Exploring Peripheral Blood-Derived Extracellular Vesicles as Biomarkers: Implications for Chronic Chagas Disease with Viral Infection or Transplantation
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Healthy Controls, Patients, Clinical Samples, and Laboratory Diagnosis
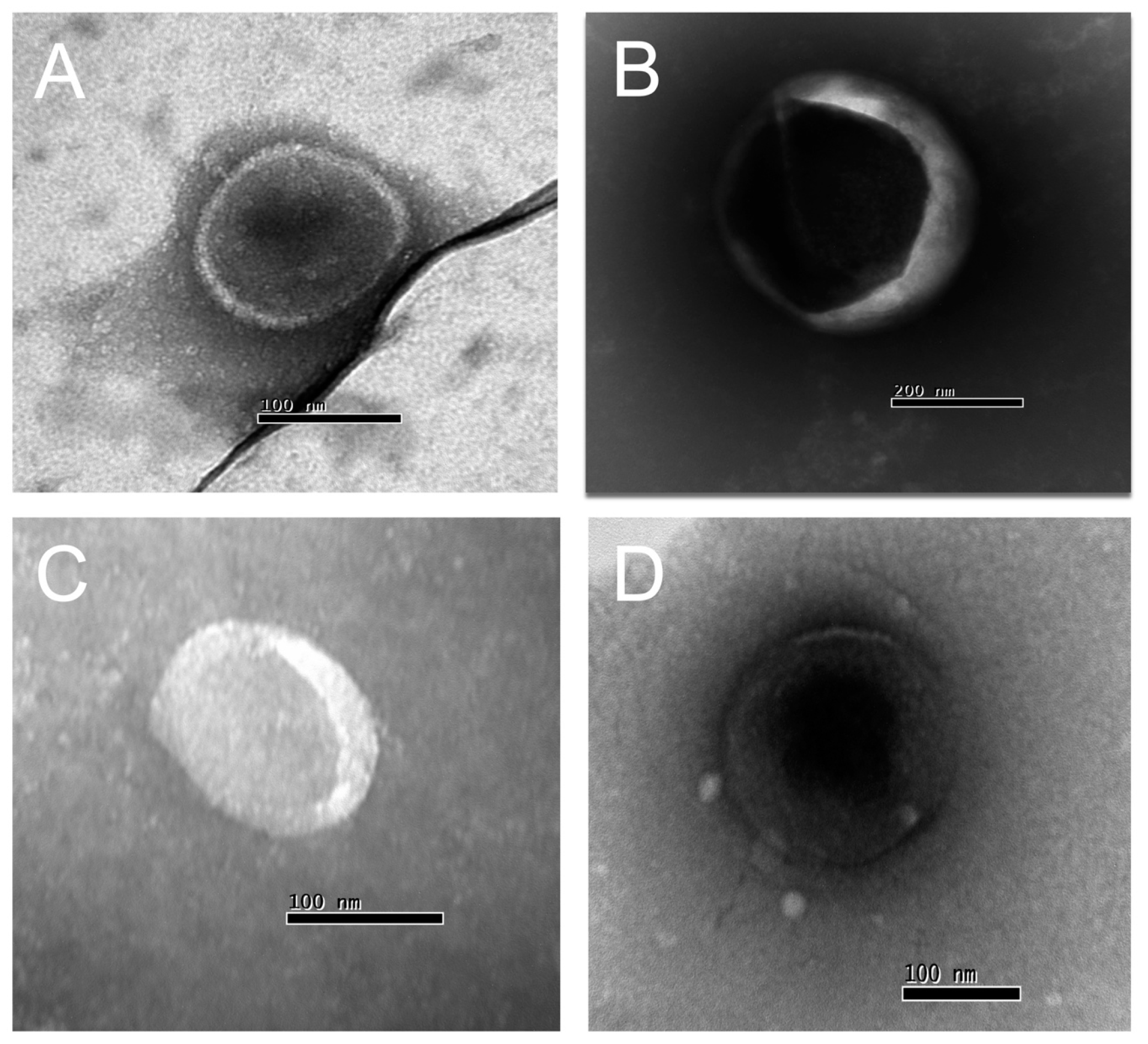
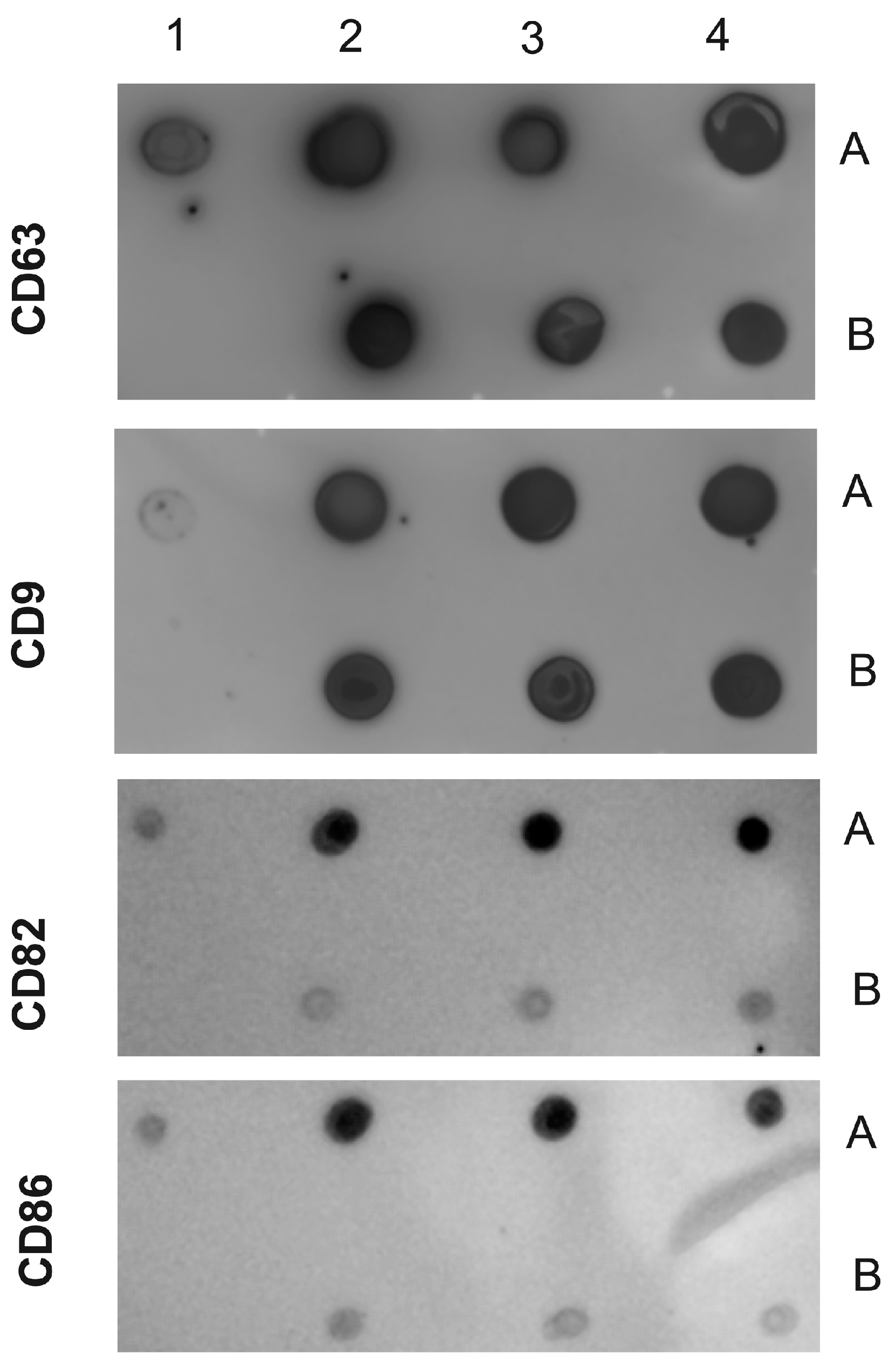
2.3. Isolation and Characterization of EVs from Human Plasma Samples from Healthy Controls and Patients
2.4. Nanoparticle Tracking Analysis (NTA)
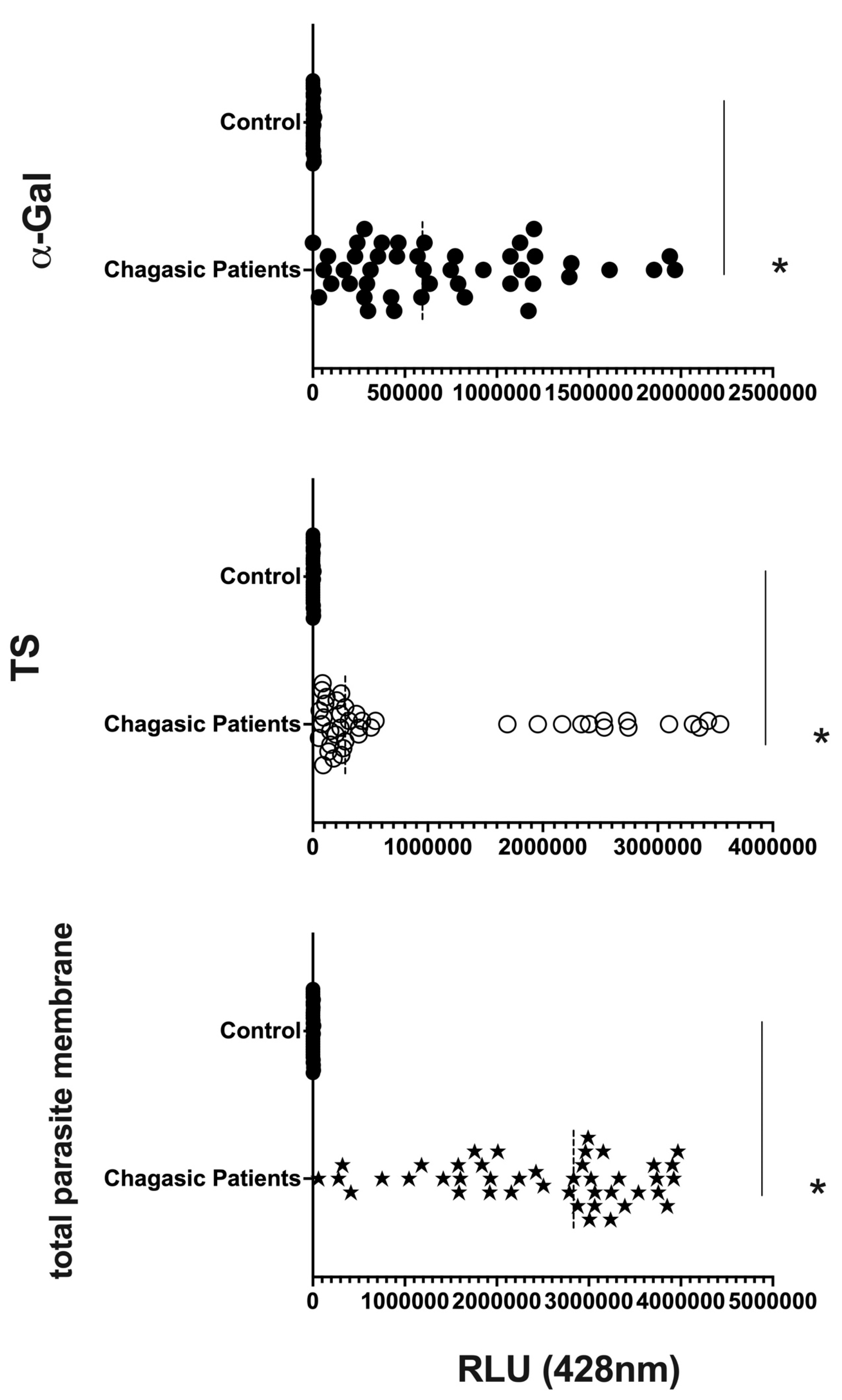
2.5. Chemiluminescent Enzyme-Linked Immunosorbent Assay (CL-ELISA) for the Detection of α-Gal, TS, and Parasite Membrane Molecules on EVs from Human Plasma from Healthy Individuals (Controls) and Patients
2.6. Data Analysis
3. Results
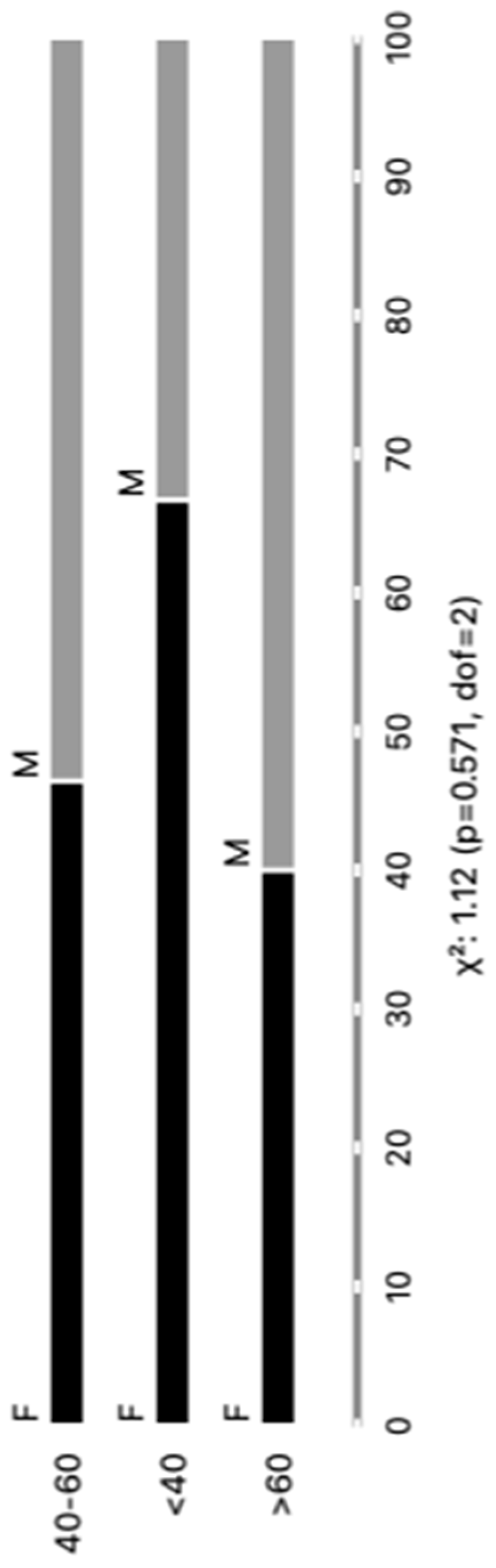
3.1. Distribution of Patients with CCD and Controls
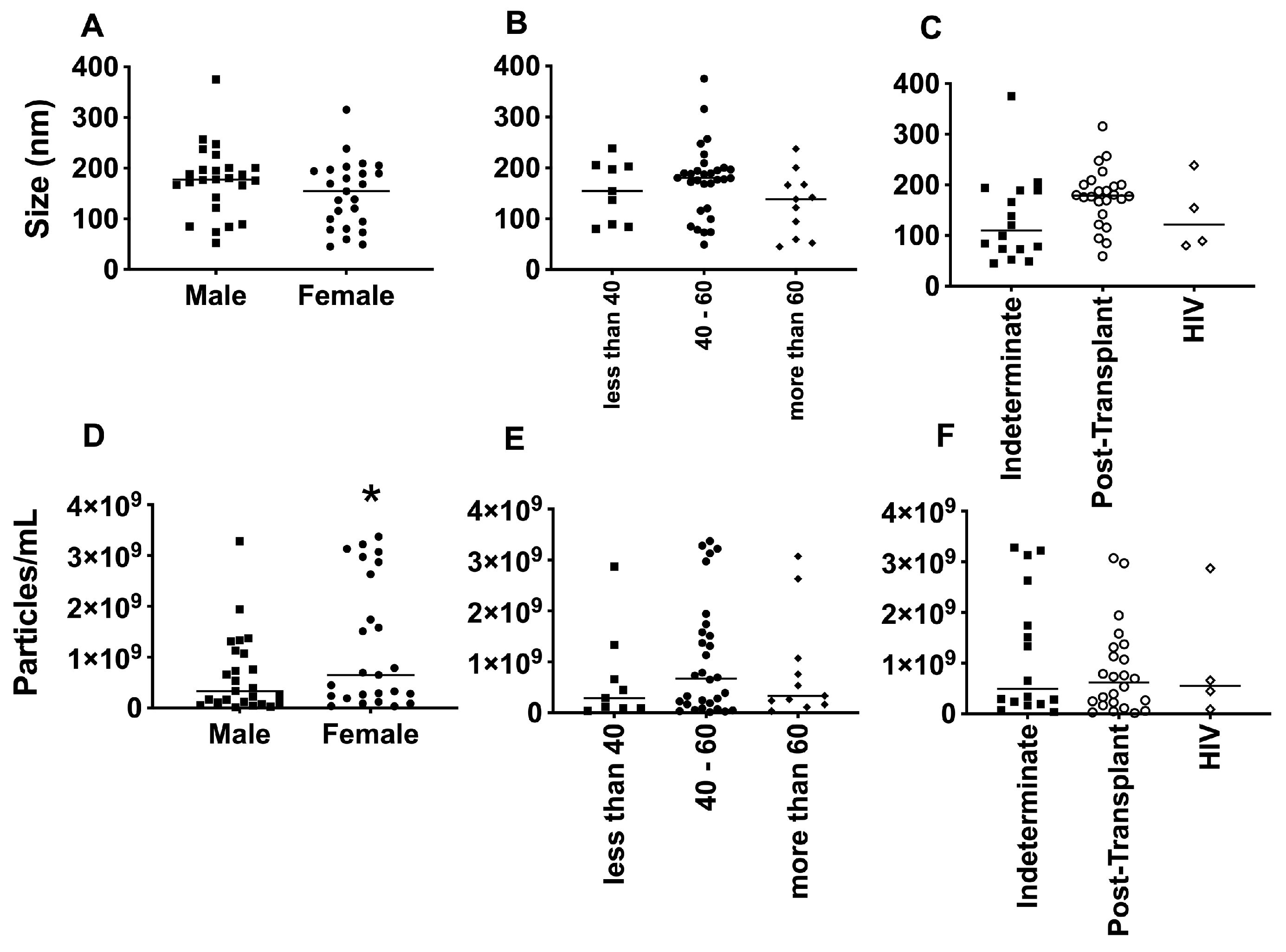
3.2. EV Size Did Not Differ Much among Groups, but Clusters of Patients Can Be Observed
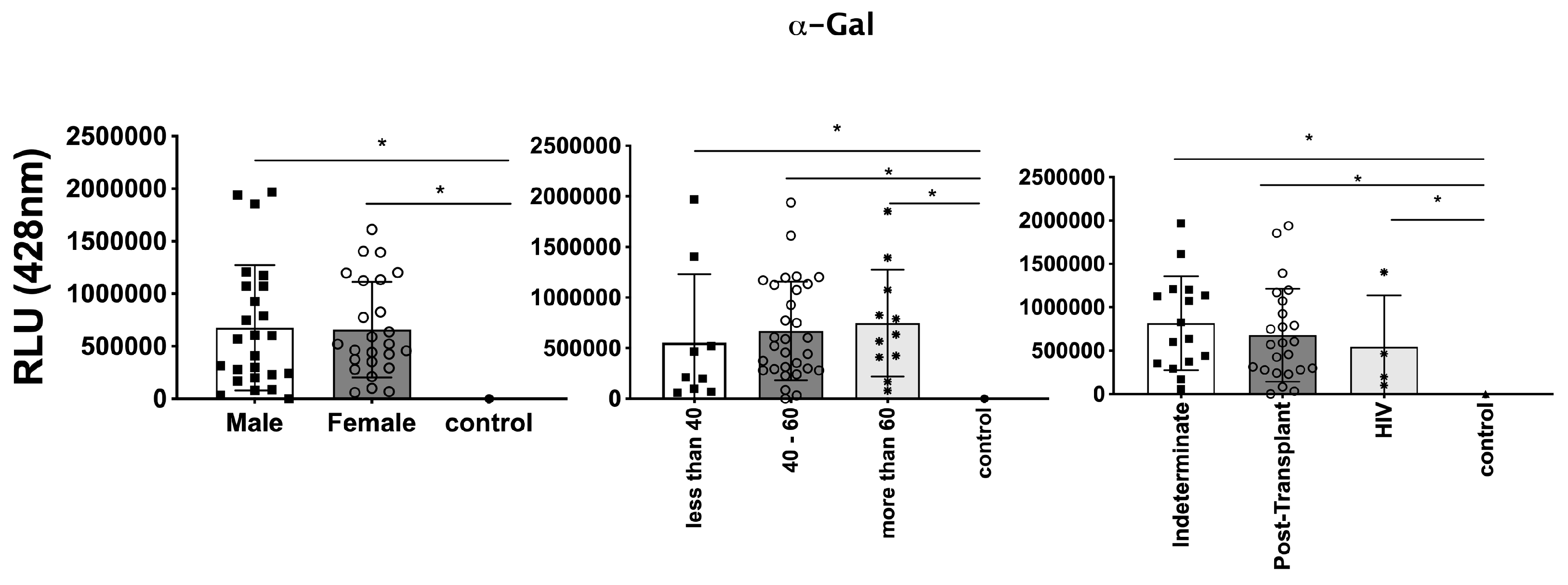
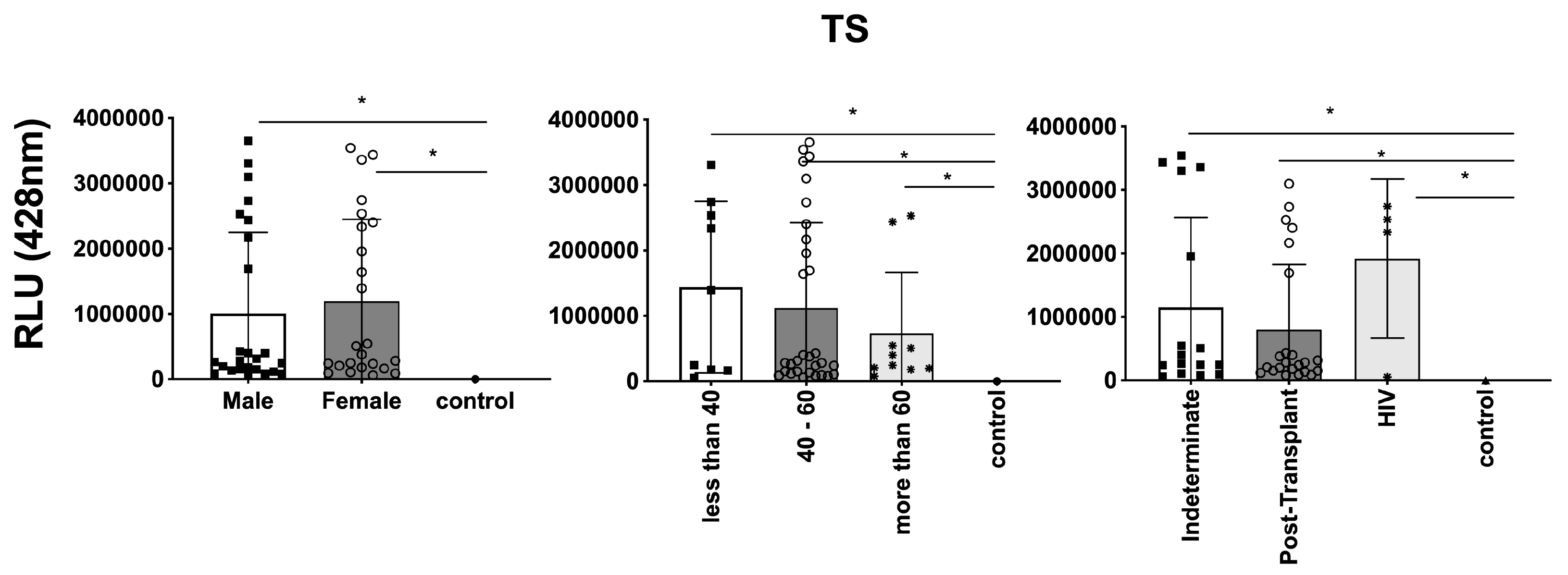
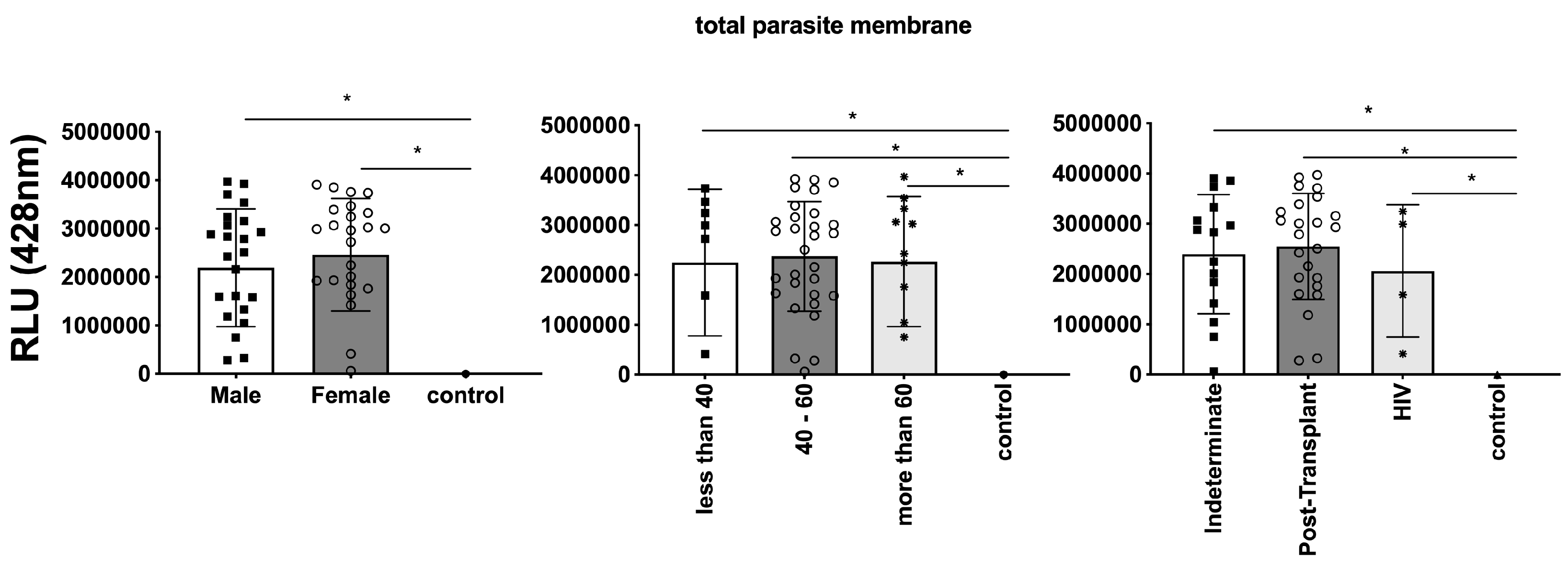
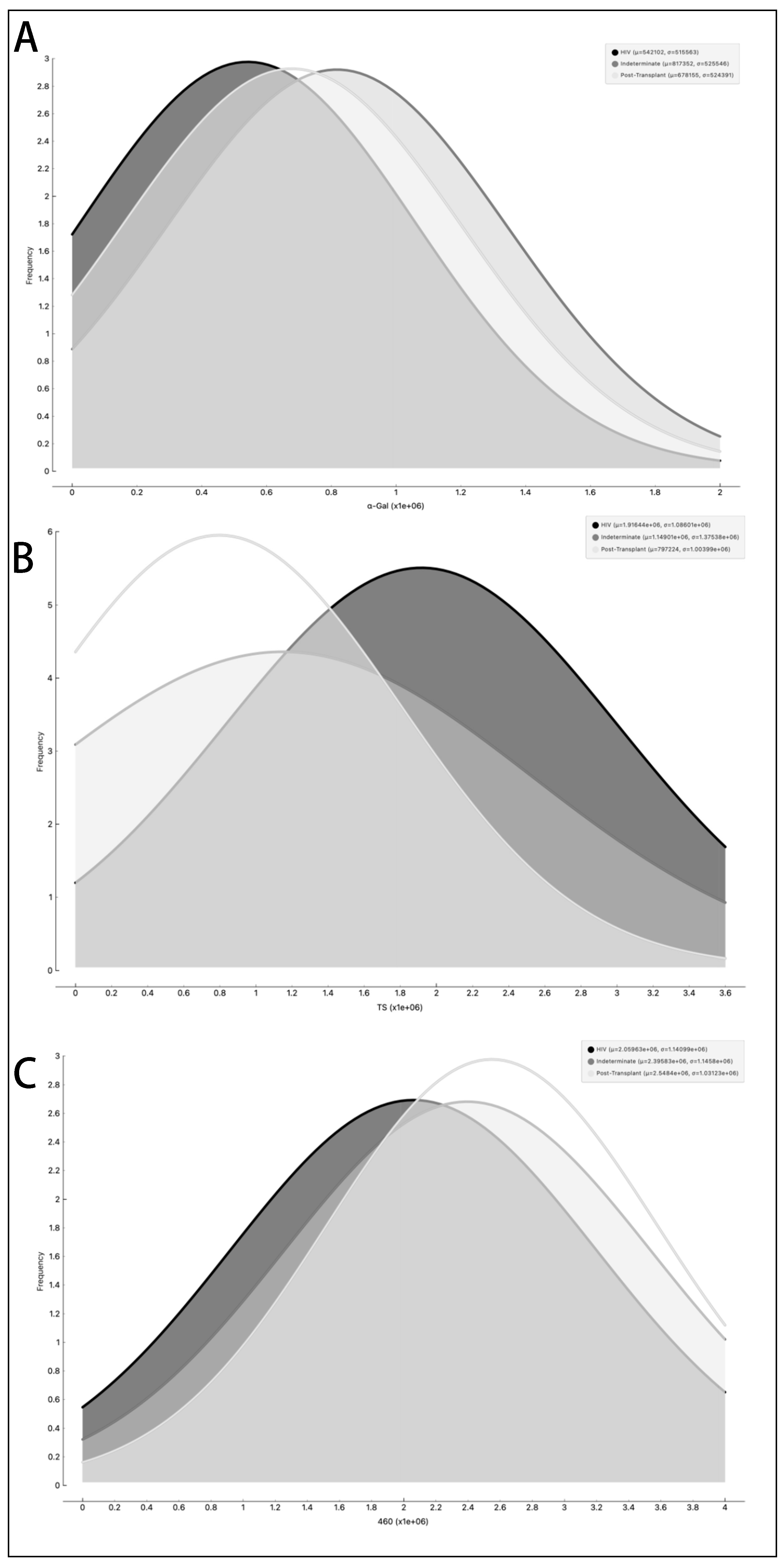
3.3. Differential Expression of Parasite Molecules Demonstrates Variability across Distinct Age Groups and Immunosuppression Categories
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCD | Chronic Chagas Disease |
| CL-ELISA | Chemiluminescent Enzyme-Linked Immunosorbent Assay |
| DNA | Deoxyribonucleic Acid |
| EDTA | Ethylenediaminetetraacetic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EV | Extracellular Vesicle |
| FAM | Fluorescein Amine |
| GPI | Glycosylphosphatidylinositol |
| HIV | Human Immunodeficiency Virus |
| HRP | Horseradish Peroxidase |
| IFN-y | Interferon gamma |
| MGB | Minor Groove Binder |
| NFQ | Nonfluorescent Quencher |
| NTA | Nanoparticle Tracking Analysis |
| PBS | Phosphate Buffer Solution |
| PCR | Polymerase Chain Reaction |
| qPCR | Quantitative Polymerase Chain Reaction |
| RLU | Relative Light Units |
| T. cruzi | Trypanosoma cruzi |
| TS | Trans-sialidase |
References
- Dias, J.C.; Silveira, A.C.; Schofield, C.J. The impact of Chagas disease control in Latin America: A review. Mem. Inst. Oswaldo Cruz 2002, 97, 603–612. [Google Scholar] [CrossRef] [PubMed]
- De Fuentes-Vicente, J.A.; Santos-Hernandez, N.G.; Ruiz-Castillejos, C.; Espinoza-Medinilla, E.E.; Flores-Villegas, A.L.; de Alba-Alvarado, M.; Cabrera-Bravo, M.; Moreno-Rodriguez, A.; Vidal-Lopez, D.G. What Do You Need to Know before Studying Chagas Disease? A Beginner’s Guide. Trop. Med. Infect. Dis. 2023, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Perez-Molina, J.A.; Perez, A.M.; Norman, F.F.; Monge-Maillo, B.; Lopez-Velez, R. Old and new challenges in Chagas disease. Lancet Infect. Dis. 2015, 15, 1347–1356. [Google Scholar] [CrossRef]
- Perez-Molina, J.A.; Molina, I. Chagas disease cardiomyopathy treatment remains a challenge—Authors’ reply. Lancet 2018, 391, 2209–2210. [Google Scholar] [CrossRef] [PubMed]
- Porta, E.O.J.; Kalesh, K.; Steel, P.G. Navigating drug repurposing for Chagas disease: Advances, challenges, and opportunities. Front. Pharmacol. 2023, 14, 1233253. [Google Scholar] [CrossRef] [PubMed]
- Abras, A.; Ballart, C.; Fernandez-Arevalo, A.; Pinazo, M.J.; Gascon, J.; Munoz, C.; Gallego, M. Worldwide Control and Management of Chagas Disease in a New Era of Globalization: A Close Look at Congenital Trypanosoma cruzi Infection. Clin. Microbiol. Rev. 2022, 35, e0015221. [Google Scholar] [CrossRef]
- Angheben, A.; Boix, L.; Buonfrate, D.; Gobbi, F.; Bisoffi, Z.; Pupella, S.; Gandini, G.; Aprili, G. Chagas disease and transfusion medicine: A perspective from non-endemic countries. Blood Transfus. 2015, 13, 540–550. [Google Scholar] [CrossRef]
- Assal, A.; Corbi, C. Chagas disease and blood transfusion: An emerging issue in non-endemic countries. Transfus. Clin. Biol. 2011, 18, 286–291. [Google Scholar] [CrossRef]
- Gascon, J.; Bern, C.; Pinazo, M.J. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010, 115, 22–27. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Gascon, J. The importance of the multidisciplinary approach to deal with the new epidemiological scenario of Chagas disease (global health). Acta Trop. 2015, 151, 16–20. [Google Scholar] [CrossRef]
- Monge-Maillo, B.; Lopez-Velez, R. Challenges in the management of Chagas disease in Latin-American migrants in Europe. Clin. Microbiol. Infect. 2017, 23, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.P.; Bastos, C.; Araújo, E.; Mascarenhas, A.V.; Martins Netto, E.; Grassi, F.; Silva, M.; Tatto, E.; Mendonça, J.; Araújo, R.F.; et al. Acute Chagas disease outbreak associated with oral transmission. Rev. Soc. Bras. Med. Trop. 2008, 41, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, A.; Gilabert, J.A. Oral transmission of Chagas disease from a One Health approach: A systematic review. Trop. Med. Int. Health 2023, 28, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Bonney, K.M.; Luthringer, D.J.; Kim, S.A.; Garg, N.J.; Engman, D.M. Pathology and Pathogenesis of Chagas Heart Disease. Annu. Rev. Pathol. 2019, 14, 421–447. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, R.M.; Mediano, M.F.F.; Mendes, F.S.; Sperandio da Silva, G.M.; Veloso, H.H.; Sangenis, L.H.C.; da Silva, P.S.; Mazzoli-Rocha, F.; Sousa, A.S.; Holanda, M.T.; et al. Chagas heart disease: An overview of diagnosis, manifestations, treatment, and care. World J. Cardiol. 2021, 13, 654–675. [Google Scholar] [CrossRef] [PubMed]
- Tanowitz, H.B.; Machado, F.S.; Spray, D.C.; Friedman, J.M.; Weiss, O.S.; Lora, J.N.; Nagajyothi, J.; Moraes, D.N.; Garg, N.J.; Nunes, M.C.; et al. Developments in the management of Chagas cardiomyopathy. Expert Rev. Cardiovasc. Ther. 2015, 13, 1393–1409. [Google Scholar] [CrossRef]
- Malik, L.H.; Singh, G.D.; Amsterdam, E.A. The Epidemiology, Clinical Manifestations, and Management of Chagas Heart Disease. Clin. Cardiol. 2015, 38, 565–569. [Google Scholar] [CrossRef]
- Steverding, D. The history of Chagas disease. Parasit Vectors 2014, 7, 317. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, A.; Marcondes de Rezende, J. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef]
- Rego, N.; Libisch, M.G.; Rovira, C.; Tosar, J.P.; Robello, C. Comparative microRNA profiling of Trypanosoma cruzi infected human cells. Front. Cell. Infect. Microbiol. 2023, 13, 1187375. [Google Scholar] [CrossRef]
- Trochine, A.; Bellora, N.; Nizovoy, P.; Duran, R.; Greif, G.; de Garcia, V.; Batthyany, C.; Robello, C.; Libkind, D. Genomic and proteomic analysis of Tausonia pullulans reveals a key role for a GH15 glucoamylase in starch hydrolysis. Appl. Microbiol. Biotechnol. 2022, 106, 4655–4667. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Viraque, F.; Chiribao, M.L.; Libisch, M.G.; Robello, C. Genome-wide chromatin interaction map for Trypanosoma cruzi. Nat. Microbiol. 2023, 8, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.N.; Cabeza, M.S.; Robello, C.; Guerrero, S.A.; Iglesias, A.A.; Arias, D.G. Biochemical characterization of GAF domain of free-R-methionine sulfoxide reductase from Trypanosoma cruzi. Biochimie 2023, 213, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Viraque, F.; Greif, G.; Berna, L.; Robello, C. Nanopore Long Read DNA Sequencing of Protozoan Parasites: Hybrid Genome Assembly of Trypanosoma cruzi. Methods Mol. Biol. 2021, 2369, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Berna, L.; Greif, G.; Pita, S.; Faral-Tello, P.; Diaz-Viraque, F.; Souza, R.C.M.; Vallejo, G.A.; Alvarez-Valin, F.; Robello, C. Maxicircle architecture and evolutionary insights into Trypanosoma cruzi complex. PLoS Negl. Trop. Dis. 2021, 15, e0009719. [Google Scholar] [CrossRef] [PubMed]
- Romer, G.; Bracco, L.A.; Ricci, A.D.; Balouz, V.; Berna, L.; Villar, J.C.; Ramsey, J.M.; Nolan, M.S.; Torrico, F.; Kesper, N.; et al. Deep serological profiling of the Trypanosoma cruzi TSSA antigen reveals different epitopes and modes of recognition by Chagas disease patients. PLoS Negl. Trop. Dis. 2023, 17, e0011542. [Google Scholar] [CrossRef]
- Callejas-Hernandez, F.; Robello, C.; Requena, J.M. Editorial: Protozoan parasites in the multi-omics era: Present and future. Front. Cell. Infect. Microbiol. 2023, 13, 1281638. [Google Scholar] [CrossRef]
- Schenkman, S.; Eichinger, D.; Pereira, M.E.; Nussenzweig, V. Structural and functional properties of Trypanosoma trans-sialidase. Annu. Rev. Microbiol. 1994, 48, 499–523. [Google Scholar] [CrossRef]
- Schenkman, S.; Eichinger, D. Trypanosoma cruzi trans-sialidase and cell invasion. Parasitol. Today 1993, 9, 218–222. [Google Scholar] [CrossRef]
- Torrecilhas, A.C.; Soares, R.P.; Schenkman, S.; Fernández-Prada, C.; Olivier, M. Extracellular Vesicles in Trypanosomatids: Host Cell Communication. Front. Cell. Infect. Microbiol. 2020, 10, 602502. [Google Scholar] [CrossRef] [PubMed]
- Garcez, E.M.; Gomes, N.; Moraes, A.S.; Pogue, R.; Uenishi, R.H.; Hecht, M.; Carvalho, J.L. Extracellular vesicles in the context of chagas disease—A systematic review. Acta Trop. 2023, 242, 106899. [Google Scholar] [CrossRef]
- Camargo, M.M.; Almeida, I.C.; Pereira, M.E.; Ferguson, M.A.; Travassos, L.R.; Gazzinelli, R.T. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J. Immunol. 1997, 158, 5890–5901. [Google Scholar] [CrossRef]
- Campos, M.A.; Almeida, I.C.; Takeuchi, O.; Akira, S.; Valente, E.P.; Procópio, D.O.; Travassos, L.R.; Smith, J.A.; Golenbock, D.T.; Gazzinelli, R.T. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 2001, 167, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Rosinha, G.M.; Almeida, I.C.; Salgueiro, X.S.; Jarvis, B.W.; Splitter, G.A.; Qureshi, N.; Bruna-Romero, O.; Gazzinelli, R.T.; Oliveira, S.C. Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect. Immun. 2004, 72, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.O.; Souza, R.T.; Cordero, E.M.; Maldonado, D.C.; Cortez, C.; Marini, M.M.; Ferreira, E.R.; Bayer-Santos, E.; Almeida, I.C.; Yoshida, N.; et al. Molecular Characterization of a Novel Family of Trypanosoma cruzi Surface Membrane Proteins (TcSMP) Involved in Mammalian Host Cell Invasion. PLoS Negl. Trop. Dis. 2015, 9, e0004216. [Google Scholar] [CrossRef]
- Monteiro, A.C.; Schmitz, V.; Svensjo, E.; Gazzinelli, R.T.; Almeida, I.C.; Todorov, A.; de Arruda, L.B.; Torrecilhas, A.C.; Pesquero, J.B.; Morrot, A.; et al. Cooperative activation of TLR2 and bradykinin B2 receptor is required for induction of type 1 immunity in a mouse model of subcutaneous infection by Trypanosoma cruzi. J. Immunol. 2006, 177, 6325–6335. [Google Scholar] [CrossRef]
- Procópio, D.O.; Almeida, I.C.; Torrecilhas, A.C.; Cardoso, J.E.; Teyton, L.; Travassos, L.R.; Bendelac, A.; Gazzinelli, R.T. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J. Immunol. 2002, 169, 3926–3933. [Google Scholar] [CrossRef]
- Almeida, I.C.; Gazzinelli, R.; Ferguson, M.A.; Travassos, L.R. Trypanosoma cruzi mucins: Potential functions of a complex structure. Mem. Inst. Oswaldo Cruz 1999, 94 (Suppl. S1), 173–176. [Google Scholar] [CrossRef][Green Version]
- Almeida, I.C.; Gazzinelli, R.T. Proinflammatory activity of glycosylphosphatidylinositol anchors derived from Trypanosoma cruzi: Structural and functional analyses. J. Leukoc. Biol. 2001, 70, 467–477. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, Chapter 3, Unit 3.22. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Soares, R.P.; Xander, P.; Costa, A.O.; Marcilla, A.; Menezes-Neto, A.; Del Portillo, H.; Witwer, K.; Wauben, M.; Nolte-T Hoen, E.; Olivier, M.; et al. Highlights of the São Paulo ISEV workshop on extracellular vesicles in cross-kingdom communication. J. Extracell. Vesicles 2017, 6, 1407213. [Google Scholar] [CrossRef]
- Trocoli Torrecilhas, A.C.; Tonelli, R.R.; Pavanelli, W.R.; da Silva, J.S.; Schumacher, R.I.; de Souza, W.; Silva, N.C.E.; de Almeida Abrahamsohn, I.; Colli, W.; Manso Alves, M.J. Trypanosoma cruzi: Parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009, 11, 29–39. [Google Scholar] [CrossRef]
- Torrecilhas, A.C.; Schumacher, R.I.; Alves, M.J.; Colli, W. Vesicles as carriers of virulence factors in parasitic protozoan diseases. Microbes Infect. 2012, 14, 1465–1474. [Google Scholar] [CrossRef]
- Almeida, I.C.; Camargo, M.M.; Procópio, D.O.; Silva, L.S.; Mehlert, A.; Travassos, L.R.; Gazzinelli, R.T.; Ferguson, M.A. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 2000, 19, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Madeira, R.P.; Dal’Mas Romera, L.M.; de Cássia Buck, P.; Mady, C.; Ianni, B.M.; Torrecilhas, A.C. New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response. J. Immunol. Res. 2021, 2021, 6650670. [Google Scholar] [CrossRef] [PubMed]
- Madeira, R.P.; Meneghetti, P.; de Barros, L.A.; de Cassia Buck, P.; Mady, C.; Ianni, B.M.; Fernandez-Becerra, C.; Torrecilhas, A.C. Isolation and molecular characterization of circulating extracellular vesicles from blood of chronic Chagas disease patients. Cell Biol. Int. 2022, 46, 883–894. [Google Scholar] [CrossRef]
- Cortes-Serra, N.; Gualdron-Lopez, M.; Pinazo, M.J.; Torrecilhas, A.C.; Fernandez-Becerra, C. Extracellular Vesicles in Trypanosoma cruzi Infection: Immunomodulatory Effects and Future Perspectives as Potential Control Tools against Chagas Disease. J. Immunol. Res. 2022, 2022, 5230603. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Serra, N.; Mendes, M.T.; Mazagatos, C.; Segui-Barber, J.; Ellis, C.C.; Ballart, C.; Garcia-Alvarez, A.; Gállego, M.; Gascon, J.; Almeida, I.C.; et al. Plasma-Derived Extracellular Vesicles as Potential Biomarkers in Heart Transplant Patient with Chronic Chagas Disease. Emerg. Infect. Dis. 2020, 26, 1846–1851. [Google Scholar] [CrossRef]
- Fernandez-Becerra, C.; Xander, P.; Alfandari, D.; Dong, G.; Aparici-Herraiz, I.; Rosenhek-Goldian, I.; Shokouhy, M.; Gualdron-Lopez, M.; Lozano, N.; Cortes-Serra, N.; et al. Guidelines for the purification and characterization of extracellular vesicles of parasites. J. Extracell. Biol. 2023, 2, e117. [Google Scholar] [CrossRef]
- Silva, V.O.; Maia, M.M.; Torrecilhas, A.C.; Taniwaki, N.N.; Namiyama, G.M.; Oliveira, K.C.; Ribeiro, K.S.; Toledo, M.D.S.; Xander, P.; Pereira-Chioccola, V.L. Extracellular vesicles isolated from Toxoplasma gondii induce host immune response. Parasite Immunol. 2018, 40, e12571. [Google Scholar] [CrossRef]
- Soekmadji, C.; Li, B.; Huang, Y.; Wang, H.; An, T.; Liu, C.; Pan, W.; Chen, J.; Cheung, L.; Falcon-Perez, J.M.; et al. The future of Extracellular Vesicles as Theranostics—An ISEV meeting report. J. Extracell. Vesicles 2020, 9, 1809766. [Google Scholar] [CrossRef]
- Alonso-Padilla, J.; Cortes-Serra, N.; Pinazo, M.J.; Bottazzi, M.E.; Abril, M.; Barreira, F.; Sosa-Estani, S.; Hotez, P.J.; Gascon, J. Response to letter to the editor: ‘Strategies to enhance access to diagnosis and treatment for Chagas disease patients in Latin America’. Expert. Rev. Anti Infect. Ther. 2019, 17, 673–675. [Google Scholar] [CrossRef]
- Cortes-Serra, N.; Losada-Galvan, I.; Pinazo, M.J.; Fernandez-Becerra, C.; Gascon, J.; Alonso-Padilla, J. State-of-the-art in host-derived biomarkers of Chagas disease prognosis and early evaluation of anti-Trypanosoma cruzi treatment response. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165758. [Google Scholar] [CrossRef]
- Marcilla, A.; Martin-Jaular, L.; Trelis, M.; de Menezes-Neto, A.; Osuna, A.; Bernal, D.; Fernandez-Becerra, C.; Almeida, I.C.; Del Portillo, H.A. Extracellular vesicles in parasitic diseases. J. Extracell. Vesicles 2014, 3, 25040. [Google Scholar] [CrossRef]
- Marin-Neto, J.A.; Cunha-Neto, E.; Maciel, B.C.; Simoes, M.V. Pathogenesis of chronic Chagas heart disease. Circulation 2007, 115, 1109–1123. [Google Scholar] [CrossRef]
- Ribeirao, M.; Pereira-Chioccola, V.L.; Renia, L.; Augusto Fragata Filho, A.; Schenkman, S.; Rodrigues, M.M. Chagasic patients develop a type 1 immune response to Trypanosoma cruzi trans-sialidase. Parasite Immunol. 2000, 22, 49–53. [Google Scholar] [CrossRef]
- Vazquez-Chagoyan, J.C.; Gupta, S.; Garg, N.J. Vaccine development against Trypanosoma cruzi and Chagas disease. Adv. Parasitol. 2011, 75, 121–146. [Google Scholar] [CrossRef]
- Chaparro Padilla, A.; Weber Aracena, L.; Realini Fuentes, O.; Albers Busquetts, D.; Hernandez Rios, M.; Ramirez Lobos, V.; Pascual La Rocca, A.; Nart Molina, J.; Beltran Varas, V.; Acuna-Gallardo, S.; et al. Molecular signatures of extracellular vesicles in oral fluids of periodontitis patients. Oral. Dis. 2020, 26, 1318–1325. [Google Scholar] [CrossRef]
- Reuken, P.A.; Stallmach, A.; Pletz, M.W.; Brandt, C.; Andreas, N.; Hahnfeld, S.; Loffler, B.; Baumgart, S.; Kamradt, T.; Bauer, M. Severe clinical relapse in an immunocompromised host with persistent SARS-CoV-2 infection. Leukemia 2021, 35, 920–923. [Google Scholar] [CrossRef]
- Bammert, T.D.; Hijmans, J.G.; Kavlich, P.J.; Lincenberg, G.M.; Reiakvam, W.R.; Fay, R.T.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Influence of sex on the number of circulating endothelial microparticles and microRNA expression in middle-aged adults. Exp. Physiol. 2017, 102, 894–900. [Google Scholar] [CrossRef]
- Toth, B.; Nikolajek, K.; Rank, A.; Nieuwland, R.; Lohse, P.; Pihusch, V.; Friese, K.; Thaler, C.J. Gender-specific and menstrual cycle dependent differences in circulating microparticles. Platelets 2007, 18, 515–521. [Google Scholar] [CrossRef]
- Portillo, S.; Zepeda, B.G.; Iniguez, E.; Olivas, J.J.; Karimi, N.H.; Moreira, O.C.; Marques, A.F.; Michael, K.; Maldonado, R.A.; Almeida, I.C. A prophylactic alpha-Gal-based glycovaccine effectively protects against murine acute Chagas disease. NPJ Vaccines 2019, 4, 13. [Google Scholar] [CrossRef]
- Brito, C.R.; McKay, C.S.; Azevedo, M.A.; Santos, L.C.; Venuto, A.P.; Nunes, D.F.; D’Avila, D.A.; Rodrigues da Cunha, G.M.; Almeida, I.C.; Gazzinelli, R.T.; et al. Virus-like Particle Display of the alpha-Gal Epitope for the Diagnostic Assessment of Chagas Disease. ACS Infect. Dis. 2016, 2, 917–922. [Google Scholar] [CrossRef]
- Schenkman, S.; Kurosaki, T.; Ravetch, J.V.; Nussenzweig, V. Evidence for the participation of the Ssp-3 antigen in the invasion of nonphagocytic mammalian cells by Trypanosoma cruzi. J. Exp. Med. 1992, 175, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Pereira-Chioccola, V.L.; Ribeirao, M.; Schenkman, S.; Rodrigues, M.M. Trans-sialidase delivered as a naked DNA vaccine elicits an immunological response similar to a Trypanosoma cruzi infection. Braz. J. Med. Biol. Res. 1999, 32, 235–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, aging and successful aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, T.; Wu, G.Y. Cytomegalovirus Hepatitis in Immunocompetent and Immunocompromised Hosts. J. Clin. Transl. Hepatol. 2021, 9, 106–115. [Google Scholar] [CrossRef]
- Urquiza, J.; Cevallos, C.; Elizalde, M.M.; Delpino, M.V.; Quarleri, J. Priming Astrocytes With HIV-Induced Reactive Oxygen Species Enhances Their Trypanosoma cruzi Infection. Front. Microbiol. 2020, 11, 563320. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeira, R.P.; Meneghetti, P.; Lozano, N.; Namiyama, G.M.; Pereira-Chioccola, V.L.; Torrecilhas, A.C. Exploring Peripheral Blood-Derived Extracellular Vesicles as Biomarkers: Implications for Chronic Chagas Disease with Viral Infection or Transplantation. Microorganisms 2024, 12, 116. https://doi.org/10.3390/microorganisms12010116
Madeira RP, Meneghetti P, Lozano N, Namiyama GM, Pereira-Chioccola VL, Torrecilhas AC. Exploring Peripheral Blood-Derived Extracellular Vesicles as Biomarkers: Implications for Chronic Chagas Disease with Viral Infection or Transplantation. Microorganisms. 2024; 12(1):116. https://doi.org/10.3390/microorganisms12010116
Chicago/Turabian StyleMadeira, Rafael Pedro, Paula Meneghetti, Nicholy Lozano, Gislene M. Namiyama, Vera Lucia Pereira-Chioccola, and Ana Claudia Torrecilhas. 2024. "Exploring Peripheral Blood-Derived Extracellular Vesicles as Biomarkers: Implications for Chronic Chagas Disease with Viral Infection or Transplantation" Microorganisms 12, no. 1: 116. https://doi.org/10.3390/microorganisms12010116
APA StyleMadeira, R. P., Meneghetti, P., Lozano, N., Namiyama, G. M., Pereira-Chioccola, V. L., & Torrecilhas, A. C. (2024). Exploring Peripheral Blood-Derived Extracellular Vesicles as Biomarkers: Implications for Chronic Chagas Disease with Viral Infection or Transplantation. Microorganisms, 12(1), 116. https://doi.org/10.3390/microorganisms12010116







