Prevalence and Association of Campylobacter spp., Salmonella spp., and Blastocystis sp. in Poultry
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and qPCR
2.3. Statistical Analysis
3. Results
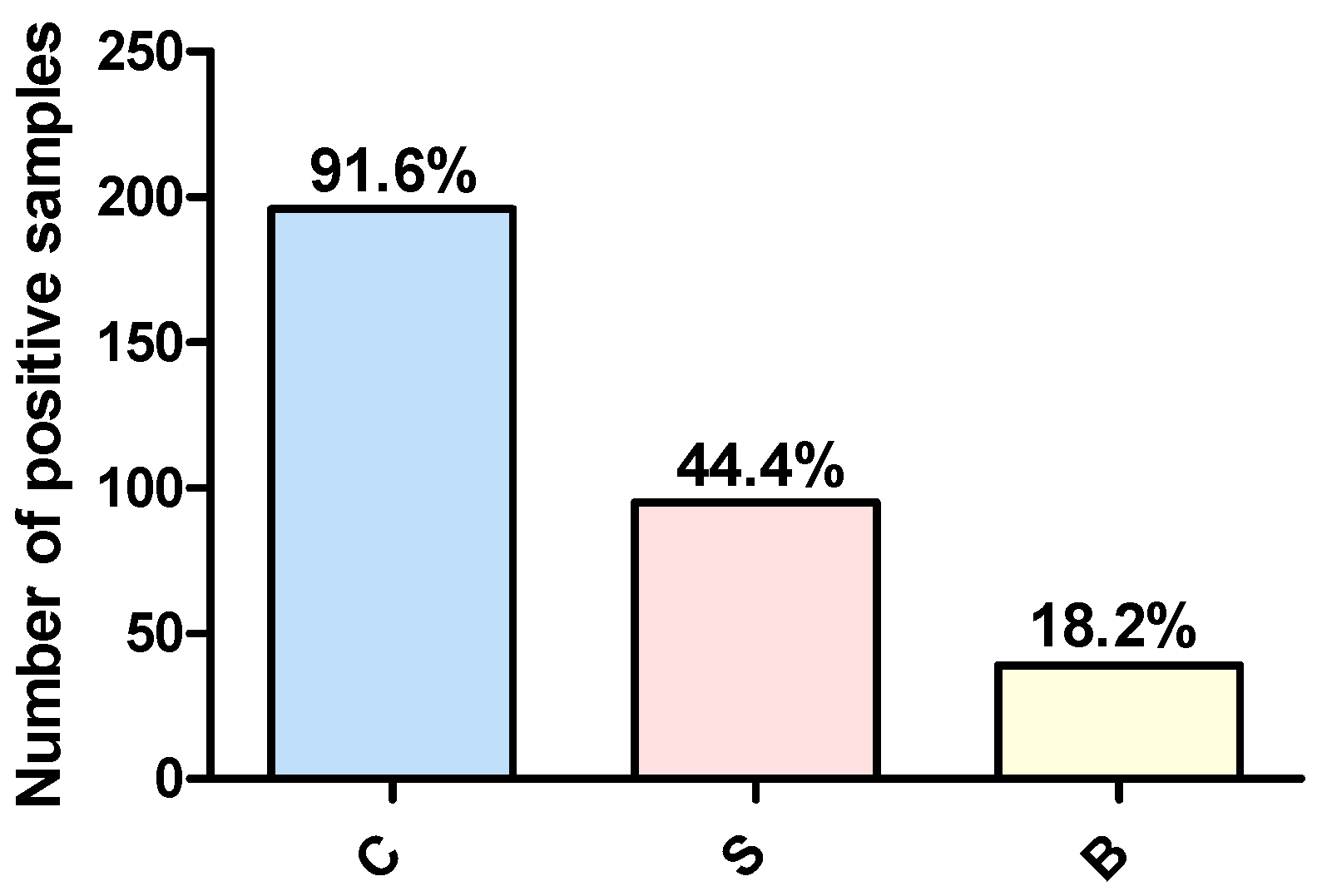
3.1. Prevalence of Campylobacter spp., Salmonella spp., and Blastocystis sp. in Poultry Samples
3.2. Assessment of the Association between Campylobacter spp., Salmonella spp., and Blastocystis sp. Infections in Poultry Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention Control). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar]
- Mortensen, N.P.; Kuijf, M.L.; Ang, C.W.; Schiellerup, P.; Krogfelt, K.A.; Jacobs, B.C.; van Belkum, A.; Endtz, H.P.; Bergman, M.P. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 2009, 11, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.R.; Zegarra, J.A.; López-Gatell, H.; Sejvar, J.; Arzate, F.; Waterman, S.; Núñez, A.S.; López, B.; Weiss, J.; Cruz, R.Q.; et al. Binational outbreak of Guillain-Barré syndrome associated with Campylobacter jejuni infection, Mexico and USA, 2011. Epidemiol. Infect. 2014, 142, 1089–1099. [Google Scholar] [CrossRef]
- Skarp, C.P.A.; Hänninen, M.L.; Rautelin, H.I.K. Campylobacteriosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 103–109. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority) Panel on Biological Hazards. Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010, 8, 1437. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008—Part A: Campylobacter and Salmonella prevalence estimates. EFSA J. 2010, 8, 1503. [Google Scholar]
- Hue, O.; Le Bouquin, S.; Laisney, M.J.; Allain, V.; Lalande, F.; Petetin, I.; Rouxel, S.; Quesne, S.; Gloaguen, P.Y.; Picherot, M.; et al. Prevalence of and risk factors for Campylobacter spp. contamination of broiler chicken carcasses at the slaughterhouse. Food Microbiol. 2010, 27, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; Abd El-Aziz, N.K.; Samir, M.; El-Naenaeey, E.Y.; Abo Remela, E.M.; Mosbah, R.A.; Bendary, M.M. Genetic diversity of Campylobacter jejuni isolated from avian and human sources in Egypt. Front. Microbiol. 2019, 10, 2353. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El Hofy, F.I.; Ammar, A.M.; Abd El Tawab, A.A.; Hefny, A.A. ERIC-PCR genotyping of some Campylobacter jejuni isolates of chicken and human origin in Egypt. Vector Borne Zoonotic Dis. 2015, 15, 713–717. [Google Scholar] [CrossRef]
- Abushahba, M.F.N.; Ahmed, S.O.; Ibrahim, A.A.; Mosa, H.A. Prevalence of zoonotic species of Campylobacter in broiler chicken and humans in Assiut Governorate, Egypt. Approaches Poult. Dairy Vet. Sci. 2018, 3, 260–268. [Google Scholar] [CrossRef]
- Mohammed, A.N.; Abdel Aziz, S.A.A. The prevalence of Campylobacter species in broiler flocks and their environment: Assessing the efficiency of chitosan/zinc oxide nanocomposite for adopting control strategy. Environ. Sci. Pollut. Res. Int. 2019, 26, 30177–30187. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef]
- Hublin, J.S.Y.; Maloney, J.G.; Santin, M. Blastocystis in domesticated and wild mammals and birds. Res. Vet. Sci. 2021, 135, 260–282. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K. Some aspects of control of salmonella infection in poultry for minimising contamination in the food chain. Worlds Poult. Sci. J. 2014, 70, 519–530. [Google Scholar] [CrossRef]
- Tan, K.S.; Mirza, H.; Teo, J.D.; Wu, B.; Macary, P.A. Current views on the clinical relevance of Blastocystis spp. Curr. Infect. Dis. Rep. 2010, 12, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Greige, S.; El Safadi, D.; Bécu, N.; Gantois, N.; Pereira, B.; Chabé, M.; Benamrouz-Vanneste, S.; Certad, G.; El Hage, R.; Chemaly, M.; et al. Prevalence and subtype distribution of Blastocystis sp. isolates from poultry in Lebanon and evidence of zoonotic potential. Parasites Vectors 2018, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, I.; Poirier, P.; Viscogliosi, E.; Dionigia, M.; Texier, C.; Delbac, F.; Alaoui, H.E. Blastocystis, an unrecognized parasite: An overview of pathogenesis and diagnosis. Ther. Adv. Infect. Dis. 2013, 1, 167–178. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Report of the task force on zoonoses data collection on the analysis of the baseline survey on the report of the prevalence of Salmonella in broiler flocks of Gallus gallus, in the EU, 2005–2006, Part A. EFSA J. 2007, 98, 1–95. [Google Scholar]
- Le Bouquin, S.; Allain, V.; Rouxel, S.; Petetin, I.; Picherot, M.; Michel, V.; Chemaly, M. Prevalence and risk factors for Salmonella spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 2010, 97, 245–251. [Google Scholar] [CrossRef]
- Huneau-Salaün, A.; Chemaly, M.; Le Bouquin, S.; Lalande, F.; Petetin, I.; Rouxel, S.; Michel, V.; Fravalo, P.; Rose, N. Risk factors for Salmonella enterica subsp. enterica contamination in 519 French laying hen flocks at the end of the laying period. Prev. Vet. Med. 2009, 89, 51–58. [Google Scholar] [CrossRef]
- Aury, K.; Chemaly, M.; Petetin, I.; Rouxel, S.; Picherot, M.; Michel, V.; Le Bouquin, S. Prevalence and risk factors for Salmonella enterica subsp. enterica contamination in French breeding and fattening turkey flocks at the end of the rearing period. Prev. Vet. Med. 2010, 94, 84–93. [Google Scholar]
- Abdel Rahman, M.; EL-Jakee, J.; Nasef, S. Prevalence of Salmonella from layers and layer breeders farms. Anim. Health Res. J. 2014, 2, 420–432. [Google Scholar]
- Soliman, S.; Seida, A.; El-Fakar, S.; Youssef, Y.; El-Jakee, J. Salmonella infection in Broiler flocks in Egypt. Biosci. Res. 2018, 15, 1925–1930. [Google Scholar]
- Kaoud, H.A.; El-Babbly, M.A.; El-Iraqi, K.G.; Khalil, M.M. Prevalence of Salmonella spp in some broiler farms in different Egyptian Governorates. J. Vet. Med. Res. 2018, 25, 164–173. [Google Scholar] [CrossRef]
- Sayed, F.G.; Galal, L.A.; Elossily, N.A.; Ahmad, R.A. Prevalence of Blastocystis spp in house and farm raised chicken in Assiut. Al-Azhar Assiut Med. J. 2015, 13, 442–449. [Google Scholar]
- Mokhtar, A.; Youssef, A. Subtype analysis of Blastocystis sp. isolated from domestic mammals and poultry and its relation to transmission to their in-contact humans in Ismailia governorate, Egypt. Parasitol. United J. 2018, 11, 90–98. [Google Scholar] [CrossRef]
- Skånseng, B.; Kaldhusdal, M.; Rudi, K. Comparison of chicken gut colonisation by the pathogens Campylobacter jejuni and Clostridium perfringens by real-time quantitative PCR. Mol. Cell. Probes 2006, 20, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, A.; Fravalo, P.; Yergeau, É.; Arsenault, J.; Lahaye, L.; Letellier, A. Chicken caecal microbiome modifications induced by Campylobacter jejuni colonization and by a non-antibiotic feed additive. PLoS ONE 2015, 10, e0131978. [Google Scholar] [CrossRef]
- Awad, W.A.; Dublecz, F.; Hess, C.; Dublecz, K.; Khayal, B.; Aschenbach, J.R.; Hess, M. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult. Sci. 2016, 95, 2259–2265. [Google Scholar] [CrossRef]
- Axelsson-Olsson, D.; Waldenström, J.; Broman, T.; Olsen, B.; Holmberg, M. Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni. Appl. Environ. Microbiol. 2005, 71, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Axelsson-Olsson, D.; Ellström, P.; Waldenström, J.; Haemig, P.D.; Brudin, L.; Olsen, B. Acanthamoeba-Campylobacter coculture as a novel method for enrichment of Campylobacter species. Appl. Environ. Microbiol. 2007, 73, 6864–6869. [Google Scholar] [CrossRef] [PubMed]
- Axelsson-Olsson, D.; Olofsson, J.; Svensson, L.; Griekspoor, P.; Waldenström, J.; Ellström, P.; Olsen, B. Amoebae and algae can prolong the survival of Campylobacter species in co-culture. Exp. Parasitol. 2010, 126, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, F.; Scherwitzel, M.; Paulsen, P.; Szostak, M.P. Survival of Campylobacter jejuni under conditions of atmospheric oxygen tension with the support of Pseudomonas spp. Appl. Environ. Microbiol. 2010, 76, 5911–5917. [Google Scholar] [CrossRef] [PubMed]
- Bui, X.T.; Winding, A.; Qvortrup, K.; Wolff, A.; Bang, D.D.; Creuzenet, C. Survival of Campylobacter jejuni in co-culture with Acanthamoeba castellanii: Role of amoeba-mediated depletion of dissolved oxygen. Environ. Microbiol. 2012, 14, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Karki, A.B.; Ballard, K.; Harper, C.; Sheaff, R.J.; Fakhr, M.K. Staphylococcus aureus enhances biofilm formation, aerotolerance, and survival of Campylobacter strains isolated from retail meats. Sci. Rep. 2021, 11, 13837. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority) Panel on Biological Hazards. Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, e05596.
- Greige, S.; Rivoal, K.; Osman, M.; Safadi, D.E.; Dabboussi, F.; Hage, R.E.; Viscogliosi, E.; Hamze, M.; Chemaly, M. Prevalence and genetic diversity of Campylobacter spp. in the production chain of broiler chickens in Lebanon and its association with the intestinal protozoan Blastocystis sp. Poult. Sci. 2019, 98, 5883–5891. [Google Scholar] [CrossRef]
- Naguib, D.; Gantois, N.; Desramaut, J.; Arafat, N.; Even, G.; Certad, G.; Chabé, M.; Viscogliosi, E. Prevalence, Subtype Distribution and Zoonotic Significance of Blastocystis sp. Isolates from Poultry, Cattle and Pets in Northern Egypt. Microorganisms 2022, 10, 2259. [Google Scholar] [CrossRef]
- Anis, N.; Bonifait, L.; Quesne, S.; Baugé, L.; Chemaly, M.; Guyard-Nicodème, M. Simultaneous Detection of Salmonella spp. and Quantification of Campylobacter spp. in a Real-Time Duplex PCR: Myth or Reality? Pathogens 2023, 12, 338. [Google Scholar] [CrossRef]
- Stern, N.J.; Bailey, J.S.; Blankenship, L.C.; Cox, N.A.; McHan, F. Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 1988, 32, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.A.; Simpson, J.M.; Lovell, M.A. Intestinal colonisation in the chicken by food-poisoning Salmonella serotypes; microbial characteristics associated with faecal excretion. Avian Pathol. 1988, 17, 571–588. [Google Scholar] [CrossRef]
- Anis, N.; Bonifait, L.; Quesne, S.; Baugé, L.; Yassine, W.; Guyard-Nicodème, M.; Chemaly, M. Survival of Campylobacter jejuni co-cultured with Salmonella spp. in aerobic conditions. Pathogens 2022, 11, 812. [Google Scholar] [CrossRef]
- Rouxel, S.; Le Gall, F.; Laisney, M.J.; Bohnert, M.; Fravalo, P.; Chemaly, M. Investigating the effect of co-infected birds by Salmonella and Campylobacter. In Proceedings of the International Symposium Salmonella and Salmonellosis, Saint-Malo, France, 24–26 September 2018. [Google Scholar]
- Deng, L.; Wojciech, L.; Gascoigne, N.R.J.; Peng, G.; Tan, K.S.W. New insights into the interactions between Blastocystis, the gut microbiota, and host immunity. PLoS Pathog. 2021, 17, e1009253. [Google Scholar] [CrossRef] [PubMed]
- Yason, J.A.; Liang, Y.R.; Png, C.W.; Zhang, Y.; Tan, K.S.W. Interactions between a pathogenic Blastocystis subtype and gut microbiota: In vitro and in vivo studies. Microbiome 2019, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Sodhi, N.; Chenu, J.W.; Cox, J.M.; Riordan, S.M.; Mitchell, H.M. The interplay between Campylobacter and Helicobacter species and other gastrointestinal microbiota of commercial broiler chickens. Gut Pathog. 2014, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Mann, E.; Dzieciol, M.; Hess, C.; Schmitz-Esser, S.; Wagner, M.; Hess, M. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni Infection. Front. Cell. Infect. Microbiol. 2016, 6, 154. [Google Scholar] [CrossRef]
- Nourrisson, C.; Scanzi, J.; Pereira, B.; NkoudMongo, C.; Wawrzyniak, I.; Cian, A.; Viscogliosi, E.; Livrelli, V.; Delbac, F.; Dapoigny, M.; et al. Blastocystis is associated with decrease of fecal microbiota protective bacteria: Comparative analysis between patients with irritable bowel syndrome and control subjects. PLoS ONE 2014, 9, e111868. [Google Scholar] [CrossRef]
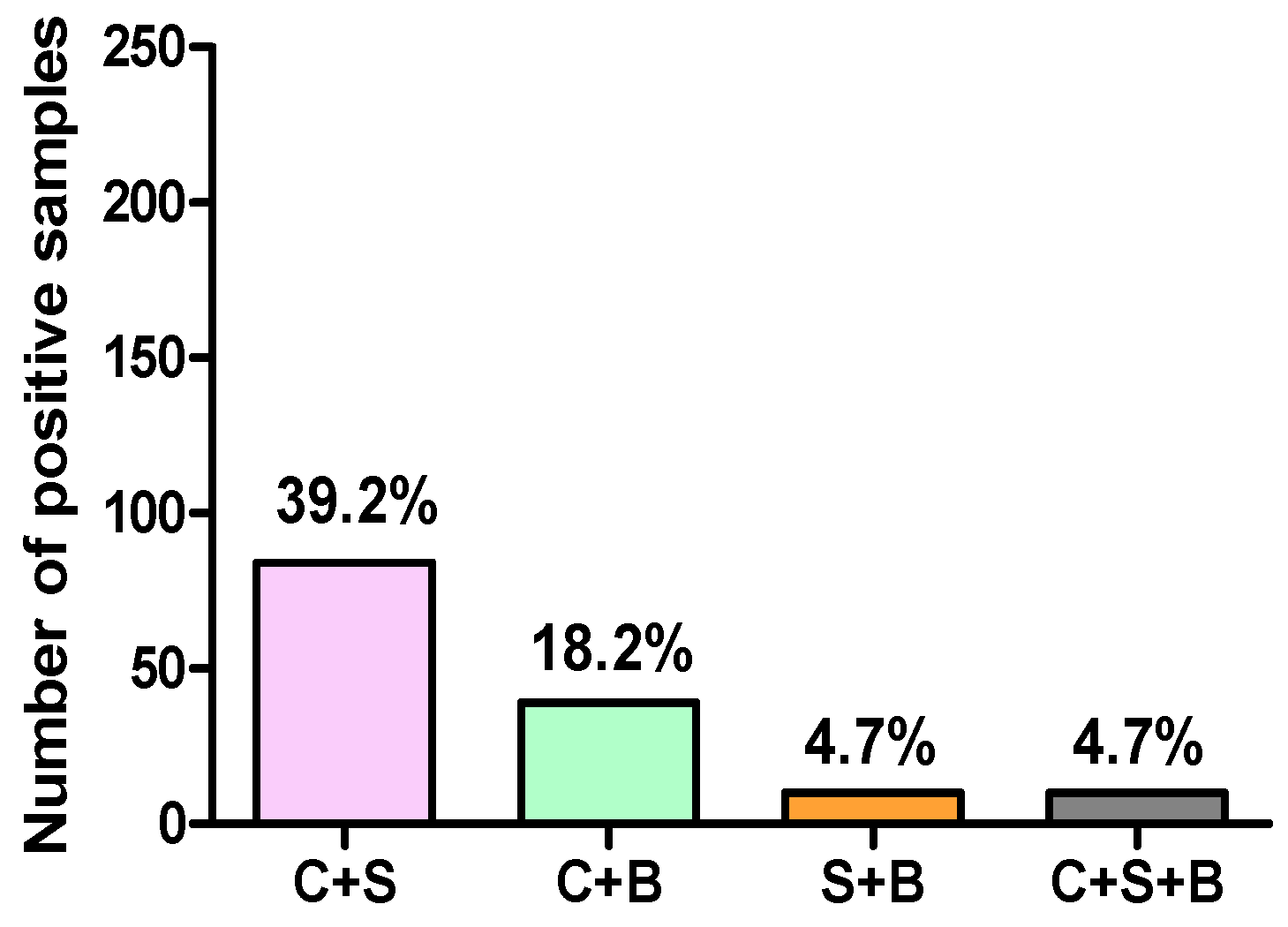
| Broilers | Layers | Breeders | ||
|---|---|---|---|---|
| Age (days) | 14–116 | 132–510 | 194–539 | |
| Chicken breeds | Ross; Cobb; Hubbard; Sasso; Baladi | White; Brown | Cobb; Hubbard; White | |
| Number of samples | Farm | 49 | 22 | 16 |
| Live bird market | 127 | 0 | 0 | |
| Total number of samples | 214 | |||
| Number of Positive Samples | |||||
|---|---|---|---|---|---|
| Origin of the Samples | Poultry Type | Number of Samples | C | S | B |
| Farm | Broilers | 49 | 44 | 22 | 9 |
| Layers | 22 | 20 | 11 | 4 | |
| Breeders | 16 | 14 | 9 | 1 | |
| Live bird market | Broilers | 127 | 118 | 53 | 25 |
| Number of Positive Samples | ||||||
|---|---|---|---|---|---|---|
| Origin of the Samples | Poultry Type | Number of Samples | C + S | C + B | S + B | C + S + B |
| Farm | Broilers | 49 | 19 | 9 | 1 | 1 |
| Layers | 22 | 11 | 4 | 2 | 2 | |
| Breeders | 16 | 8 | 1 | 0 | 0 | |
| Live bird market | Broilers | 127 | 46 | 25 | 7 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guyard-Nicodème, M.; Anis, N.; Naguib, D.; Viscogliosi, E.; Chemaly, M. Prevalence and Association of Campylobacter spp., Salmonella spp., and Blastocystis sp. in Poultry. Microorganisms 2023, 11, 1983. https://doi.org/10.3390/microorganisms11081983
Guyard-Nicodème M, Anis N, Naguib D, Viscogliosi E, Chemaly M. Prevalence and Association of Campylobacter spp., Salmonella spp., and Blastocystis sp. in Poultry. Microorganisms. 2023; 11(8):1983. https://doi.org/10.3390/microorganisms11081983
Chicago/Turabian StyleGuyard-Nicodème, Muriel, Nagham Anis, Doaa Naguib, Eric Viscogliosi, and Marianne Chemaly. 2023. "Prevalence and Association of Campylobacter spp., Salmonella spp., and Blastocystis sp. in Poultry" Microorganisms 11, no. 8: 1983. https://doi.org/10.3390/microorganisms11081983
APA StyleGuyard-Nicodème, M., Anis, N., Naguib, D., Viscogliosi, E., & Chemaly, M. (2023). Prevalence and Association of Campylobacter spp., Salmonella spp., and Blastocystis sp. in Poultry. Microorganisms, 11(8), 1983. https://doi.org/10.3390/microorganisms11081983







