Chryseobacterium herbae Isolated from the Rhizospheric Soil of Pyrola calliantha H. Andres in Segrila Mountain on the Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Cultivation
2.2. Phylogenetic and Genotypic Analysis
2.3. Physiological, Biochemical, and Chemotaxonomic Analysis
2.4. Genome Analyses
2.5. Shutgun Proteomics Analyses
2.6. Pigment Analyses
3. Results and Discussion
3.1. Phylogenetic and Genotypic Analysis
3.2. Physiological, Biochemical, and Chemotaxonomic Analysis
3.3. Genome Analyses
3.4. Shut-Gun Proteomics Results
4. Description of Chryseobacterium herbae sp. nov.
Chryseobacterium herbae (her’bae. L. gen. n. herbae, of a herb)
5. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García-López, M.; Meier-Kolthoff, J.P.; Tindall, B.J.; Gronow, S.; Woyke, T.; Kyrpides, N.C.; Hahnke, R.L.; Göker, M. Analysis of 1000 Type-Strain Genomes Improves Taxonomic Classification of Bacteroidetes. Front. Microbiol. 2019, 10, 2083. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yu, S.M. Chryseobacterium salivictor sp. nov., a Plant-Growth-Promoting Bacterium Isolated from Freshwater. Antonie van Leeuwenhoek 2020, 113, 989–995. [Google Scholar] [CrossRef]
- Kumar, V.; Patial, V.; Thakur, V.; Singh, R.; Singh, D. Genomics Assisted Characterization of Plant Growth-Promoting and Metabolite Producing Psychrotolerant Himalayan Chryseobacterium Cucumeris PCH239. Arch. Microbiol. 2023, 205, 108. [Google Scholar] [CrossRef]
- Montero-Calasanz, M.D.C.; Göker, M.; Rohde, M.; Spröer, C.; Schumann, P.; Busse, H.J.; Schmid, M.; Tindall, B.J.; Klenk, H.P.; Camacho, M. Chryseobacterium hispalense sp. nov., a Plantgrowth- Promoting Bacterium Isolated from a Rainwater Pond in an Olive Plant Nursery, and Emended Descriptions of Chryseobacterium defluvii, Chryseobacterium indologenes, Chryseobacterium wanjuense and Chryseobacterium gregarium. Int. J. Syst. Evol. Microbiol. 2013, 63, 4386–4395. [Google Scholar] [CrossRef]
- Dardanelli, M.S.; Manyani, H.; González-Barroso, S.; Rodríguez-Carvajal, M.A.; Gil-Serrano, A.M.; Espuny, M.R.; López-Baena, F.J.; Bellogín, R.A.; Megías, M.; Ollero, F.J. Effect of the Presence of the Plant Growth Promoting Rhizobacterium (PGPR) Chryseobacterium balustinum Aur9 and Salt Stress in the Pattern of Flavonoids Exuded by Soybean Roots. Plant Soil 2010, 328, 483–493. [Google Scholar] [CrossRef]
- Anderson, M.; Habiger, J. Characterization and Identification of Productivity-Associated Rhizobacteria in Wheat. Appl. Environ. Microbiol. 2012, 78, 4434–4446. [Google Scholar] [CrossRef]
- Xiang, X.; Yi, X.; Zheng, W.; Li, Y.; Zhang, C.; Wang, X.; Chen, Z.; Huang, M.; Ying, G.G. Enhanced Biodegradation of Thiamethoxam with a Novel Polyvinyl Alcohol (PVA)/Sodium Alginate (SA)/Biochar Immobilized Chryseobacterium sp. H5. J. Hazard. Mater. 2023, 443, 130247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, W.-J.; Chen, S.-F.; Lei, Q.; Li, J.; Bhatt, P.; Mishra, S.; Chen, S. Cellular Response and Molecular Mechanism of Glyphosate Degradation by Chryseobacterium sp. Y16C. J. Agric. Food Chem. 2023, 71, 6650–6661. [Google Scholar] [CrossRef]
- Dahal, R.H.; Chaudhary, D.K.; Kim, D.U.; Pandey, R.P.; Kim, J. Chryseobacterium antibioticum sp. nov. with Antimicrobial Activity against Gram-Negative Bacteria, Isolated from Arctic Soil. J. Antibiot. 2021, 74, 115–123. [Google Scholar] [CrossRef]
- Kaur, H.; Mohan, B.; Hallur, V.; Raj, A.; Basude, M.; Mavuduru, R.S.; Taneja, N. Increased Recognition of Chryseobacterium Species as an Emerging Cause of Nosocomial Urinary Tract Infection Following Introduction of Matrix-Assisted Laser Desorption/Ionisation-Time of Flight for Bacterial Identification. Indian J. Med. Microbiol. 2017, 35, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Abdalhamid, B.; Elhadi, N.; Alsamman, K.; Aljindan, R. Chryseobacterium Gleum Pneumonia in an Infant with Nephrotic Syndrome. IDCases 2016, 5, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Mirza, I.A.; Khalid, A.; Hameed, F.; Imtiaz, A.; Ashfaq, A.; Tariq, A. Chryseobacterium Indologenes as a Novel Cause of Bacteremia in a Neonate. J. Coll. Phys. Surg. Pak. 2019, 29, 375–378. [Google Scholar] [CrossRef]
- Loch, T.P.; Faisal, M. Chryseobacterium Aahli sp. nov., Isolated from Lake Trout (Salvelinus namaycush) and Brown Trout (Salmo trutta), and Emended Descriptions of Chryseobacterium ginsenosidimutans and Chryseobacterium gregarium. Int. J. Syst. Evol. Microbiol. 2014, 64, 1573–1579. [Google Scholar] [CrossRef]
- Kämpfer, P.; Fallschissel, K.; Avendaño-Herrera, R. Chryseobacterium chaponense sp. nov., Isolated from Farmed Atlantic Salmon (Salmo salar). Int. J. Syst. Evol. Microbiol. 2011, 61, 497–501. [Google Scholar] [CrossRef]
- Zamora, L.; Fernández-Garayzábal, J.F.; Palacios, M.A.; Sánchez-Porro, C.; Svensson-Stadler, L.A.; Domínguez, L.; Moore, E.R.; Ventosa, A.; Vela, A.I. Chryseobacterium oncorhynchi sp. nov., Isolated from Rainbow Trout (Oncorhynchus mykiss). Syst. Appl. Microbiol. 2012, 35, 24–29. [Google Scholar] [CrossRef]
- Kirk, K.E.; Hoffman, J.A.; Smith, K.A.; Strahan, B.L.; Failor, K.C.; Krebs, J.E.; Gale, A.N.; Do, T.D.; Sontag, T.C.; Batties, A.M.; et al. Chryseobacterium angstadtii sp. nov., Isolated from a Newt Tank. Int. J. Syst. Evol. Microbiol. 2013, 63, 4777–4783. [Google Scholar] [CrossRef] [PubMed]
- Kook, M.; Son, H.M.; Ngo, H.T.T.; Yi, T.H. Chryseobacterium camelliae sp. nov., Isolated from Green Tea. Int. J. Syst. Evol. Microbiol. 2014, 64, 851–857. [Google Scholar] [CrossRef]
- Lin, S.Y.; Hameed, A.; Liu, Y.C.; Hsu, Y.H.; Hsieh, Y.T.; Lai, W.A.; Young, C.C. Chryseobacterium endophyticum sp. nov., Isolated from a Maize Leaf. Int. J. Syst. Evol. Microbiol. 2017, 67, 570–575. [Google Scholar] [CrossRef]
- Kämpfer, P.; Poppel, M.T.; Wilharm, G.; Busse, H.J.; McInroy, J.A.; Glaeser, S.P. Chryseobacterium gallinarum sp. nov., Isolated from a Chicken, and Chryseobacterium contaminans sp. nov., Isolated as a Contaminant from a Rhizosphere Sample. Int. J. Syst. Evol. Microbiol. 2014, 64, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lee, K.S.; Shin, D.S.; Han, J.H.; Park, K.S.; Lee, C.H.; Park, K.H.; Kim, S.B. Four New Species of Chryseobacterium from the Rhizosphere of Coastal Sand Dune Plants, Chryseobacterium elymi sp. nov., Chryseobacterium hagamense sp. nov., Chryseobacterium lathyri sp. nov. and Chryseobacterium rhizosphaerae sp. nov. Syst. Appl. Microbiol. 2010, 33, 122–127. [Google Scholar] [CrossRef]
- Nguyen, N.L.; Kim, Y.J.; Hoang, V.A.; Yang, D.C. Chryseobacterium ginsengisoli sp. nov., Isolated from the Rhizosphere of Ginseng and Emended Description of Chryseobacterium gleum. Int. J. Syst. Evol. Microbiol. 2013, 63, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, X.; Kahaer, M.; Tian, T.; Sun, Y. Chryseobacterium endalhagicum sp. nov., Isolated from Seed of Leguminous Plant. Int. J. Syst. Evol. Microbiol. 2021, 71, 005077. [Google Scholar] [CrossRef]
- Kim, K.K.; Bae, H.S.; Schumann, P.; Lee, S.T. Chryseobacterium daecheongense sp. nov., Isolated from Freshwater Lake Sediment. Int. J. Syst. Evol. Microbiol. 2005, 55, 133–138. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kang, S.J.; Oh, T.K. Chryseobacterium daeguense sp. nov., Isolated from Wastewater of a Textile Dye Works. Int. J. Syst. Evol. Microbiol. 2007, 57, 1355–1359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kämpfer, P.; Dreyer, U.; Neef, A.; Dott, W.; Busse, H.J. Chryseobacterium defluvii sp. nov., Isolated from Wastewater. Int. J. Syst. Evol. Microbiol. 2003, 53, 93–97. [Google Scholar] [CrossRef]
- Pires, C.; Carvalho, M.F.; De Marco, P.; Magan, N.; Castro, P.M.L. Chryseobacterium palustre sp. nov. and Chryseobacterium humi sp. nov., Isolated from Industrially Contaminated Sediments. Int. J. Syst. Evol. Microbiol. 2010, 60, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huo, Y.Y.; Li, Z.Y.; Wang, C.S.; Oren, A.; Xu, X.W. Chryseobacterium profundimaris sp. nov., a New Member of the Family Flavobacteriaceae Isolated from Deep-Sea Sediment. Antonie Van Leeuwenhoek 2015, 107, 979–989. [Google Scholar] [CrossRef]
- Heidler von Heilborn, D.; Nover, L.L.; Weber, M.; Hölzl, G.; Gisch, N.; Waldhans, C.; Mittler, M.; Kreyenschmidt, J.; Woehle, C.; Hüttel, B.; et al. Polar Lipid Characterization and Description of Chryseobacterium capnotolerans sp. nov., Isolated from High CO2-Containing Atmosphere and Emended Descriptions of the Genus Chryseobacterium, and the Species C. Balustinum, C. Daecheongense, C. Formosense, C. Gleum, C. Indologenes, C. Joostei, C. Scophthalmum and C. Ureilyticum. Int. J. Syst. Evol. Microbiol. 2022, 72, 005372. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.Q.; Peng, Y.; Huang, C.P.; Zhang, C.; Wu, F.Z. Effects of Streams on Lignin Degradation during Foliar Litter Decomposition in an Alpine Forest. Chin. J. Plant Ecol. 2016, 40, 893. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, X.; Wang, R.; Liu, R.; Shao, X.; Li, J.; Wei, Y. Linkages and Key Factors between Soil Bacterial and Fungal Communities along an Altitudinal Gradient of Different Slopes on Mount Segrila, Tibet, China. Front. Microbiol. 2022, 13, 1024198. [Google Scholar] [CrossRef]
- Chen, Q.; Lei, T.; Wu, Y.; Si, G.; Xi, C.; Zhang, G. Comparison of Soil Organic Matter Transformation Processes in Different Alpine Ecosystems in the Qinghai-Tibet Plateau. J. Geophys. Res. Biogeosci. 2019, 124, 33–45. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yu, Z.; Shen, G.; Cheng, H.; Tao, S. Composition and Diversity of Soil Microbial Communities in the Alpine Wetland and Alpine Forest Ecosystems on the Tibetan Plateau. Sci. Total Environ. 2020, 747, 141358. [Google Scholar] [CrossRef]
- He, C.; Liu, J.; Ke, T.; Luo, Y.; Zhang, S.; Mao, T.; Li, Z.; Qin, X.; Jin, S. Pyrolae Herba: A Review on Its Botany, Traditional Uses, Phytochemistry, Pharmacology and Quality Control. J. Ethnopharmacol. 2022, 298, 115584. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.M. A Manual for the Practical Study of the Root-Nodule Bacteria; CAB International: Wallingford, UK, 1970. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Pruesse, E.; Peplies, J.; Glöckner, F.O. SINA: Accurate High-Throughput Multiple Sequence Alignment of Ribosomal RNA Genes. Bioinformatics 2012, 28, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.A.; Krieg, N.R. Methods for General and Molecular Bacteriology. Am. Soc. Microbiol. 1989. [Google Scholar]
- Rahman, A.; Sitepu, I.R.; Tang, S.-Y.; Hashidoko, Y. Salkowski’s Reagent Test as a Primary Screening Index for Functionalities of Rhizobacteria Isolated from Wild Dipterocarp Saplings Growing Naturally on Medium-Strongly Acidic Tropical Peat Soil. Biosci. Biotechnol. Biochem. 2010, 74, 2202–2208. [Google Scholar] [CrossRef]
- Libbert, E.; Silhengst, P. Interactions between Plants and Epiphytic Bacteria Regarding Their Auxin Metabolism: VIII. Transfer of 14C-Indoleacetic Acid from Epiphytic Bacteria to Corn Coleotiles. Physiol. Plant 1970, 23, 480–487. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Halebian, S.; Harris, B.; Finegold, S.M.; Rolfe, R.D. Rapid Method That Aids in Distinguishing Gram-Positive from Gram-Negative Anaerobic Bacteria. J. Clin. Microbiol. 1981, 13, 444–448. [Google Scholar] [CrossRef]
- Tindall, B.J.; Sikorski, J.; Smibert, R.A.; Krieg, N.R. Phenotypic Characterization and the Principles of Comparative Systematics. In Methods for General and Molecular Microbiology, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 330–393. [Google Scholar]
- Vandamme, P.; Vancanneyt, M.; Pot, B.; Mels, L.; Hoste, B.; Dewettinck, D.; Vlaes, L.; Van Den Borre, C.; Higgins, R.; Hommez, J. Polyphasic Taxonomic Study of the Emended Genus Arcobacter with Arcobacter butzleri comb. nov. and Arcobacter skirrowii sp. nov., an Aerotolerant Bacterium Isolated from Veterinary Specimens. Int. J. Syst. Evol. Microbiol. 1992, 42, 344–356. [Google Scholar] [CrossRef][Green Version]
- Xu, X.-W.; Huo, Y.-Y.; Wang, C.-S.; Oren, A.; Cui, H.-L.; Vedler, E.; Wu, M. Pelagibacterium halotolerans gen. nov., sp. nov. and Pelagibacterium luteolum sp. nov., Novel Members of the Family Hyphomicrobiaceae. Int. J. Syst. Evol. Microbiol. 2011, 61, 1817–1822. [Google Scholar] [CrossRef]
- Kates, M. Techniques of Lipidology. 2. Rev. In Laboratory Techniques in Biochemistry and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Minnikin, D.E.; O’donnell, A.G.; Goodfellow, M.; Alderson, G.; Athalye, M.; Schaal, A.; Parlett, J.H. An Integrated Procedure for the Extraction of Bacterial Isoprenoid Quinones and Polar Lipids. J. Microbiol. Methods 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Marmur, J. A Procedure for the Isolation of Deoxyribonucleic Acid from Micro-Organisms. J. Mol. Biol. 1961, 3, 208–2018. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Lim, J.; Kwon, S.; Chun, J. A Large-Scale Evaluation of Algorithms to Calculate Average Nucleotide Identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.-W.; De Meyer, S. Proposed Minimal Standards for the Use of Genome Data for the Taxonomy of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A Database Tandem for Fast and Reliable Genome-Based Classification and Nomenclature of Prokaryotes. Nucleic Acids Res. 2021, 50, D801–D807. [Google Scholar] [CrossRef]
- Wilhelm, M.; Schlegl, J.; Hahne, H.; Gholami, A.M.; Lieberenz, M.; Savitski, M.M.; Ziegler, E.; Butzmann, L.; Gessulat, S.; Marx, H.; et al. Mass-Spectrometry-Based Draft of the Human Proteome. Nature 2014, 509, 582–587. [Google Scholar] [CrossRef]
- Omenn, G.S.; Lane, L.; Overall, C.M.; Pineau, C.; Packer, N.H.; Cristea, I.M.; Lindskog, C.; Weintraub, S.T.; Orchard, S.; Roehrl, M.H.A.; et al. The 2022 Report on the Human Proteome from the HUPO Human Proteome Project. J. Proteome Res. 2023, 22, 1024–1042. [Google Scholar] [CrossRef]
- Wu, C.C.; MacCoss, M.J. Shotgun Proteomics: Tools for the Analysis of Complex Biological Systems. Curr. Opin. Mol. Ther. 2002, 4, 242–250. [Google Scholar] [PubMed]
- Siddaramappa, S.; Narjala, A.; Viswanathan, V.; Maliye, C.; Lakshminarayanan, R. Phylogenetic Insights into the Diversity of Chryseobacterium Species. Access Microbiol. 2019, 1, e000019. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Montero-Calasanz, M.; Göker, M.; Rohde, M.; Spröer, C.; Schumann, P.; Busse, H.-J.; Schmid, M.; Klenk, H.-P.; Tindall, B.J.; Camacho, M. Chryseobacterium oleae sp. nov., an Efficient Plant Growth Promoting Bacterium in the Rooting Induction of Olive Tree (Olea europaea L.) Cuttings and Emended Descriptions of the Genus Chryseobacterium, C. Daecheongense, C. Gambrini, C. Gleum, C. Joostei, C. Jejuense, C. Luteum, C. Shigense, C. Taiwanense, C. Ureilyticum and C. Vrystaatense. Syst. Appl. Microbiol. 2014, 37, 342–350. [Google Scholar]
- Sang, M.K.; Kim, H.-S.; Myung, I.-S.; Ryu, C.-M.; Kim, B.S.; Kim, K.D. Chryseobacterium kwangjuense sp. nov., Isolated from Pepper (Capsicum annuum L.) Root. Int. J. Syst. Evol. Microbiol. 2013, 63, 2835–2840. [Google Scholar] [CrossRef] [PubMed]
- De Beer, H.; Hugo, C.J.; Jooste, P.J.; Willems, A.; Vancanneyt, M.; Coenye, T.; Vandamme, P.A.R. Chryseobacterium vrystaatense sp. nov., Isolated from Raw Chicken in a Chicken-Processing Plant. Int. J. Syst. Evol. Microbiol. 2005, 55, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- Klimkaitė, L.; Ragaišis, I.; Krasauskas, R.; Ružauskas, M.; Sužiedėlienė, E.; Armalytė, J. Novel Antibiotic Resistance Genes Identified by Functional Gene Library Screening in Stenotrophomonas maltophilia and Chryseobacterium spp. Bacteria of Soil Origin. Int. J. Mol. Sci. 2023, 24, 6037. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an Update of CRISRFinder, Includes a Portable Version, Enhanced Performance and Integrates Search for Cas Proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef]
- Barrangou, R.; Doudna, J.A. Applications of CRISPR Technologies in Research and Beyond. Nat. Biotechnol. 2016, 34, 933–941. [Google Scholar] [CrossRef]
- Hille, F.; Charpentier, E. CRISPR-Cas: Biology, Mechanisms and Relevance. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016, 371, 5569–5576. [Google Scholar] [CrossRef]
- Reichenbach, H.; Kohl, W.; Böttger-Vetter, A.; Achenbach, H. Flexirubin-Type Pigments in Flavobacterium. Arch. Microbiol. 1980, 126, 291–293. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhou, H.; Zhong, G.; Huo, L.; Tang, Y.J.; Zhang, Y.; Bian, X. Genome Mining and Biosynthesis of Primary Amine-Acylated Desferrioxamines in a Marine Gliding Bacterium. Org. Lett. 2020, 22, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Glavina Del Rio, T.; Abt, B.; Spring, S.; Lapidus, A.; Nolan, M.; Tice, H.; Copeland, A.; Cheng, J.F.; Chen, F.; Bruce, D.; et al. Complete Genome Sequence of Chitinophaga Pinensis Type Strain (UQM 2034). Stand. Genom. Sci. 2010, 2, 87–95. [Google Scholar] [CrossRef]
- Mohr, K.I.; Volz, C.; Jansen, R.; Wray, V.; Hoffmann, J.; Bernecker, S.; Wink, J.; Gerth, K.; Stadler, M.; Müller, R. Pinensins: The First Antifungal Lantibiotics. Angew. Chem. Int. Ed. 2015, 54, 11254–11258. [Google Scholar] [CrossRef]
- Murakami, M.; Sun, Q.; Ishida, K.; Matsuda, H.; Okino, T.; Yamaguchi, K. Microviridins, Elastase Inhibitors from the Cyanobacterium Nostoc Minutum (NIES-26). Phytochemistry 1997, 45, 1197–1202. [Google Scholar] [CrossRef]
- Rohrlack, T.; Christoffersen, K.; Kaebernick, M.; Neilan, B.A. Cyanobacterial Protease Inhibitor Microviridin J Causes a Lethal Molting Disruption in Daphnia pulicaria. Appl. Environ. Microbiol. 2004, 70, 5047–5050. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.D.; Deisenhofer, J. TonB-Dependent Receptors—Structural Perspectives. Biochim. Biophys. Acta Biomembr. 2002, 1565, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Boddu, R.S.; Perumal, O.; Divakar, K. Microbial Nitroreductases: A Versatile Tool for Biomedical and Environmental Applications. Biotechnol. Appl. Biochem. 2021, 68, 1518–1530. [Google Scholar] [CrossRef]
- Zhang, R.; Vivanco, J.M.; Shen, Q. The Unseen Rhizosphere Root–Soil–Microbe Interactions for Crop Production. Curr. Opin. Microbiol. 2017, 37, 8–14. [Google Scholar] [CrossRef]


| Percentage | ||||
|---|---|---|---|---|
| Fatty Acids | 1 | 2 a | 3 b | 4 c |
| C16:0 | 1.16 | 3.3 | 1.2 | 1.1 ± 0.3 |
| C16:0 3-OH | ND | 0.5 | Tr | 1.3 ± 0.3 |
| iso-C13:0 | 1.63 | Tr | 1.7 | 1.1 ± 0.4 |
| iso-C15:0 | 42.82 | 38.5 | 45.4 | 41.8 ± 1.4 |
| iso-C15:0 3-OH | 3.79 | 1.6 | 4.2 | 2.7 ± 0.3 |
| iso-C16:0 | 0.82 | 3.9 | ND | ND |
| iso-C16:0 3-OH | 1.18 | 2.3 | ND | Tr |
| iso-C17:0 | 0.71 | 1.3 | 1.4 | Tr |
| iso-C17:0 3-OH | 21.03 | 8.7 | 10.8 | 15.4 ± 1.8 |
| iso-C17:1 ω9c | ND | ND | 11.6 | 19.7 ± 2.3 |
| anteiso-C15:0 | 1.24 | 4.8 | Tr | 1.7 ± 0.7 |
| Summed features 3 | 8.3 | 14.7 | 15.4 | 9.1 ± 0.9 |
| Summed features 9 | 12.43 | 14.8 | ND | NA |
| Characteristic | 1 | 2 a | 3 b | 4 c |
|---|---|---|---|---|
| Color of colonies | orange | yellow | yellow | yellow |
| Gram-stain | − | − | − | − |
| Growth temperature (°C) | 10–30 | 5–35 | 10–38 | 4–32 |
| Optimal growth (°C) | 25 | − | 28–38 | 5 |
| pH range for growth | 5.0–9.0 | 5.0–8.0 | 6.0–8.0 | nm |
| Optimal | 6.0 | 7.0–8.0 | nm | |
| Growth in NaCl (%w/v) | 0–1.8 | 0–1 | 1–3 | 1–2 |
| Oxidase | − | + | nm | + |
| Catalase | + | + | nm | + |
| Motility | − | − | − | − |
| Esculin | − | + | + | + |
| Starch | + | + | + | − |
| Tween 40 | + | + | nm | + |
| Tween 80 | + | nm | + | + |
| β-Galactosidase | − | − | − | nm |
| Nitrate reduction | − | − | − | |
| Indole production | + | + | + | nm |
| D-Glucose fermentation | − | + | nm | + |
| Urease | − | − | − | + |
| Mannitol | w | + | + | nm |
| D-Maltose | − | + | + | nm |
| Mannose | w | + | nm | + |
| DNA G+C content (mol%) * | 37.9 | 38.2 | 40.2 | 37.1 |
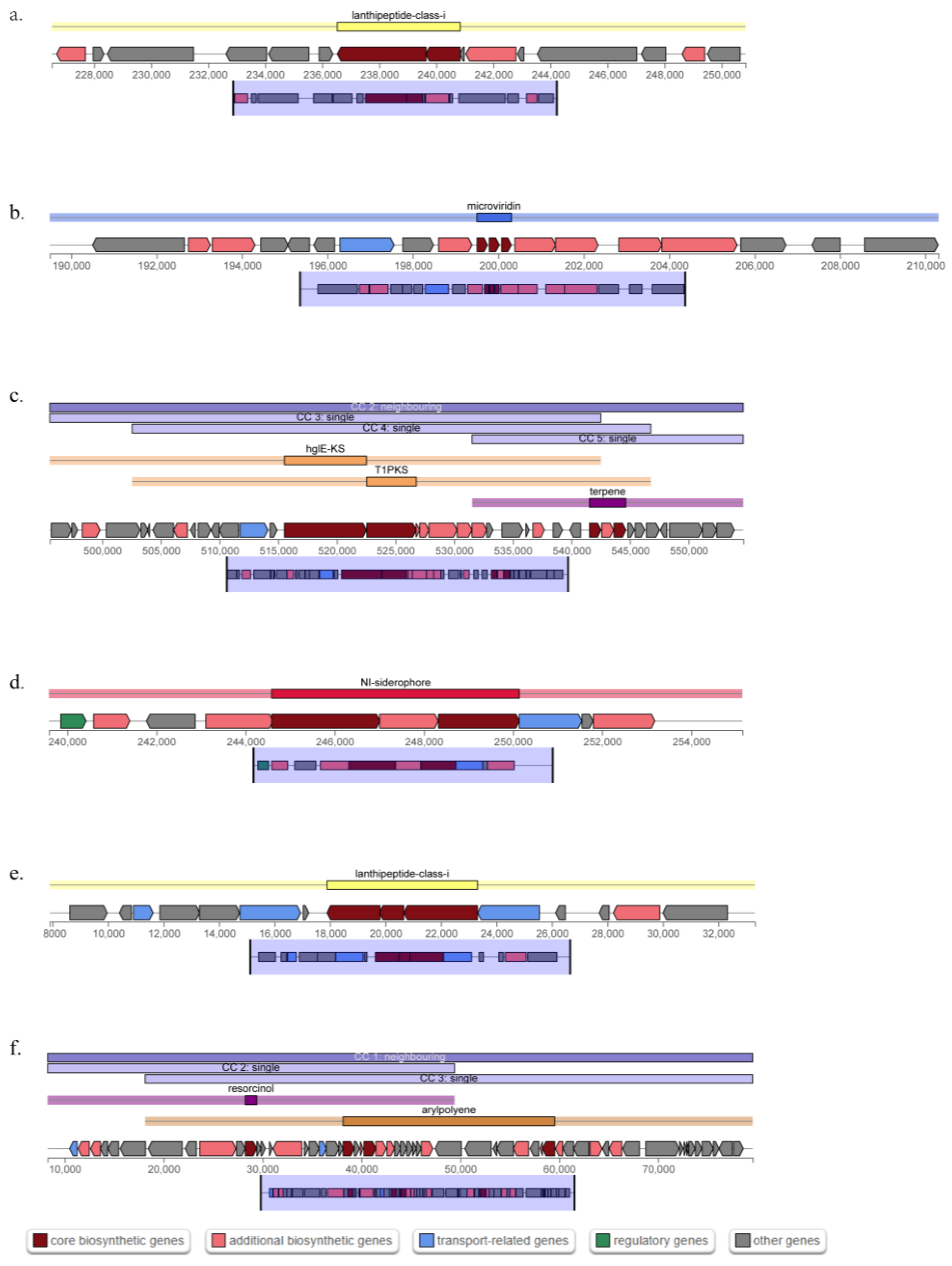
| Region | Type | From-To | Most Similar Known Cluster | Similarity | |
|---|---|---|---|---|---|
| Region 1.1 | lanthipeptide-class-i | 226,529 250,834 | |||
| Region 2.1 | microviridin | 189,501 210,305 | |||
| Region 2.2 | hglE-KS, T1PKS, terpene | 495,527 554,653 | |||
| Region 6.1 | NI-siderophore | 239,589 255,146 | fulvivirgamide A2/fulvivirgamide B2/fulvivirgamide B3/fulvivirgamide B4 | Other | 33% |
| Region 7.1 | lanthipeptide-class-i | 7895 33,311 | pinensins | RiPP | 17% |
| Region 8.1 | resorcinol, arylpolyene | 8270 79,537 | flexirubin | Polyketide | 75% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, Y.; Kong, D.; Ma, Q.; Li, Y.; Xing, Z.; Ruan, Z. Chryseobacterium herbae Isolated from the Rhizospheric Soil of Pyrola calliantha H. Andres in Segrila Mountain on the Tibetan Plateau. Microorganisms 2023, 11, 2017. https://doi.org/10.3390/microorganisms11082017
Zhang L, Wang Y, Kong D, Ma Q, Li Y, Xing Z, Ruan Z. Chryseobacterium herbae Isolated from the Rhizospheric Soil of Pyrola calliantha H. Andres in Segrila Mountain on the Tibetan Plateau. Microorganisms. 2023; 11(8):2017. https://doi.org/10.3390/microorganisms11082017
Chicago/Turabian StyleZhang, Li, Yan Wang, Delong Kong, Qingyun Ma, Yan Li, Zhen Xing, and Zhiyong Ruan. 2023. "Chryseobacterium herbae Isolated from the Rhizospheric Soil of Pyrola calliantha H. Andres in Segrila Mountain on the Tibetan Plateau" Microorganisms 11, no. 8: 2017. https://doi.org/10.3390/microorganisms11082017
APA StyleZhang, L., Wang, Y., Kong, D., Ma, Q., Li, Y., Xing, Z., & Ruan, Z. (2023). Chryseobacterium herbae Isolated from the Rhizospheric Soil of Pyrola calliantha H. Andres in Segrila Mountain on the Tibetan Plateau. Microorganisms, 11(8), 2017. https://doi.org/10.3390/microorganisms11082017





