Comparative Microbiome Analysis of Three Epidemiologically Important Tick Species in Latvia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Design and Tick Sampling
2.2. DNA Extraction and Tick Species Identification
2.3. Library Preparation and Sequencing
2.4. 16S rRNA Amplicon Sequence Analysis
2.5. Statistical Analysis
3. Results
3.1. Tick Sample Characteristics
3.2. Microbial Diversity and Microbiome Composition of Three Tick Species
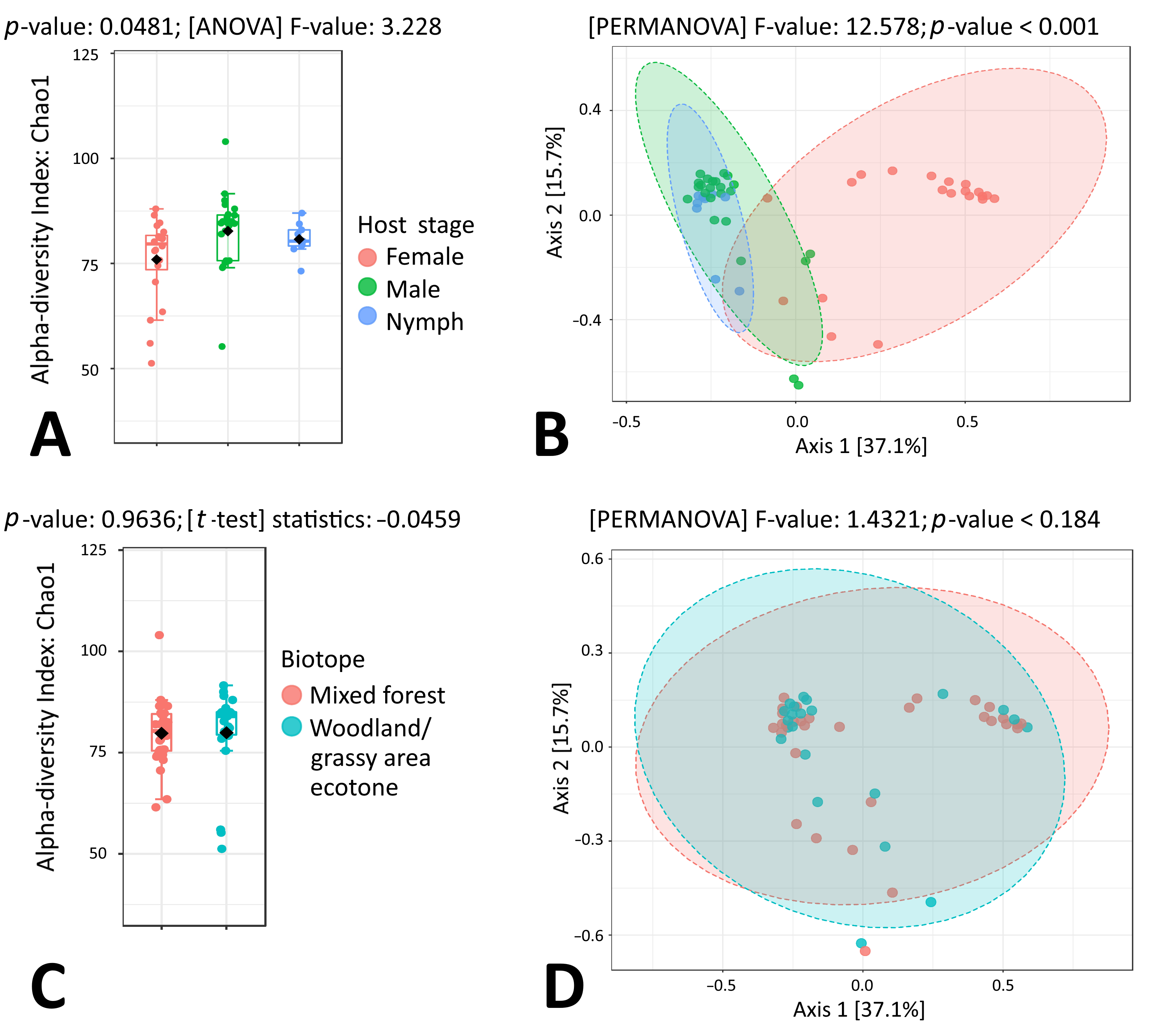
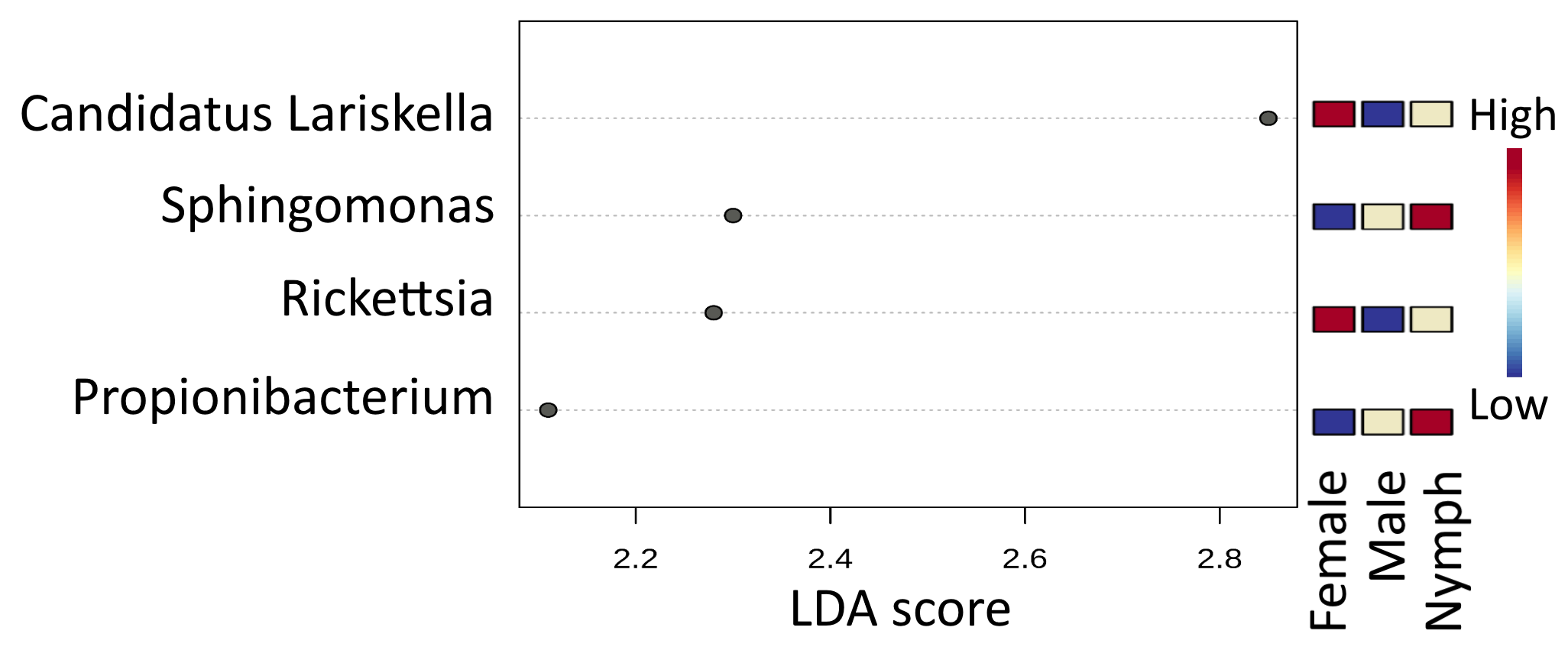
3.3. Microbiome Composition of I. ricinus Ticks
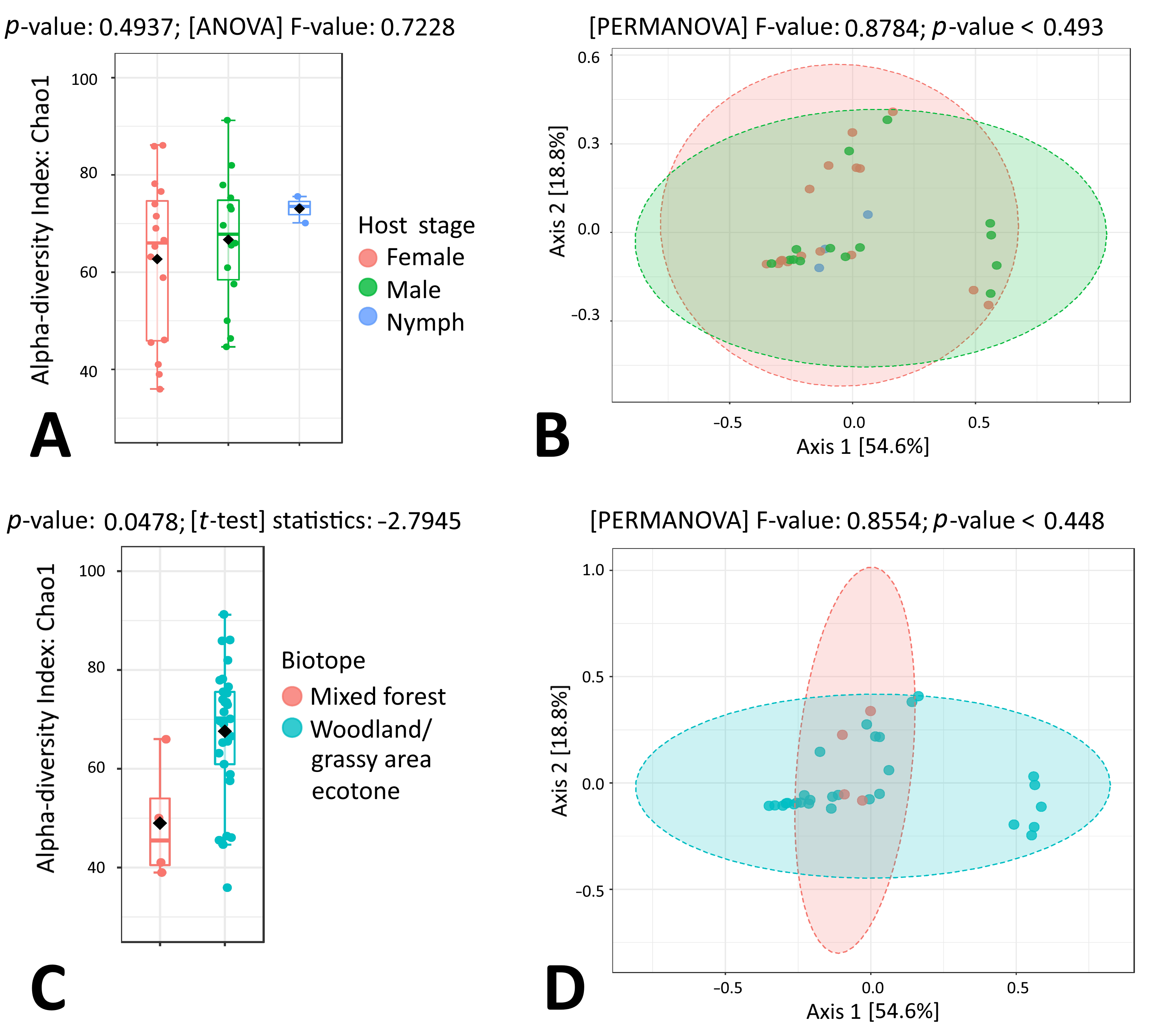
3.4. Microbiome Composition of I. persulcatus Ticks
3.5. Microbiome Composition of D. reticulatus Ticks
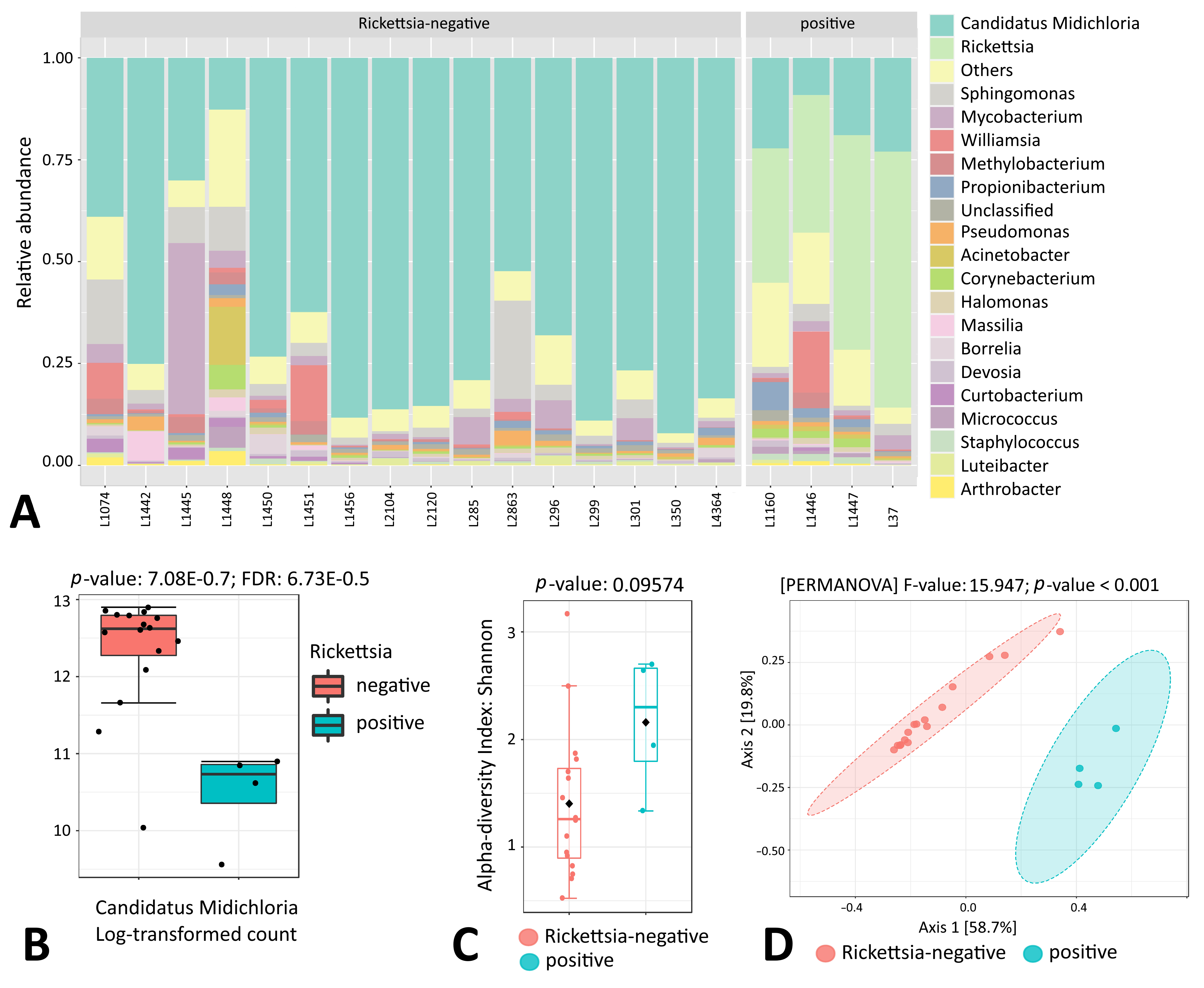
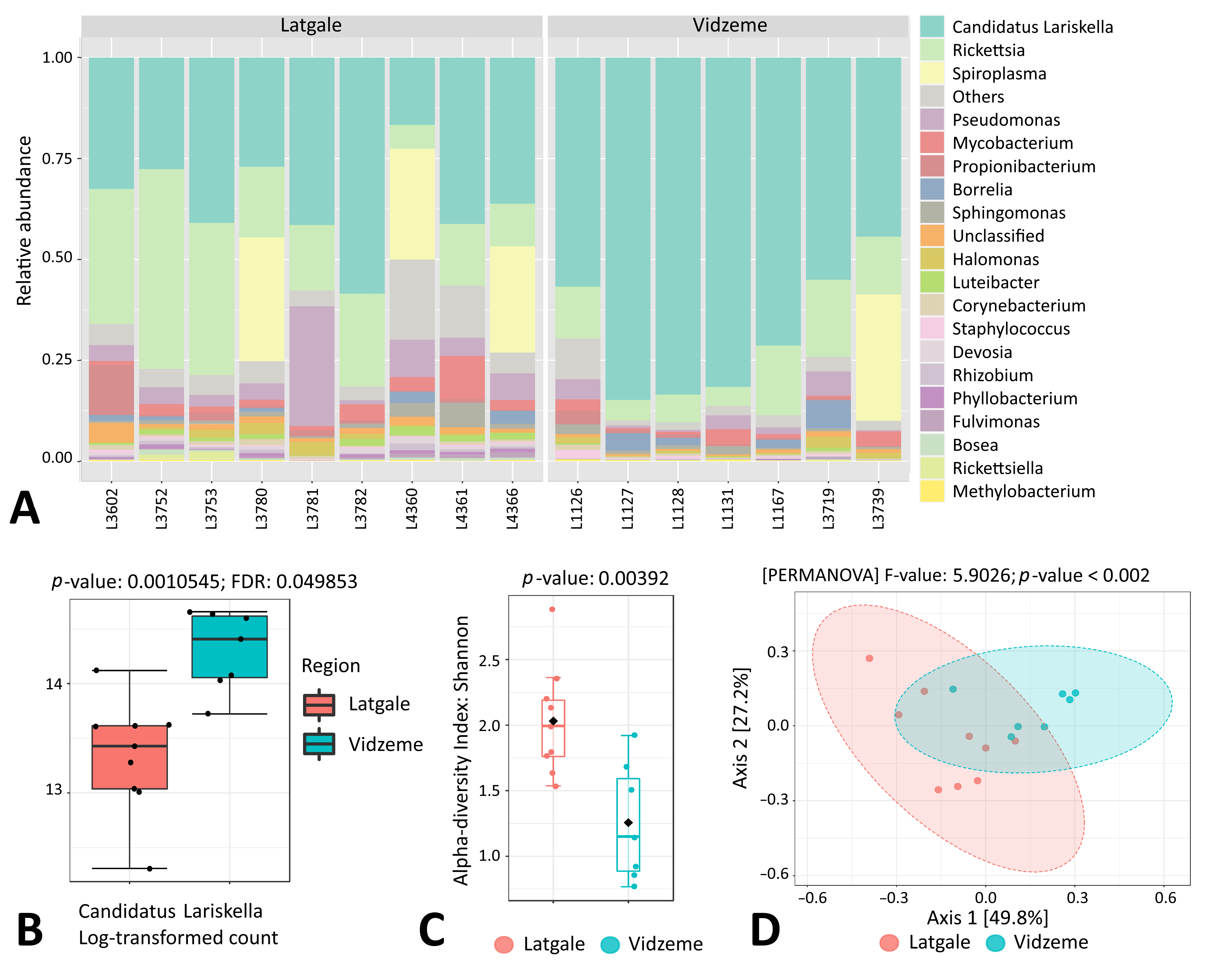
3.6. Endosymbiont Species Abundance in Female Ixodes Ticks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narasimhan:, S.; Fikrig, E. Tick Microbiome: The Force Within. Trends Parasitol. 2015, 31, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.A.; Spacek, D.; Snyder, M.P. High-Throughput Sequencing Technologies. Mol. Cell 2015, 58, 586–597. [Google Scholar] [CrossRef]
- Pollet, T.; Sprong, H.; Lejal, E.; Krawczyk, A.I.; Moutailler, S.; Cosson, J.-F.; Vayssier-Taussat, M.; Estrada-Peña, A. The Scale Affects Our View on the Identification and Distribution of Microbial Communities in Ticks. Parasites Vectors 2020, 13, 36. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Binetruy, F.; Hernández-Jarguín, A.M.; Duron, O. The Tick Microbiome: Why Non-Pathogenic Microorganisms Matter in Tick Biology and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 236. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Pollet, T. Update on the Intricate Tango between Tick Microbiomes and Tick-Borne Pathogens. Parasite Immunol. 2021, 43, e12813. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Swei, A.; Abouneameh, S.; Pal, U.; Pedra, J.H.F.; Fikrig, E. Grappling with the Tick Microbiome. Trends Parasitol. 2021, 37, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Aivelo, T.; Norberg, A.; Tschirren, B. Bacterial Microbiota Composition of Ixodes Ricinus Ticks: The Role of Environmental Variation, Tick Characteristics and Microbial Interactions. PeerJ 2019, 7, e8217. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Cabezas-Cruz, A.; Pollet, T.; Vayssier-Taussat, M.; Cosson, J.-F. High Throughput Sequencing and Network Analysis Disentangle the Microbial Communities of Ticks and Hosts Within and Between Ecosystems. Front. Cell. Infect. Microbiol. 2018, 8, 236. [Google Scholar] [CrossRef]
- Aivelo, T.; Lemoine, M.; Tschirren, B. Elevational Changes in Bacterial Microbiota Structure and Diversity in an Arthropod-Disease Vector. Microb. Ecol. 2022, 84, 868–878. [Google Scholar] [CrossRef]
- Brinkerhoff, R.J.; Clark, C.; Ocasio, K.; Gauthier, D.T.; Hynes, W.L. Factors Affecting the Microbiome of Ixodes Scapularis and Amblyomma Americanum. PLoS ONE 2020, 15, e0232398. [Google Scholar] [CrossRef]
- Batool, M.; Blazier, J.C.; Rogovska, Y.V.; Wang, J.; Liu, S.; Nebogatkin, I.V.; Rogovskyy, A.S. Metagenomic Analysis of Individually Analyzed Ticks from Eastern Europe Demonstrates Regional and Sex-Dependent Differences in the Microbiota of Ixodes Ricinus. Ticks Tick-Borne Dis. 2021, 12, 101768. [Google Scholar] [CrossRef]
- Van Treuren, W.; Ponnusamy, L.; Brinkerhoff, R.J.; Gonzalez, A.; Parobek, C.M.; Juliano, J.J.; Andreadis, T.G.; Falco, R.C.; Ziegler, L.B.; Hathaway, N.; et al. Variation in the Microbiota of Ixodes Ticks with Regard to Geography, Species, and Sex. Appl. Environ. Microbiol. 2015, 81, 6200–6209. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, A.I.; Röttjers, L.; Fonville, M.; Takumi, K.; Takken, W.; Faust, K.; Sprong, H. Quantitative Microbial Population Study Reveals Geographical Differences in Bacterial Symbionts of Ixodes Ricinus. Microbiome 2022, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.T.; Elshahed, M.S.; Sundstrom, K.D.; Little, S.E.; Youssef, N.H. Influence of Tick Sex and Geographic Region on the Microbiome of Dermacentor Variabilis Collected from Dogs and Cats across the United States. Ticks Tick-Borne Dis. 2022, 13, 102002. [Google Scholar] [CrossRef] [PubMed]
- Capligina, V.; Seleznova, M.; Akopjana, S.; Freimane, L.; Lazovska, M.; Krumins, R.; Kivrane, A.; Namina, A.; Aleinikova, D.; Kimsis, J.; et al. Large-Scale Countrywide Screening for Tick-Borne Pathogens in Field-Collected Ticks in Latvia during 2017–2019. Parasites Vectors 2020, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Filippova, N.A. Fauna SSSR Paukoobraznye. Arachnida Class: Ixodid Ticks of the Subfamily Ixodinae; Nauka: Leningrad, Russia, 1977. [Google Scholar]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. (Eds.) Ticks of Europe and North Africa; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-63759-4. [Google Scholar]
- Sprong, H.; Fonville, M.; Docters van Leeuwen, A.; Devillers, E.; Ibañez-Justicia, A.; Stroo, A.; Hansford, K.; Cull, B.; Medlock, J.; Heyman, P.; et al. Detection of Pathogens in Dermacentor Reticulatus in Northwestern Europe: Evaluation of a High-Throughput Array. Heliyon 2019, 5, e01270. [Google Scholar] [CrossRef]
- Goecks, J.; Nekrutenko, A.; Taylor, J. Galaxy Team Galaxy: A Comprehensive Approach for Supporting Accessible, Reproducible, and Transparent Computational Research in the Life Sciences. Genome Biol. 2010, 11, R86. [Google Scholar] [CrossRef]
- Kosakovsky Pond, S.; Wadhawan, S.; Chiaromonte, F.; Ananda, G.; Chung, W.-Y.; Taylor, J.; Nekrutenko, A. Windshield Splatter Analysis with the Galaxy Metagenomic Pipeline. Genome Res. 2009, 19, 2144–2153. [Google Scholar] [CrossRef]
- Jing, G.; Sun, Z.; Wang, H.; Gong, Y.; Huang, S.; Ning, K.; Xu, J.; Su, X. Parallel-META 3: Comprehensive Taxonomical and Functional Analysis Platform for Efficient Comparison of Microbial Communities. Sci. Rep. 2017, 7, 40371. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A Web-Based Tool for Comprehensive Statistical, Visual and Meta-Analysis of Microbiome Data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef] [PubMed]
- Chicana, B.; Couper, L.I.; Kwan, J.Y.; Tahiraj, E.; Swei, A. Comparative Microbiome Profiles of Sympatric Tick Species from the Far-Western United States. Insects 2019, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.Y.; Griggs, R.; Chicana, B.; Miller, C.; Swei, A. Vertical vs. Horizontal Transmission of the Microbiome in a Key Disease Vector, Ixodes Pacificus. Mol. Ecol. 2017, 26, 6578–6589. [Google Scholar] [CrossRef]
- Carpi, G.; Cagnacci, F.; Wittekindt, N.E.; Zhao, F.; Qi, J.; Tomsho, L.P.; Drautz, D.I.; Rizzoli, A.; Schuster, S.C. Metagenomic Profile of the Bacterial Communities Associated with Ixodes Ricinus Ticks. PLoS ONE 2011, 6, e25604. [Google Scholar] [CrossRef]
- de Carvalho, I.L.; Santos, N.; Soares, T.; Zé-Zé, L.; Núncio, M.S. Francisella-like Endosymbiont in Dermacentor Reticulatus Collected in Portugal. Vector Borne Zoonotic Dis. Larchmt. N 2011, 11, 185–188. [Google Scholar] [CrossRef]
- Sassera, D.; Beninati, T.; Bandi, C.; Bouman, E.A.P.; Sacchi, L.; Fabbi, M.; Lo, N. “Candidatus Midichloria Mitochondrii”, an Endosymbiont of the Tick Ixodes Ricinus with a Unique Intramitochondrial Lifestyle. Int. J. Syst. Evol. Microbiol. 2006, 56, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Lejal, E.; Chiquet, J.; Aubert, J.; Robin, S.; Estrada-Peña, A.; Rue, O.; Midoux, C.; Mariadassou, M.; Bailly, X.; Cougoul, A.; et al. Temporal Patterns in Ixodes Ricinus Microbial Communities: An Insight into Tick-Borne Microbe Interactions. Microbiome 2021, 9, 153. [Google Scholar] [CrossRef]
- René-Martellet, M.; Minard, G.; Massot, R.; Tran Van, V.; Valiente Moro, C.; Chabanne, L.; Mavingui, P. Bacterial Microbiota Associated with Rhipicephalus Sanguineus (s.l.) Ticks from France, Senegal and Arizona. Parasites Vectors 2017, 10, 416. [Google Scholar] [CrossRef]
- Budachetri, K.; Kumar, D.; Crispell, G.; Beck, C.; Dasch, G.; Karim, S. The Tick Endosymbiont Candidatus Midichloria Mitochondrii and Selenoproteins Are Essential for the Growth of Rickettsia Parkeri in the Gulf Coast Tick Vector. Microbiome 2018, 6, 141. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Hayes, S.; Mavros, A.J. Nonpathogenic Rickettsiae in Dermacentor Andersoni: A Limiting Factor for the Distribution of Rickettsia Rickettsii. In Conference on Rickettsiae and Rickettsial Diseases (1980: Rocky Mountain Laboratories); Rickettsiae and rickettsial diseases; Academic Press: New York, NY, USA, 1981; pp. 585–594. ISBN 0-12-143150-9. [Google Scholar]
- Macaluso, K.R.; Sonenshine, D.E.; Ceraul, S.M.; Azad, A.F. Rickettsial Infection in Dermacentor Variabilis (Acari: Ixodidae) Inhibits Transovarial Transmission of a Second Rickettsia. J. Med. Entomol. 2002, 39, 809–813. [Google Scholar] [CrossRef]
- Matsuura, Y.; Kikuchi, Y.; Meng, X.Y.; Koga, R.; Fukatsu, T. Novel Clade of Alphaproteobacterial Endosymbionts Associated with Stinkbugs and Other Arthropods. Appl. Environ. Microbiol. 2012, 78, 4149–4156. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.I.; Ivanov, L.I.; Nishikawa, M.; Saito, R.; Sidel’nikov, I.N.; Zdanovskaia, N.I.; Mokretsova, E.V.; Tarasevich, I.V.; Suzuki, H. Microorganism “Montezuma” of the order Rickettsiales: The potential causative agent of tick-borne disease in the Far East of Russia. Zhurnal Mikrobiol. Epidemiol. I Immunobiol. 2004, 1, 7–13. [Google Scholar]
- Kurilshikov, A.; Livanova, N.N.; Fomenko, N.V.; Tupikin, A.E.; Rar, V.A.; Kabilov, M.R.; Livanov, S.G.; Tikunova, N.V. Comparative Metagenomic Profiling of Symbiotic Bacterial Communities Associated with Ixodes Persulcatus, Ixodes Pavlovskyi and Dermacentor Reticulatus Ticks. PLoS ONE 2015, 10, e0131413. [Google Scholar] [CrossRef]
- Eremeeva, M.E.; Oliveira, A.; Robinson, J.B.; Ribakova, N.; Tokarevich, N.K.; Dasch, G.A. Prevalence of Bacterial Agents in Ixodes Persulcatus Ticks from the Vologda Province of Russia. Ann. N. Y. Acad. Sci. 2006, 1078, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Binetruy, F.; Noël, V.; Cremaschi, J.; McCoy, K.D.; Arnathau, C.; Plantard, O.; Goolsby, J.; Pérez de León, A.A.; Heylen, D.J.A.; et al. Evolutionary Changes in Symbiont Community Structure in Ticks. Mol. Ecol. 2017, 26, 2905–2921. [Google Scholar] [CrossRef] [PubMed]
- Eremeeva, M.E.; Oliveira, A.; Moriarity, J.; Robinson, J.B.; Tokarevich, N.K.; Antyukova, L.P.; Pyanyh, V.A.; Emeljanova, O.N.; Ignatjeva, V.N.; Buzinov, R.; et al. Detection and Identification of Bacterial Agents in Ixodes Persulcatus Schulze Ticks from the North Western Region of Russia. Vector Borne Zoonotic Dis. Larchmt. N 2007, 7, 426–436. [Google Scholar] [CrossRef]
- Mukhacheva, T.A.; Kovalev, S.Y. Bacteria of the Family ‘Candidatus Midichloriaceae’ in Sympatric Zones of Ixodes Ticks: Genetic Evidence for Vertical Transmission. Microb. Ecol. 2017, 74, 185–193. [Google Scholar] [CrossRef]



| Tick Species | Tick Stage | No (%) of Samples | Biotope, No. (%) of Tick Samples | |
|---|---|---|---|---|
| Mixed Forest | Woodland/Grassy Area Ecotone | |||
| I. ricinus | Female | 20 (37.7) | 14 (70.0) | 6 (30.0) |
| Male | 24 (45.3) | 10 (41.7) | 14 (58.3) | |
| Nymph | 9 (17.0) | 7 (77.8) | 2 (22.2) | |
| Total | 53 | 31 (58.5) | 22 (41.5) | |
| I. persulcatus | Female | 16 (40.0) | 10 (62.5) | 6 (37.5) |
| Male | 17 (42.5) | 7 (41.2) | 10 (58.8) | |
| Nymph | 7 (17.5) | 4 (57.1) | 3 (42.9) | |
| Total | 40 | 21 (52.5) | 19 (47.5) | |
| D. reticulatus | Female | 16 (48.5) | 2 (12.5) | 14 (87.5) |
| Male | 14 (42.4) | 2 (14.3) | 12 (85.7) | |
| Nymph | 3 (9.1) | 0 | 3 (100.0) | |
| Total | 33 | 4 (12.1) | 29 (87.9) | |
| Total | 126 | 56 | 70 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namina, A.; Kazarina, A.; Lazovska, M.; Akopjana, S.; Ulanova, V.; Kivrane, A.; Freimane, L.; Sadovska, D.; Kimsis, J.; Bormane, A.; et al. Comparative Microbiome Analysis of Three Epidemiologically Important Tick Species in Latvia. Microorganisms 2023, 11, 1970. https://doi.org/10.3390/microorganisms11081970
Namina A, Kazarina A, Lazovska M, Akopjana S, Ulanova V, Kivrane A, Freimane L, Sadovska D, Kimsis J, Bormane A, et al. Comparative Microbiome Analysis of Three Epidemiologically Important Tick Species in Latvia. Microorganisms. 2023; 11(8):1970. https://doi.org/10.3390/microorganisms11081970
Chicago/Turabian StyleNamina, Agne, Alisa Kazarina, Marija Lazovska, Sarmite Akopjana, Viktorija Ulanova, Agnija Kivrane, Lauma Freimane, Darja Sadovska, Janis Kimsis, Antra Bormane, and et al. 2023. "Comparative Microbiome Analysis of Three Epidemiologically Important Tick Species in Latvia" Microorganisms 11, no. 8: 1970. https://doi.org/10.3390/microorganisms11081970
APA StyleNamina, A., Kazarina, A., Lazovska, M., Akopjana, S., Ulanova, V., Kivrane, A., Freimane, L., Sadovska, D., Kimsis, J., Bormane, A., Capligina, V., & Ranka, R. (2023). Comparative Microbiome Analysis of Three Epidemiologically Important Tick Species in Latvia. Microorganisms, 11(8), 1970. https://doi.org/10.3390/microorganisms11081970





