Reduced Arbuscular Mycorrhizal Fungi (AMF) Diversity in Light and Moderate Fire Sites in Taiga Forests, Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Site Overview
2.2. Sample Plot
2.3. Soil Sample Collection and Experimental Method
2.4. Soil Physical and Chemical Properties Analysis
2.5. DNA Extraction and Arbuscular Mycorrhizal Fungi Sequencing
2.6. Data Processing
3. Results
3.1. Analysis of the Difference in Physicochemical Properties of Soils in the Fire Intensities
3.2. Differential Analysis of Soil Arbuscular Mycorrhizal Fungi Diversity in the Fire Sites
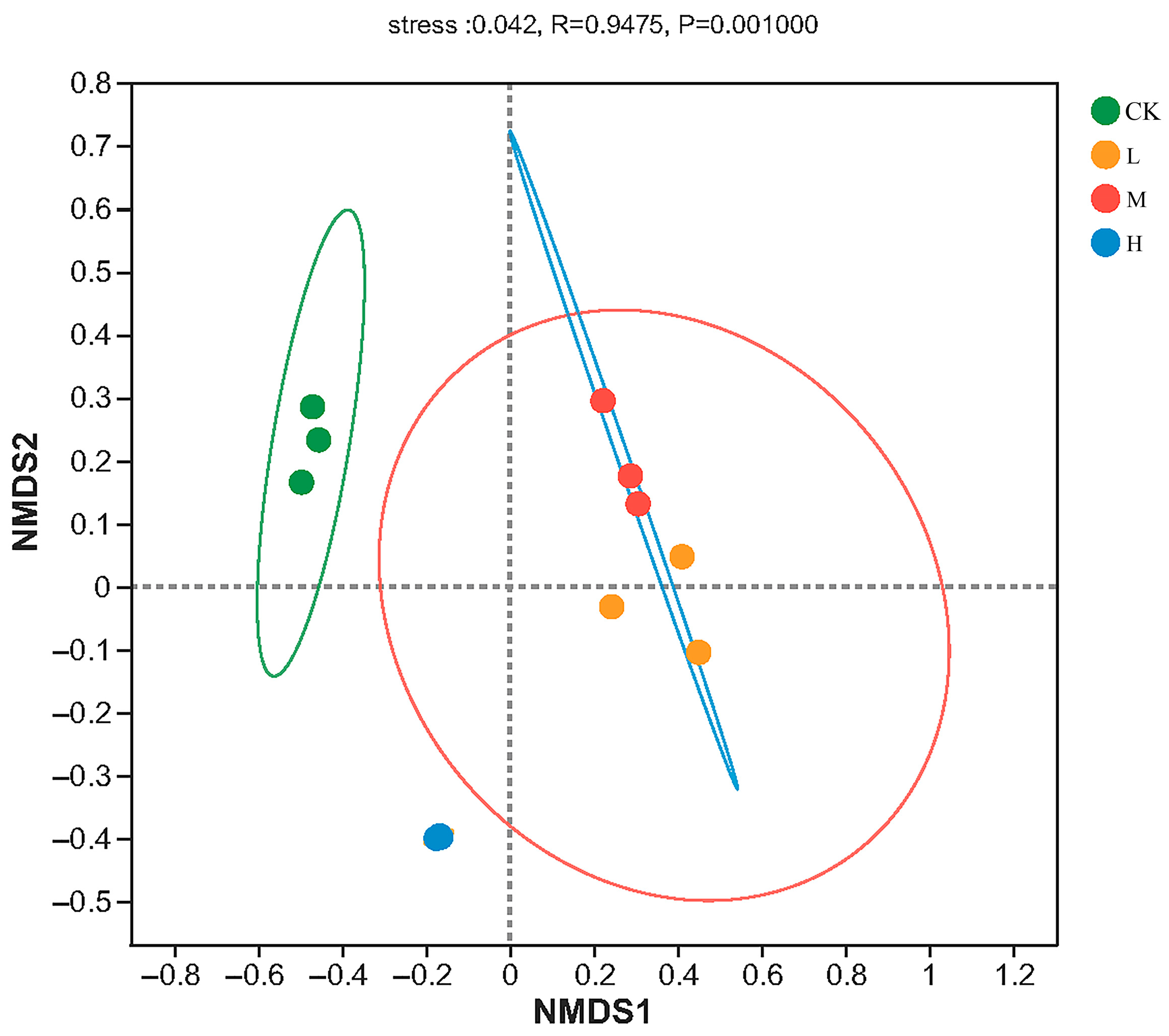
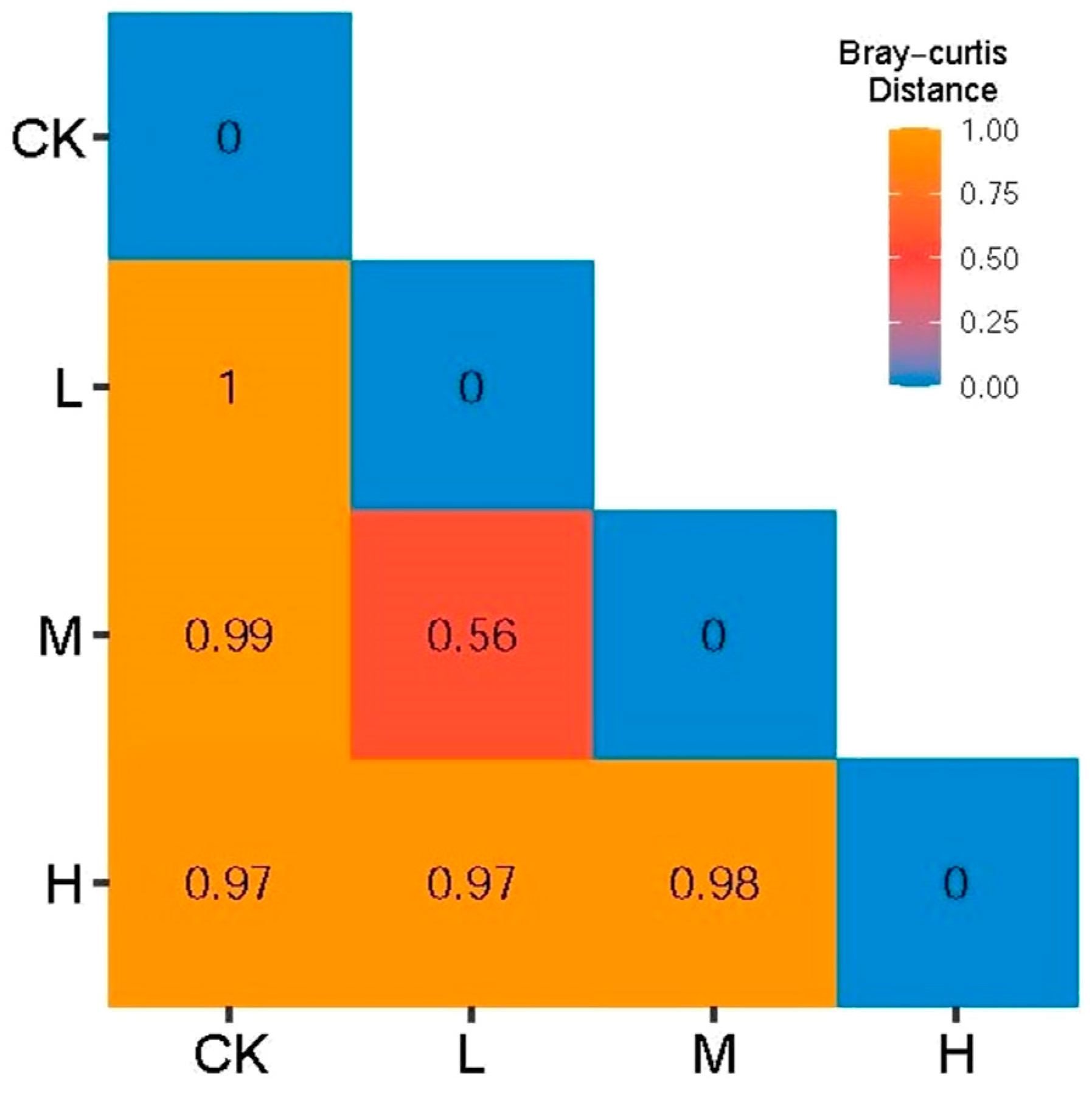
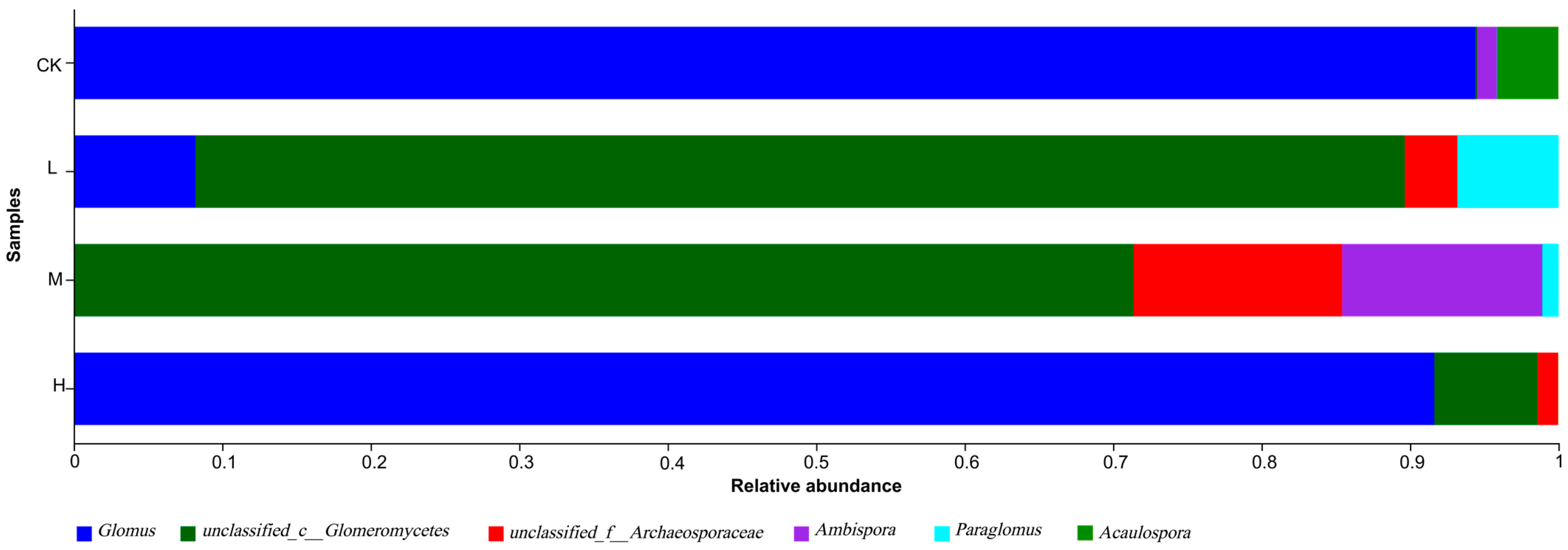
3.3. Analysis of Differences in the Structural Composition of Arbuscular Mycorrhizal Fungi Communities in Fire Trails
3.4. Correlation Analysis of Factors Influencing the Structure and Diversity of Arbuscular Mycorrhizal Fungi Communities at the Fire Sites
4. Discussion
4.1. Effect of Fire on Soil Nutrient Content
4.2. Effect of Fire on Soil Arbuscular Mycorrhizal Fungi Diversity
4.3. Effect of Different Intensities of Fire on the Structural Composition of Arbuscular Mycorrhizal Fungi Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hart, S.; Deluca, T.H.; Newman, G.S.; Mackenzie, M.D.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2005, 220, 166–184. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A review of the effects of forest fire on soil properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Hamman, S.T.; Burke, I.C.; Knapp, E.E. Soil nutrients and microbial activity after early and late season prescribed burns in a Sierra Nevada mixed conifer forest. For. Ecol. Manag. 2008, 256, 367–374. [Google Scholar] [CrossRef]
- Treseder, K.K.; Mack, M.C.; Cross, A. Relationships among fires, Fungi, and soil dynamics in Alaskan boreal forests. Ecol. Appl. 2004, 14, 1826–1838. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Zhang, L.P.; Wei, M.F.; Zhen, L. Effect of arbuscular mycorrhizal fungi, organic fertilizer and soil sterilization on maize growth. Acta Ecol. Sin. 2011, 31, 192–196. [Google Scholar] [CrossRef]
- Ajmal, R.; Ashis, B. Fire-induced geochemical changes in soil: Implication for the element cycling. Sci. Total Environ. 2023, 868, 161714. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Orchid mycorrhizas. In Mycorrhizal Symbiosis, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 349–375. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Balestrini, R.; Brunetti, C.; Chitarra, W.; Nerva, L. Photosynthetic Traits and Nitrogen Uptake in Crops: Which Is the Role of Arbuscular Mycorrhizal Fungi? Plants 2020, 9, 1105. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Z.; Xu, X.; Wang, X. Arbuscular Mycorrhizal Fungi Associated with Roots Reveal High Diversity Levels at Different Elevations in Tropical Montane Rainforests. Diversity 2022, 14, 587. [Google Scholar] [CrossRef]
- Djighaly, P.I.; Ngom, D.; Diagne, N.; Fall, D.; Ngom, M.; Diouf, D.; Hocher, V.; Laplaze, L.; Champion, A.; Farrant, J.M.; et al. Effect of Casuarina Plantations Inoculated with Arbuscular Mycorrhizal Fungi and Frankia on the Diversity of Herbaceous Vegetation in Saline Environments in Senegal. Diversity 2020, 12, 293. [Google Scholar] [CrossRef]
- Yin, Z.P.; Zhang, Y.; Hu, N.; Shi, Y.C.; Li, T.; Zhao, Z.W. Differential responses of 23 maize cultivar seedlings to an arbuscular mycorrhizal fungus when grown in a metal-polluted soil. Sci. Total Environ. 2021, 789, 14805. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.; Waller, L.; Lekberg, Y. Cascading effects of fire retardant on plant-microbe interactions, community composition, and invasion. Ecol. Appl. 2016, 26, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Nouhra, E.; Goto, B.T.; Berbara, R.L.; Urcelay, C. Effects of fire on arbuscular mycorrhizal fungi in the Mountain Chaco Forest. For. Ecol. Manag. 2014, 315, 86–94. [Google Scholar] [CrossRef]
- Moura, J.B.; Souza, R.F.; Vieira-Júnior, W.G.; Lucas, L.S.; Santos, J.M.; Silva, S.D.E.; Marin, C. Effects of a megafire on the arbuscular mycorrhizal fungal community and parameters in the Brazilian Cerrado ecosystem. For. Syst. 2022, 31, e001. [Google Scholar] [CrossRef]
- Mirzaei, J.; Heydari, M.; Omidipour, R.; Jafarian, N.; Carcaillet, C. Decrease in Soil Functionalities and Herbs’ Diversity, but Not That of Arbuscular Mycorrhizal Fungi, Linked to Short Fire Interval in Semi-Arid Oak Forest Ecosystem, West Iran. Plants 2023, 12, 1112. [Google Scholar] [CrossRef]
- Pattinson, G.S.; Hammill, K.A.; Sutton, B.G.; Mcgee, P.A. Simulated fire reduces the density of arbuscular mycorrhizal fungi at the soil surface. Mycol. Res. 1999, 103, 491–496. [Google Scholar] [CrossRef]
- Miozzi, L.; Vaira, A.M.; Catoni, M.; Fiorilli, V.; Acctotto, G.P.; Lanfranco, L. Arbuscular Mycorrhizal Symbiosis: Plant Friend or Foe in the Fight Against Viruses? Front. Microbiol. 2019, 10, 1238. [Google Scholar] [CrossRef]
- Wu, S.; Shi, Z.; Chen, X.; Gao, J.; Wang, X. Arbuscular mycorrhizal fungi increase crop yields by improving biomass under rainfed condition: A meta-analysis. PeerJ 2022, 10, e12861. [Google Scholar] [CrossRef]
- Bedini, A.; Mercy, L.; Schneider, C.; Franken, P.; Lucic-Mercy, E. Unraveling the initial plant hormone signaling, metabolic mechanisms and plant defense triggering the endomycorrhizal symbiosis behavior. Front. Plant Sci. 2018, 9, 1800. [Google Scholar] [CrossRef]
- Luo, Q.Y.; Wang, X.J.; Li, Y.Y.; Lin, S.S.; Sun, L.; Wang, Q.; Wang, Q.; Jin, L. Mechanism of biological control to plant diseases using arbuscular mycorrhizal fungi. Acta Ecol. Sin. 2013, 33, 5997–6005. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Luan, L.; Hu, K.J.; Liu, M.Q.; Chem, Z.Y.; Geisen, S.; Chen, X.Y.; Li, H.X.; Xu, Q.S.; Bonkowski, M.; et al. Trophic interactions as determinants of the arbuscular mycorrhizal fungal community with cascading plant-promoting consequences. Microbiome 2020, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, T.; Li, S.; Liu, B. Modeling Optimal Forest Rotation Age for Carbon Sequestration in the Great Khingan Mountains of Northeast China. Forests 2022, 13, 838. [Google Scholar] [CrossRef]
- Zhong, C.; Guo, M.; Zhou, F.; Li, J.; Yu, F.; Guo, F.; Li, W. Forest succession trajectories after fires in valleys and on slopes in the Greater Khingan Mountains, China. J. For. Res. 2023, 34, 623–640. [Google Scholar] [CrossRef]
- Xiang, X.J.; Shi, Y.; Yang, J.; Kong, J.J.; Lin, X.G.; Zhang, H.Y.; Zeng, J.; Chu, H.Y. Rapid recovery of soil bacterial communities after wildfire in a Chinese boreal forest. Sci. Rep. 2014, 4, 3829. [Google Scholar] [CrossRef]
- Huang, W.; Hu, Y.; Chang, Y.; Liu, M.; Li, Y.; Ren, B.; Shi, S. Effects of Fire Severity and Topography on Soil Black Carbon Accumulation in Boreal Forest of Northeast China. Forests 2018, 9, 408. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Li, M.S.; Liu, X.L.; Yin, W.P.; Li, G.F.; Mu, L.Q.; Cui, X.Y.; Cheng, Z.C. Soil bacterial community composition and diversity of typical permafrost in Greater Khingan Mountains. Microbiol. China 2020, 47, 2759–2770. [Google Scholar] [CrossRef]
- Yang, L.B.; Jiang, Y.B.; Zhou, T.; Cui, F.X.; Zhu, D.G.; Xu, F. Effects of litter fall on soil fungal diversity under snow cover in the Greater Xing’an Mountains. Res. Environ. Sci. 2022, 35, 1037–1044. [Google Scholar] [CrossRef]
- Yue, X.; Dun, X.J.; Cui, D.J.; Guo, X.D.; Song, X.Y.; Liu, L.; Wang, L.L.; Jiang, C.; Xu, J.W.; Li, S.M. Effects of Different Fire Intensities on Forest Soil Nutrients. Shandong Agric. Sci. 2018, 50, 72–76. [Google Scholar] [CrossRef]
- Shang, W.; Wu, X.D.; Zhao, L.; Yue, G.Y.; Zhao, Y.H.; Qiao, Y.P.; Li, Y.Q. Seasonal variations in labile soil organic matter fractions in permafrost soils with different vegetation types in the Central Qinghai-Tibet Plateau. Catena 2016, 137, 670–678. [Google Scholar] [CrossRef]
- Kim, H.M.; Jung, J.Y.; Yergeau, E.; Hwang, C.Y.; Hinzman, L.; Nam, S.; Hong, S.G.; Kim, O.S.; Chun, J.; Lee, Y.K. Bacterial community structure and soil properties of a subarctic tundra soil in Council, Alaska. FEMS Microbiol. Ecol. 2014, 89, 465–475. [Google Scholar] [CrossRef]
- Wear, E.K.; Wilbanks, E.G.; Nelson, C.E.; Carlson, C.A. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environ. Microbiol. 2018, 20, 2709–2726. [Google Scholar] [CrossRef] [PubMed]
- Ade, L.J.; Hu, L.; Zi, H.B.; Wang, C.T.; Lerdau, M.; Dong, S.K. Efect of snowpack on the soil bacteria of alpine meadows in the Qinghai-Tibetan Plateau of China. Catena 2018, 164, 13–22. [Google Scholar] [CrossRef]
- Hu, W.G.; Zhang, Q.; Li, D.Y.; Cheng, G.; Mu, J.; Wu, Q.B.; Niu, F.; An, L.Z.; Feng, H.Y. Diversity and community structure of fungi through a permafrost core profle from the Qinghai-Tibet Plateau of China. J. Basic Microbiol. 2015, 54, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Xue, L.; Li, Q.; Chen, H. Effects of a Wildfire on Selected Physical, Chemical and Biochemical Soil Properties in a Pinus massoniana Forest in South China. Forests 2014, 5, 2947–2966. [Google Scholar] [CrossRef]
- Johnson, D.L.; Curtis, P.S. Effects of forest management on soil C and N storage: Meta analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Wang, C.; Morrissey, E.M.; Mau, R.L.; Hayer, M.; Pineiro, J.; Mack, M.C.; Marks, J.C.; Bell, S.L.; Miller, S.N.; Schwartz, E.; et al. The temperature sensitivity of soil: Microbial biodiversity, growth, and carbon mineralization. ISME J. 2021, 15, 2738–2747. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, L.P.; Wang, X.; Wei, X.R.; Gao, H.L.; Zhang, Y.J.; Cheng, Z.M. Effects of wildfire and topography on soil nutrients in a semiarid restored grassland. Plant Soil 2018, 428, 123–136. [Google Scholar] [CrossRef]
- Mcintosh, P.D.; Laffan, M.D.; Hewitt, A.E. The role of fire and nutrient loss in the genesis of the forest soils of Tasmania and southern New Zealand. For. Ecol. Manag. 2005, 220, 185–215. [Google Scholar] [CrossRef]
- Kugbe, J.; Fosu, M.; Vlek, P.L. Impact of season, fuel load and vegetation cover on fire mediated nutrient losses across sa-vanna agro-ecosystems: The case of northern Ghana. Nutr. Cycl. Agroecosyst. 2015, 102, 113–136. [Google Scholar] [CrossRef]
- Romanya, J.; Khanna, P.K.; Raison, R.J. Effects of slash burning on soil phosphorus fractions and sorption and desorption of phosphorus. For. Ecol. Manag. 1994, 65, 89–103. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Nix, B.; Jacobs, K.A.; Bowles, M.L. Two decades of low-severity prescribed fire increases soil nutrient availability in a Midwestern, USA oak (Quercus) forest. Geoderma 2012, 183–184, 80–91. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Berch, S.M.; Preston, C.M.; Lavkulich, L.M. Phosphorus forms and related soil chemistry of Podzolic soils on northern Vancouver Island. II. The effects of clear-cutting and burning. Can. J. For. Res 2000, 30, 1726–1741. [Google Scholar] [CrossRef]
- Kim, C.G.; Shin, K.; Joo, K.Y.; Lee, K.S.; Shin, S.S.; Choung, Y. Effects of soil conservation measures in a partially vegetated area after forest fires. Sci. Total Environ. 2008, 399, 158–164. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.J. The impact of land use on spatial and temporal distribution of soil moisture on the Loess Plateau. Acta Geogr. Sin. 2000, 55, 84–91. [Google Scholar] [CrossRef]
- Yang, L.B.; Cui, F.X.; Huang, Q.Y.; Zhu, D.G.; Xu, F. Analysis on bacterial diversity and composition of Larixg melinii fallen wood with different decomposition levels. J. Cent. South Univ. For. Technol. 2021, 41, 93–100+182. [Google Scholar] [CrossRef]
- Xiang, X.; Gibbons, S.M.; Yang, J. Arbuscular mycorrhizal fungal communities show low resistance and high resilience to wildfire disturbance. Plant Soil 2015, 397, 347–356. [Google Scholar] [CrossRef]
- Rashid, A.; Ahmed, T.; Ayub, N.; Khan, A.G. Effect of forest fire on number, viability and post-fire re-establishment of arbuscular mycorrhizae. Mycorrhiza 1997, 7, 217–220. [Google Scholar] [CrossRef]
- Zhou, S.X.; Butenschoen, O.; Barantal, S.; Handa, I.T.; Makkonen, M.; Vos, V.; Aerts, R.; Berg, M.P.; McKie, B.; Van Ruijven, J.; et al. Decomposition of leaf litter mixtures across biomes: The role of litter identity, diversity and soil fauna. J. Ecol. 2020, 108, 2283–2297. [Google Scholar] [CrossRef]
- Gałązka, A.; Niedźwiecki, J.; Grządziel, J.; Gawryjołek, K. Evaluation of Changes in Glomalin-Related Soil Proteins (GRSP) Content, Microbial Diversity and Physical Properties Depending on the Type of Soil as the Important Biotic Determinants of Soil Quality. Agronomy 2020, 10, 1279. [Google Scholar] [CrossRef]
- Li, Q.; Xiang, X.J.; Du, Y.G.; Li, Y.K.; Li, L.; Zhang, F.W.; Guo, X.W.; Cao, G.M. Arbuscular mycorrhizal fungal community structure following different grazing intensities in alpine grassland. Soil Sci. Soc. Am. J. 2020, 85, 1620–1633. [Google Scholar] [CrossRef]
- Ma, X.C.; Ceng, Q.H.; Zhang, H.G.; Bian, C.Y.; Chen, H.Y.H.; Jiang, D.L.; Xu, X. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytol. 2020, 229, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P.; Chaloner, T.M. Specialists, generalists and the shape of the ecological niche in fungi. New Phytol. 2022, 234, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.S.; Bennett, A.E. Stressed out symbiotes: Hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi. Oecologia 2016, 182, 625–641. [Google Scholar] [CrossRef]
- Wang, X.; Gao, S.H.; Chen, J.Q.; Yao, Z.W.; Zhang, X.D. Reducing soil CO2, CH4 and N2O emissions through management of harvest residues in Chinese fir plantation. For. Ecol. Manag. 2022, 511, 120140. [Google Scholar] [CrossRef]
- Wang, S.; Pan, S.; Shah, G.M.; Zhang, Z.; Yang, L.; Yang, S. Enhancement in arsenic remediation by maize (Zea mays L.) using edta in combination with arbuscular mycorrhizal fungi. Appl. Ecol. Environ. Res. 2018, 16, 5987–5999. [Google Scholar] [CrossRef]
- Cairney, J.W.G.; Bastias, B.A. Influences of Fire on Forest Soil Fungal Communities. Can. J. For. Res. 2007, 37, 207–215. [Google Scholar] [CrossRef]
- Ito, A. Mega fire emissions in Siberia: Potential supply of bioavailable iron from forests to the ocean. Biogeosciences 2011, 8, 1679–1697. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhao, X.Y.; Liu, C.L.; Bai, L.; Zhao, M.; Li, L.L. Diversity and characteristics of colonization of root-associated fungi of Vaccinium uliginosum. Sci. Rep. 2018, 8, 15283. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, J.J.; Yu, Z.H.; Li, Y.S.; Jin, J.; Liu, X.B.; Wang, G.H. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, H.W.; Li, X.Y.; Su, Z.C.; Li, X.; Xu, M.K. Effects of tillage and residue incorporation on composition and abundance of microbial communities of a fluvo-aquic soil. Eur. J. Soil Biol. 2014, 65, 70–78. [Google Scholar] [CrossRef]
- Posada, R.H.; De Prager, M.; Heredia-Abarca, G.; Sieverding, E. Effects of soil physical and chemical parameters, and farm management practices on arbuscular mycorrhizal fungi communities and diversities in coffee plantations in Colombia and Mexico. Agrofor. Syst. 2018, 92, 555–574. [Google Scholar] [CrossRef]
- Huang, R.L.; Crowther, T.W.; Sui, Y.Y.; Sun, B.; Liang, Y.T. High stabiligy and metabolic capacity of bacterial community promote the rapid reduction of easily decomposing carbon in soil. Commun. Biol. 2021, 4, 1376. [Google Scholar] [CrossRef] [PubMed]





| Fire Intensity | Burning Dead Wood Ratio | Changes in Surface Vegetation |
|---|---|---|
| L (Light fire) | ≤30% | Only some of the vegetation was burned |
| M (Moderate fire) | 30–70% | Trees were partially burned, shrubs, herbs, and other vegetation were completely burned |
| H (Heavy fire) | ≥70% | Trees, shrubs, herbs, and other vegetation were completely burned to death |
| Intensity | MBC mg/kg | MC% | pH | AK mg/kg | TN g/kg | SOC g/kg | AP mg/kg | AN mg/kg |
|---|---|---|---|---|---|---|---|---|
| CK (Control—blank) | 149.97 ± 33.56 c | 0.04 ± 0.004 c | 6.79 ± 0.03 a | 213.05 ± 19.19 a | 1.69 ± 0.01 b | 97.05 ± 3.41 b | 11.74 ± 0.69 c | 96.14 ± 11.33 b |
| L (Light fire) | 268.37 ± 23.67 b | 0.16 ± 0.002 b | 6.64 ± 0.02 a | 140.07 ± 5.2 b | 1.95 ± 0.06 b | 122.98 ± 5.96 a | 46.9 ± 0.83 a | 89.37 ± 12.31 b |
| M (Moderate fire) | 847.07 ± 369.5 a | 0.12 ± 0.006 b | 6.53 ± 0.05 a | 80.43 ± 6.38 c | 1.71 ± 0.06 b | 93.16 ± 6.49 b | 18.21 ± 0.66 c | 82.13 ± 24.22 b |
| H (Heavy fire) | 349.99 ± 12.5 b | 0.27 ± 0.03 a | 5.84 ± 0.4 b | 80.97 ± 11.17 c | 2.96 ± 0.43 a | 76.06 ± 3.24 c | 27.48 ± 1.57 b | 148.16 ± 17.29 a |
| Name | MBC | MC | pH | AK | TN | SOC | AP | AN |
|---|---|---|---|---|---|---|---|---|
| Chao1 | −0.531 | −0.422 | 0.16 | 0.446 | −0.4078 | −0.109 | −0.629 * | −0.134 |
| Shannon | −0.741 ** | −0.348 | 0.228 | 0.692 * | −0.321 | 0.021 | −0.416 | 0.006 |
| Simpson | 0.727 ** | 0.369 | −0.179 | −0.734 ** | 0.251 | −0.007 | 0.391 | −0.119 |
| Soil Factors | R2 | p-Value |
|---|---|---|
| MBC | 0.7839 | 0.004 ** |
| MC | 0.0871 | 0.681 |
| pH | 0.1062 | 0.604 |
| AK | 0.1806 | 0.414 |
| TN | 0.2325 | 0.3 |
| SOC | 0.5492 | 0.035 * |
| AP | 0.7023 | 0.006 ** |
| AN | 0.3329 | 0.158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Wu, S.; Du, J.; Liu, Y.; Sui, X.; Yang, L. Reduced Arbuscular Mycorrhizal Fungi (AMF) Diversity in Light and Moderate Fire Sites in Taiga Forests, Northeast China. Microorganisms 2023, 11, 1836. https://doi.org/10.3390/microorganisms11071836
Cheng Z, Wu S, Du J, Liu Y, Sui X, Yang L. Reduced Arbuscular Mycorrhizal Fungi (AMF) Diversity in Light and Moderate Fire Sites in Taiga Forests, Northeast China. Microorganisms. 2023; 11(7):1836. https://doi.org/10.3390/microorganisms11071836
Chicago/Turabian StyleCheng, Zhichao, Song Wu, Jun Du, Yongzhi Liu, Xin Sui, and Libin Yang. 2023. "Reduced Arbuscular Mycorrhizal Fungi (AMF) Diversity in Light and Moderate Fire Sites in Taiga Forests, Northeast China" Microorganisms 11, no. 7: 1836. https://doi.org/10.3390/microorganisms11071836
APA StyleCheng, Z., Wu, S., Du, J., Liu, Y., Sui, X., & Yang, L. (2023). Reduced Arbuscular Mycorrhizal Fungi (AMF) Diversity in Light and Moderate Fire Sites in Taiga Forests, Northeast China. Microorganisms, 11(7), 1836. https://doi.org/10.3390/microorganisms11071836







