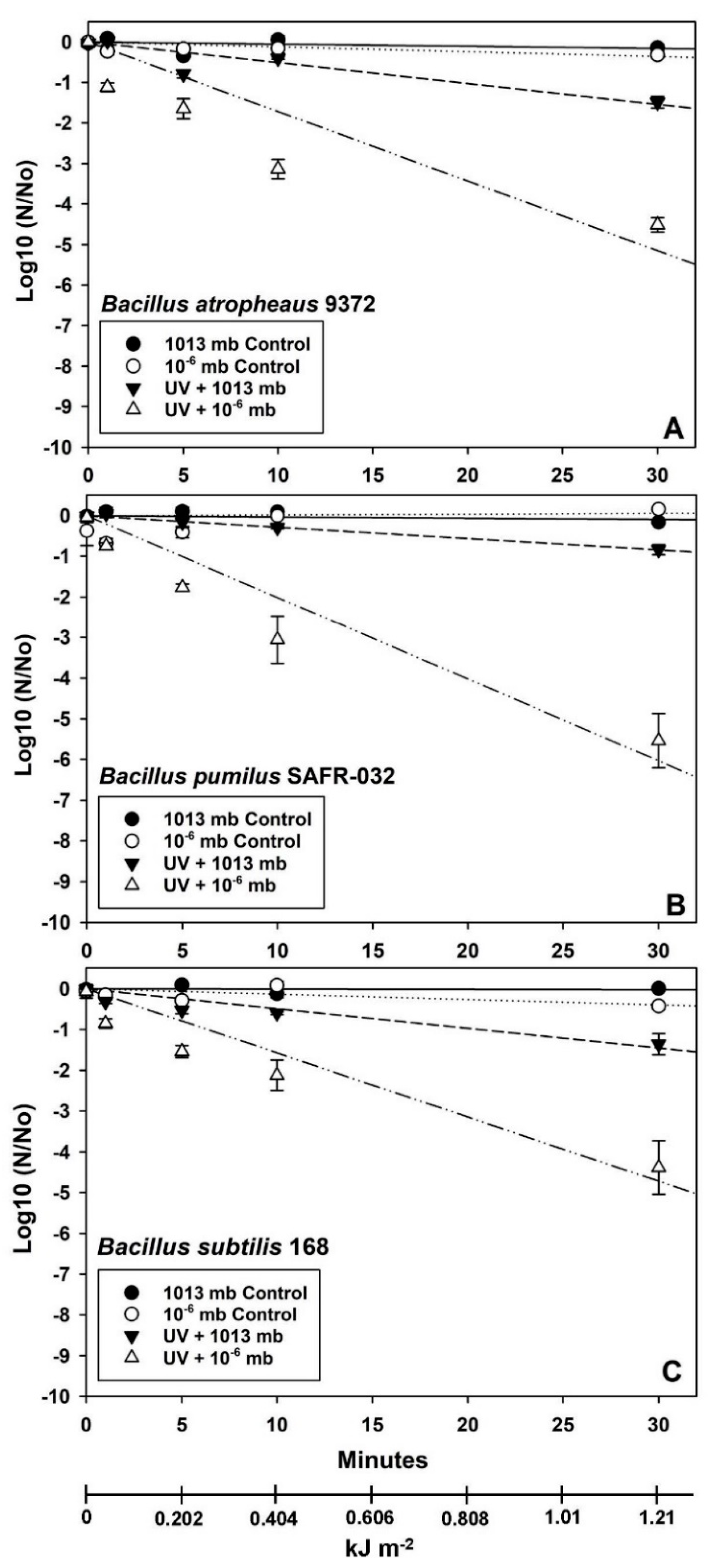
In contrast, the VAC + high UVC assays exhibited significantly greater losses of spore viability in which the biocidal effects of adding VAC to the high UV flux were similar among the three
Bacillus spp. (
Figure 5). The order of resistance to VAC + high UVC appeared to be
B. subtilis >
B. pumilus >
B. atrophaeus. Furthermore, the interactive effects of VAC + high UVC generally yielded losses of viability between −4 to −6.5 logs for all three
Bacillus spp. after only 5 min of LMO simulations.
All data for Exp-1 (
Figure 3) and Exp-2 (
Figure 4) were best represented by linear models over the plotted ranges of the independent variables. In Exp-3 (
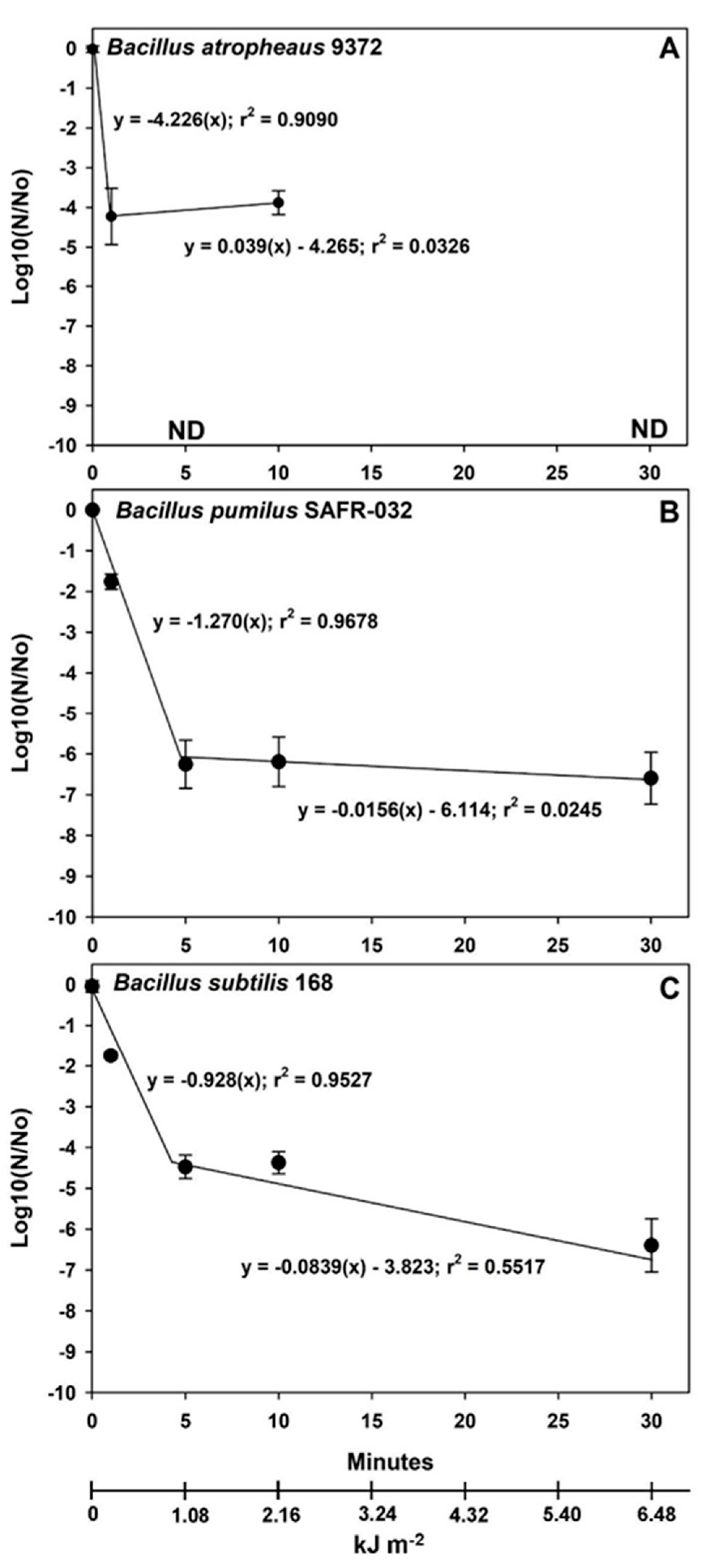
Figure 5), linear models best represented the (i) lab controls, (ii) internal PAC vacuum controls, and (iii) high UVC irradiation at lab atmospheric pressures of 1013 mbar. In contrast, the bioburden reductions for the VAC + high UV exposures followed biphasic responses in which distinct inflection points (i.e., called a spline point) were observed between the 1st phases (i.e., steep slopes) between 0 and 5 min and the 2nd phases (i.e., shallower slopes) between 5 and 30 min (
Figure 6). In general, this ‘
tailing effect’ might be represented by logarithmic decay plots that smoothly transition through the inflection points given in
Figure 6. However, a single logarithmic decay plot implies that a single biocidal factor is acting upon survival through the transition from an initial rapid inactivation rate to a more refractory inactivation process.
It is proposed here that logarithmic decay plots do not accurately represent what is occurring biologically to dried spores on simulated spacecraft materials. It is proposed that the 1st phase represents the inherent biocidal effect of the stressor(s) on spore survival (i.e., VAC + high UVC in this case) and the 2nd phase represents shadowing effects by
Bacillus spores caught in surface defects or stacked into multi-layers on the aluminum coupons (see [
22,
23,
26]).
Figure 6 gives the biphasic linear plots for the VAC + high UVC irradiation data in Exp-3. In all three
Bacillus spp., the initial 1st phase plots exhibited extremely steep slopes indicating that the inherent biocidal sensitivity of the spores to the interplanetary simulation would follow fast inactivation rates for sun-exposed spacecraft surfaces. The 2nd phases captured shadowing ‘
outliers’ that did not necessarily represent sensitivity to UVC irradiation.
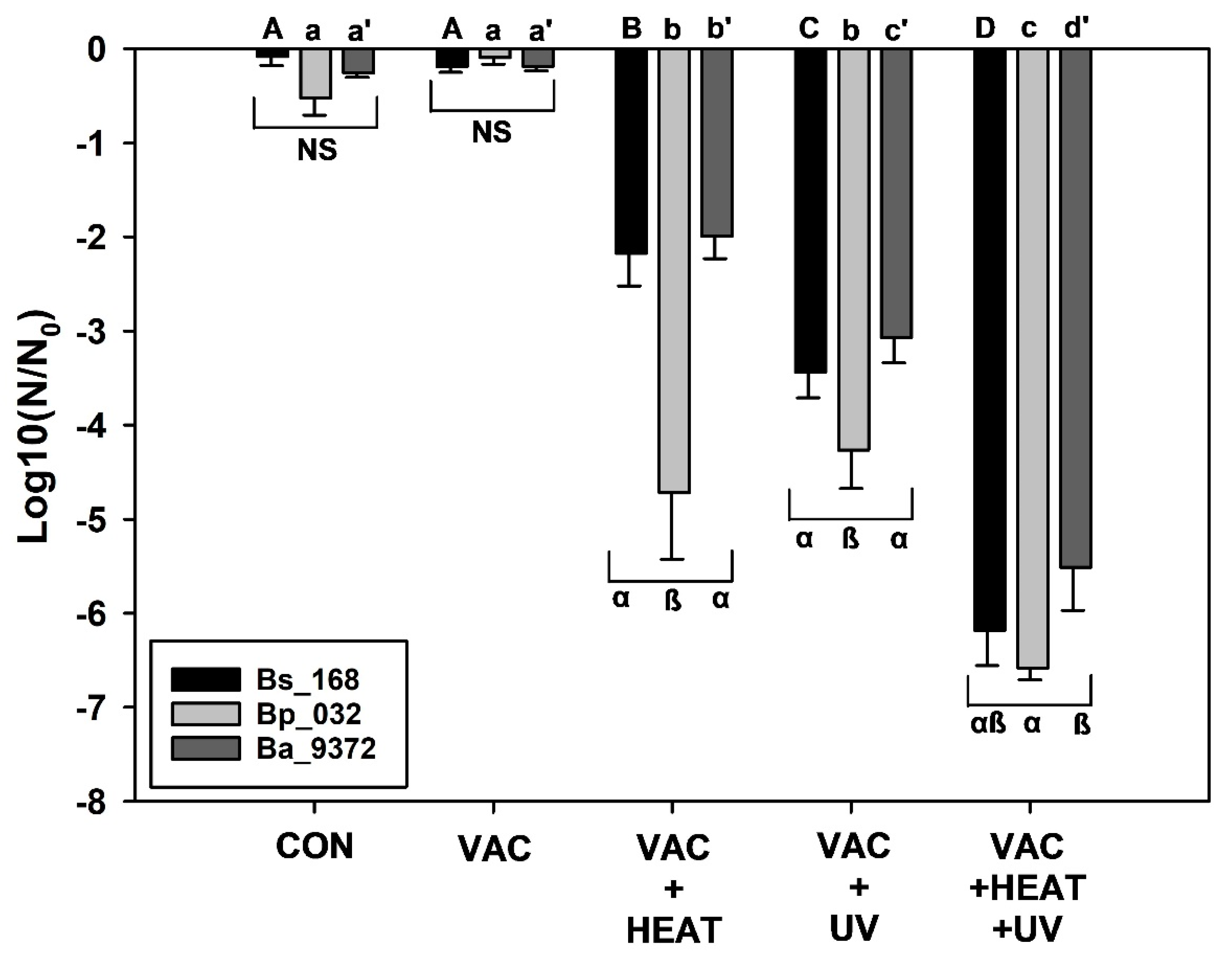
Lastly, a three-factor experiment was conducted that combined sublethal doses of VAC + HEAT + high UVC irradiation to examine the interactive effects of these space biocidal conditions on spores of three
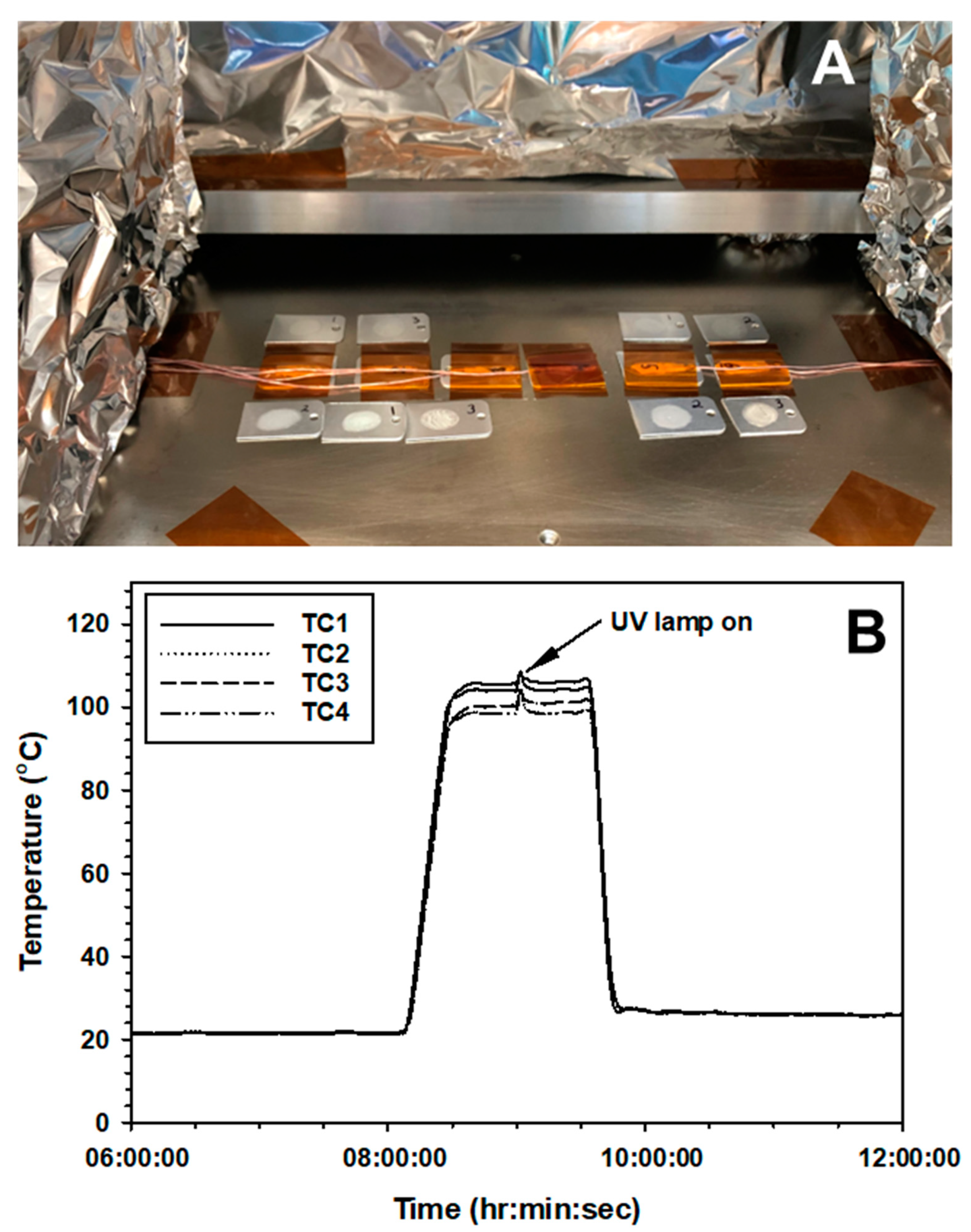
Bacillus spp. Based on the results in Exp-1, -2, and -3, sublethal doses of each parameter were chosen. The VAC exposure was for 48 h in the PAC system for all assays because VAC alone did not appear to induce any discernable biocidal effect in earlier experiments. The HEAT treatment was for only 1.25 h (with short 30 min ramps for heating and cooling; see
Figure 2B). And the high UVC irradiation exposure was for only 2 min. These short exposures for HEAT and high UVC should have yielded inactivation rates of less-than −1 log for HEAT for all three
Bacillus spp. (based on
Figure 3) and approx. −2 to −4 logs for high UVC for all three species (based on
Figure 5). However, the combinations of the three sublethal doses of the simulated space factors yielded approx. −6.5 to −7 logs of biocidal activity for all three
Bacillus spp. (
Figure 7).
A Sterility Assurance Level (SAL) can be defined as a bioburden reduction of greater than −12 logs achieved by determining the biocidal effect of a sterilizing protocol on a viable population of 1 × 10
6 spores (i.e., −6 logs) and extrapolating the inactivation rate to −12 logs [
19,
27,
28]. SAL predictions (
Table 1) for the plots in
Figure 3,
Figure 4 and
Figure 5 were generated by dividing −12 logs (i.e., the desired bioburden reduction; 1 SAL) by the negative slope values given in
Table S6. SAL values were only generated for the combinations of synergistic factors in Experiments 1, 2, and 3. However, the approach can be used for all linear models with negative slopes.
SAL predictions to achieve bioburden reductions of −12 logs exhibited very short time-steps for all three
Bacillus spp. against exposures to VAC + HEAT, VAC + low UV, and VAC + high UV (
Table 1). In Exp-1, which was on the interactive effects of VAC + HEAT (
Figure 3),
B. pumilus exhibited the shortest SAL time-step of 22.8 h to reach −12 logs. In the VAC + low UV experiment (Exp-2;
Figure 4), the SAL predictions for all three
Bacillus spp. were similar and in the range of 59.7 to 76.3 min. For Exp-3 (
Figure 5) (i.e., VAC + high UV), the 1 SAL predictions were extremely fast and on the order of 2.84 min (
B. atrophaeus), 9.45 (
B. pumilus), and 12.94 min (
B. subtilis).
SAL predictions for the synergistic interactions among the three space biocidal conditions of VAC + HEAT + UV are a bit more cumbersome to derive because the results are not amenable to generating linear models. However, as a first-order prediction, if most or all of the replicates exhibited non-detects in the VAC + HEAT + UV assays (see
Table S5), doubling the time-steps for each factor might allow a SAL prediction. Recall that for Exp-4, the VAC was held at 10
−6 mbar for 48 h, the HEAT was raised to 100 °C for 1.25 h, and the high UVC flux of 3.6 W m
−2 was applied for 2 min. A 1 SAL prediction of these three factors might be the equivalent of doubling the times for HEAT (e.g., 2.5 h) and high UVC (e.g., 4 min). VAC-only effects are ignored here because the results from all four experiments support the conclusion that VAC had little to no effect when tested alone. Thus, external spacecraft surfaces within the inner solar system would achieve hundreds to thousands of SAL exposures within a few weeks to a few months in space.
4. Discussion
Synergistic interactions among multiple space biocidal conditions provide more accurate predictions on the lethality of the interplanetary environment than single-factor experiments. However, multi-factorial assays are often difficult to complete due to rapidly increasing numbers of combinations of the biocidal factors. For example, if three factors are considered for an assay, the total number of possible combinations are eight (i.e., 2N = 23 = 8 combinations). Four factors quickly escalate to 16 combinations (i.e., 24 = 16). Furthermore, selecting the dominant factors to test often leads to significant knowledge gaps for more subtle interactions. In the current study, the interactive effects of vacuum (VAC), simulated solar heating (HEAT), and simulated solar UV irradiation (UV) were examined in various combinations to examine the potential lethality of interplanetary space on three spore-forming Bacillus spp. commonly recovered from spacecraft surfaces.
Vacuum alone was found to have no significant effect on the survival of
B. atrophaeus,
B. pumilus, or
B. subtilis over the course the short-term exposures tested here compared to the lab controls maintained at a sea level pressure of 1013 mbar (
p > 0.05) (
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7). However, longer-term exposures to space vacuum have reported between −0.5 to −2 logs for 6 and 69 months for
B. subtilis spores maintained as dried monolayers on metal coupons (see
Figure 1 and references in Schuerger et al. [
17]). All interactive assays described below were conducted under a background pressure of 0.05 (
Figure 3) to 10
−6 (
Figure 4,
Figure 5,
Figure 6 and
Figure 7) mbar maintained for 48 h. There was no evidence in the current experiments, or in the literature reviewed (see [
29,
30]), that the difference between 0.05 and 10
−6 mbar had affected the results described herein.
In contrast, in all VAC + HEAT assays (
Figure 3 and
Figure 7), the presence of vacuum increased the lethality of high temperature (i.e., 100 °C tested here) by as much as −6.5 logs for
B. pumilus and approx. −4 to −5 logs for
B. atrophaeus and
B. subtilis. Thus, the synergism between VAC + HEAT had a significantly greater effect on the UV-resistant strain of
B. pumilus SAFR-032 as compared to the other two
Bacillus spp. The results are consistent with other studies in which vacuum increased the lethality of high temperatures at 59–60 °C by −0.5 logs [
31,
32,
33] and at 100 °C by −6 logs [
17]. In one interesting study, the synergistic effects of vacuum applied during long-term heating assays against the heat-tolerant
Bacillus sp. ATCC 29669 boosted the overall lethality of high temperatures between 125 and 170 °C by several to many orders of magnitude under vacuum [
34]. However, synergism appeared to abate when vacuum and lab-air samples were exposed to 200 °C [
34]. And finally, incubating
B. subtilis spores at −193 °C (80 K) increased survival significantly compared to room temperatures of 20 °C when both were under vacuum [
31]. These data support the conclusion that the lethality of warmer temperatures increases under vacuum compared to similar tests under lab pressures closer to 1013 mbar.
The inactivation kinetics of
Bacillus spp. spores exposed to VAC + HEAT simulations followed linear models as presented in
Figure 3 here—or elsewhere [
17,
31,
32,
34]—suggesting that viable outliers do not persist beyond the linear extensions of the models when samples are exposed to high temperatures under vacuum.
Synergism was also observed between VAC + low UV simulations (
Figure 4; UV flux at 3.16 AU within the asteroid belt) and between VAC + high UV simulations (
Figure 5; UV flux at 1.36 AU close to perihelion for Mars), exhibiting as much as −5 to −7 logs of increased lethality for all three
Bacillus spp. at 1, 5, and 10 min of UV exposure. The results were consistent with a sizeable body of literature depicting synergism between UV irradiation and vacuum-tested against diverse
Bacillus spp. [
35,
36,
37,
38,
39,
40,
41]. Synergism between VAC and UV has also been reported for the non-spore-forming bacteria
Escherichia coli [
42,
43] and
Deinococcus radiodurans [
43]. The levels of increased lethality were typically several orders of magnitude in these studies depending on the lengths of exposure to the combined VAC + UV conditions.
When all three space factors were combined into one assay (Exp-4), the overall bioburden reductions reached approx. −7 logs (
Figure 7) after only brief exposures to HEAT (i.e., only 1.25 h at 100 °C) and UV (i.e., only 2 min during the heat-pulse) in a VAC background of 10
−6 mbar for 48 h.
Figure 2B depicts one example of the overall timing of these three factors. Longer times for either HEAT or UV yielded 100% inactivation for all test samples during the three-factor experiments, meaning that no detectable viable spores were recovered. Exp-4 (i.e., VAC + HEAT + UV) required sublethal doses of each parameter to test for synergism. To measure synergistic interactions with predictive knowledge on how each factor has contributed to the overall biocidal effect, only experiments with sublethal dosages can be run. For example, if a 24 h concomitant VAC + HEAT + UV simulation were conducted, how could one resolve the contribution of each factor when results yielded zero survivors?
Linear models were chosen here to represent the inactivation kinetics for combinations of VAC, HEAT, and UV exposure. In most cases, the linear models fit the data best. However, exceptions for biphasic plots for VAC + high UV are noteworthy. For spores of all three
Bacillus spp., very steep inactivation kinetics were observed for the 1st phases in the high UV exposures up to approx. 5 min followed by a tailing effect due to single-digit surviving
outliers at longer time-steps (
Figure 6;
Table S4). Schuerger et al. [
26] reported similar effects of tailing when seven
Bacillus spp. were exposed to a simulated Mars surface UVC flux, but eventually, they were able to sterilize aluminum coupons after longer exposures. Tailing is generally not observed for the heat sterilization of microbes but can be observed with UV sterilization processes due to the subtle shading effects of spores entrapped in pits, cracks, other surface defects or present as multi-layered spore aggregates [
23].
Synergism works to greatly enhance the lethality of the space environment on spacecraft bioburdens. The results presented herein focused on the interactive effects of three space conditions including vacuum, simulated solar heating, and simulated solar UV irradiation. The results were consistent across all assays, and synergism was observed in all multi-factor experiments. In addition, these results are consistent with a large body of literature; some of which have been cited above. In addition, synergism has been described between HEAT and UV exposures for the fungus
Aspergillus nidulans [
44]. Interestingly, one study on the interactions between vacuum and ionizing radiation (IRAD) demonstrated a sort of a “positive synergistic interaction” in which spores of
Bacillus megaterium,
B. subtilis var.
niger,
Clostridium sprorgenes, and
Aspergillus niger were found to survive better under γ-radiation when spores were exposed under high vacuum of approx. 10
−9 mbar versus lab air [
45]. Presumably, the γ-rays ionized the gas molecules in the lab air, forming O
−, O
2−, and/or NOx
− radicals, which contributed to the biocidal conditions within the sealed glass vessels.
The results presented here can be used to estimate Sterility Assurance Levels (SALs) for spacecraft surfaces exposed to combinations of VAC, HEAT, and UV irradiation during interplanetary transits. The medical equipment industry’s standard approach [
19,
27,
28] to estimating SALs is to estimate the times required to inactivate 1 × 10
6 spores by a specific sterilization protocol and then double those exposure times (i.e., thus achieving 10
−12 bioburden reductions; syn. here with −12 logs).
Table 1 presents SAL estimates for all linear models in
Figure 3,
Figure 4 and
Figure 5. The SALs in
Table 1 were estimated by extending the linear models to −12 logs and are similar to what can be derived following the industry standard protocol outlined above.
SAL estimates for the slowest combination of VAC + low UV (
Table 1) approached 76 min of exposure for sun-facing surfaces. The fastest times to one SAL were observed for the linear plots in
Figure 5B and 5C at 2.8 and 9.5 min for
B. atrophaeus and
B. pumilus, respectively. Overall, these inactivation kinetics are in agreement with survival rates given for the Lunar Microbial Survival (LMS) [
17] and Cruise-Phase Microbial Survival (CPMS) [
18] models. In the LMS, when all factors were combined into a single prediction per lunation (14.77 d of solar illumination in each 29.53 d lunation) for lunar spacecraft, as many as −2479 logs of bioburden reduction were possible. The value equates to an accumulation of 207 SALs (i.e., [−2479 logs] ÷ [−12 logs per SAL]) for external spacecraft surfaces on the Moon per lunation. Thus, single SALs are expected to occur over extremely short periods of time within the inner solar system (
Table 1; [
18]), and extremely high numbers of accumulated SALs can be achieved over a few weeks to months when spacecraft surfaces are exposed to vacuum, solar heating up to at least 100 °C, and solar UV irradiation.
Results suggest that the VEEGA trajectory for the Europa Clipper (EC) spacecraft will keep the EC spacecraft within the inner solar system for up to 3.5 years [
5], potentially accumulating in excess of 1.7 × 10
4 SALs on external surfaces (i.e., 207 SALs for every 14.77 d of solar illumination on the Moon in the LMS model multiplied by 3.5 years) during exposure to cis-lunar/LEO conditions or higher (i.e., closer to Venus). In addition, even after leaving the 1–2 AU environment, the EC spacecraft’s external surfaces will continue to accumulate SAL exposures throughout the mission, increasing the confidence that launched bioburdens from the Earth are unlikely to impact the mission science [
18]. Bioburden reductions on internal spacecraft surfaces will depend on the thermal conduction of heat into interior spaces [
17] and on the overall long-term effects of VAC on the lethality of the interplanetary environment [
18].
Regarding the MSR mission architecture, the best environment in which vacuum, solar heating, and solar UV will impact microbial survival is during operations in LMO for the Orbital Sample (OS) holding device and the external surfaces of the ERO spacecraft. However, no quantitative model has yet been developed to precisely model the LMO, ERO, or OS environments in regard to how quickly each phase of the MSR mission will accumulate adequate SAL values for mitigating both forward and back contamination.
And finally, what is the most biologically relevant method to represent bioburden reductions on spacecraft surfaces when the datasets are dominated by numerous
non-detect values? Retaining treatments that were 100%
non-detects in the plots acted to artificially flatten the linear models by artificially creating plotted means from randomly generated values in the datasets. For example, there are missing treatments for most of the 48 h plots for VAC + HEAT in
Figure 3. Including the
non-detects when all replicates were below the detection limit of the MPN assays would have implied that some spores survived around −6 to −7 logs depending on the random numbers used. If a treatment series like 12, 24, and 48 h (e.g.,
Figure 3) is used—and all replicates in the 48 h treatments were
non-detects—we cannot interpret the overall lethality of the VAC + HEAT exposures after 24 h. Was full lethality achieved at 26, 32, 36.7, or 48 h?
Thus, caution must be taken to not bias the plotted data by inserting randomly generated values for non-detects in treatments in which 100% of all replicates have zero recovered spores. Such a process would create mean values of randomly generated data that implied some survival was present when in fact none was detected. In contrast, random numbers were used for non-detects in treatments in which at least one replicate had an actual detection of viable spores because now there was at least one positive detection of a few viable spores and the randomly generated values between 0 and 1 were good proxies for other non-detects in the data subsets.
The analytical problem arises when using log-transformations in ANOVA to induce homogeneity of treatment means. ANOVA cannot process zero values. However, if at least one replicate in a treatment has a positive detection of spores, the other non-detect replicates can be represented by randomly generated numbers between 0 and 1. This issue became a dominant bias in these experiments because there were numerous non-detects in most of the outlying VAC + HEAT and VAC + high UV assays.