Monocyte Activation and Ageing Biomarkers in the Development of Cardiovascular Ischaemic Events or Diabetes in People with HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Plasma Biomarkers
2.3. Blood Telomere Length Measurement
2.4. DNA Methylation Analysis and Epigenetic Age Prediction
2.5. Statistical Analysis
3. Results
3.1. Description of the Study Population
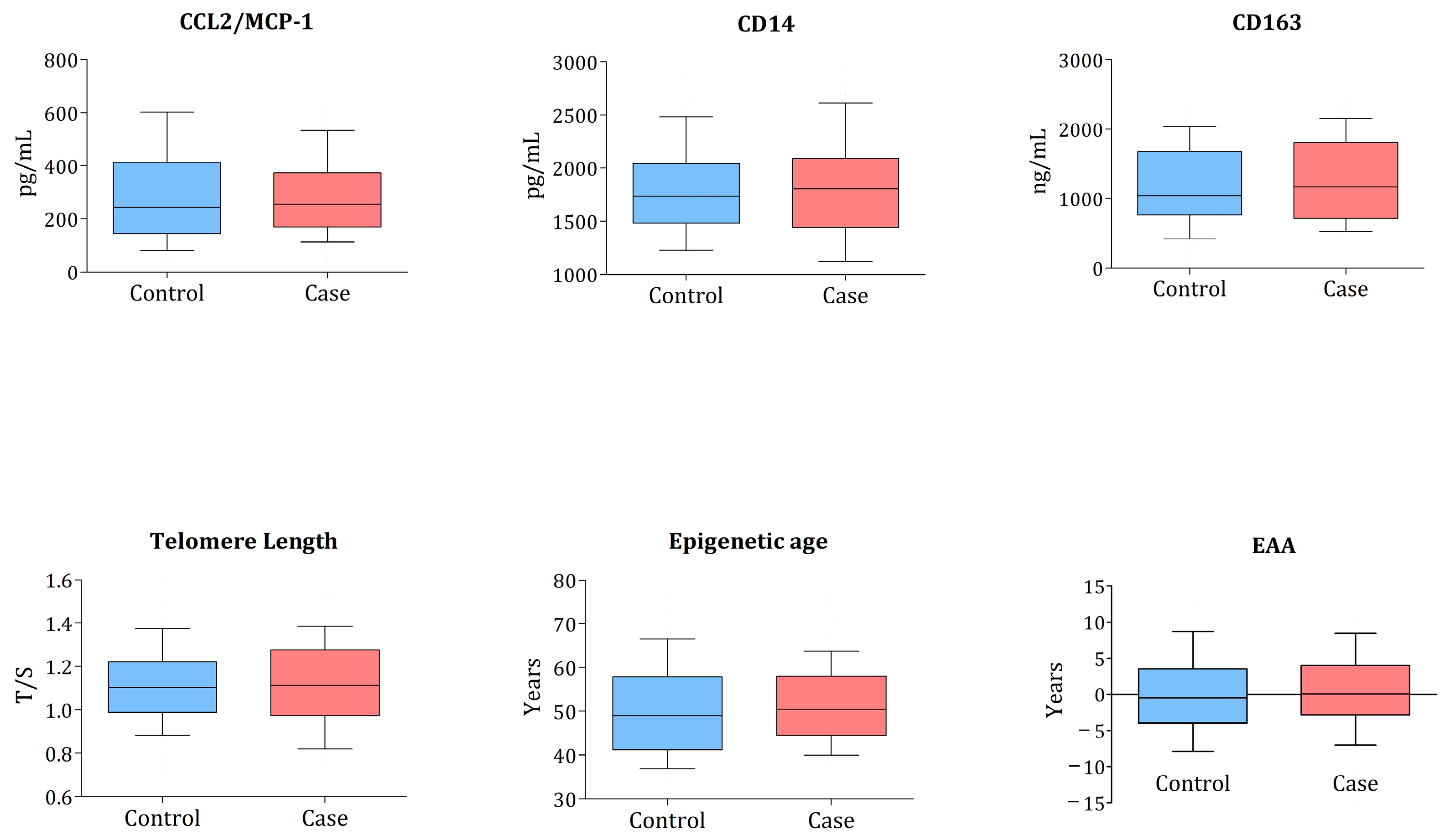
3.2. Associations between Monocyte Biomarkers, TL, and EAA with Cardiometabolic Events
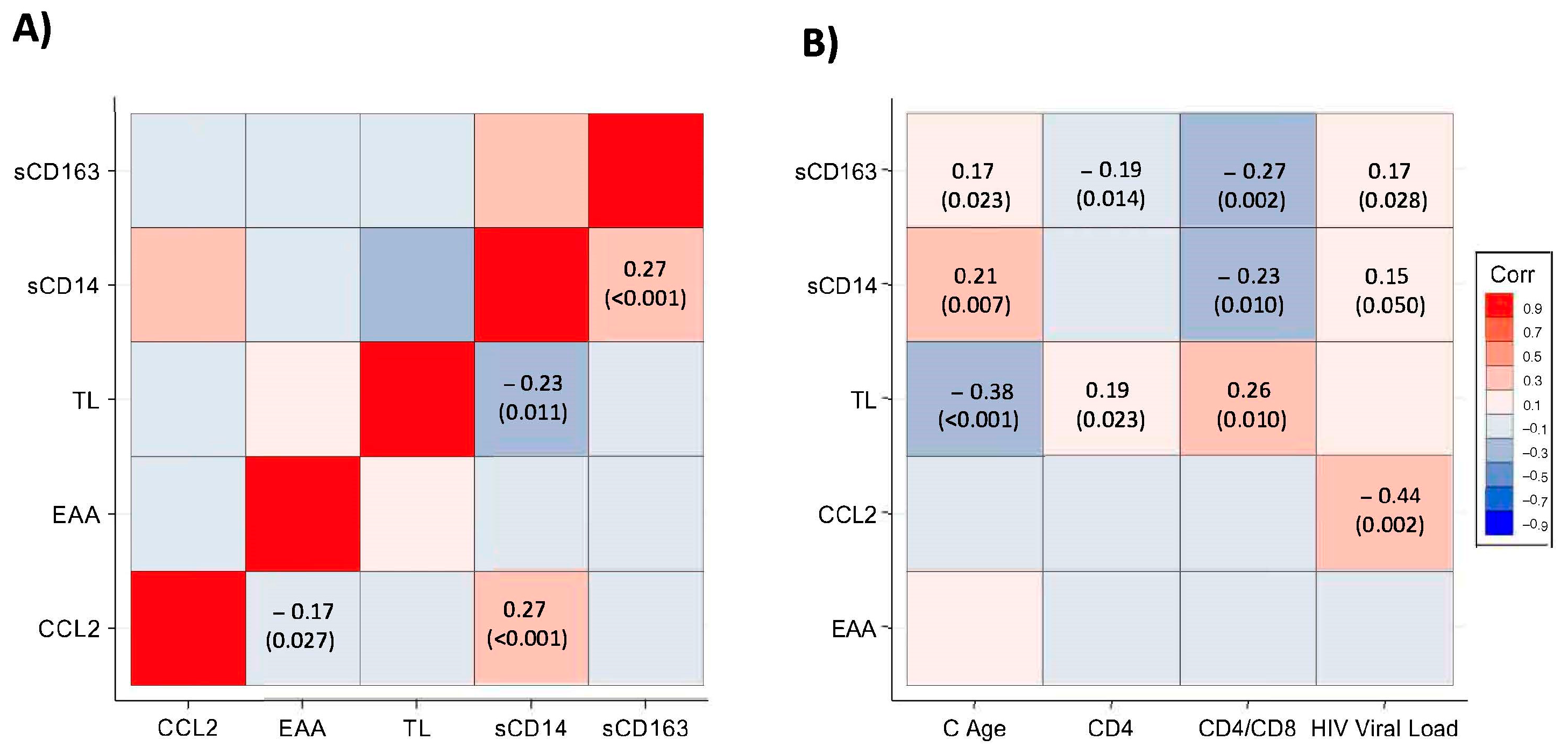
3.3. Correlations of TL, EAA, and Monocyte Biomarkers
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.S.V.; Stelzle, D.; Lee, K.K.; Beck, E.J.; Alam, S.; Clifford, S.; Longenecker, C.T.; Strachan, F.; Bagchi, S.; Whiteley, W.; et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living with HIV. Circulation 2018, 138, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern. Med. 2013, 173, 614. [Google Scholar] [CrossRef]
- Marcus, J.L.; Leyden, W.A.; Chao, C.R.; Chow, F.C.; Horberg, M.A.; Hurley, L.B.; Klein, D.B.; Quesenberry, C.P., Jr.; Towner, W.J.; Silverberg, M.J. HIV Infection and Incidence of Ischemic Stroke. AIDS 2014, 28, 1911–1919. [Google Scholar] [CrossRef]
- Feinstein, M.J.; Bahiru, E.; Achenbach, C.; Longenecker, C.T.; Hsue, P.; So-Armah, K.; Freiberg, M.S.; Lloyd-Jones, D.M. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. Am. J. Cardiol. 2016, 117, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Verdin, E.; McCune, J.M. Immunosenescence and HIV. Curr. Opin. Immunol. 2012, 24, 501–506. [Google Scholar] [CrossRef]
- Duprez, D.A.; Neuhaus, J.; Kuller, L.H.; Tracy, R.; Belloso, W.; Wit, S.D.; Drummond, F.; Lane, H.C.; Ledergerber, B.; Lundgren, J.; et al. Inflammation, Coagulation and Cardiovascular Disease in HIV-Infected Individuals. PLoS ONE 2012, 7, e44454. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, A.R.; Zheng, Y.; Bosch, R.J.; Krishnan, S.; Rodriguez, B.; Hunt, P.W.; Plants, J.; Seth, A.; Wilson, C.C.; Deeks, S.G.; et al. Soluble Markers of Inflammation and Coagulation but Not T-Cell Activation Predict Non-AIDS-Defining Morbid Events During Suppressive Antiretroviral Treatment. J. Infect. Dis. 2014, 210, 1248–1259. [Google Scholar] [CrossRef]
- McKibben, R.A.; Margolick, J.B.; Grinspoon, S.; Li, X.; Palella, F.J.; Kingsley, L.A.; Witt, M.D.; George, R.T.; Jacobson, L.P.; Budoff, M.; et al. Elevated Levels of Monocyte Activation Markers Are Associated with Subclinical Atherosclerosis in Men with and Those without HIV Infection. J. Infect. Dis. 2015, 211, 1219–1228. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Cremers, B.; Zawada, A.M.; Seiler, S.; Binder, N.; Ege, P.; Große-Dunker, G.; Heisel, I.; Hornof, F.; Jeken, J.; et al. CD14++CD16+ Monocytes Independently Predict Cardiovascular Events A Cohort Study of 951 Patients Referred for Elective Coronary Angiography. J. Am. Coll. Cardiol. 2012, 60, 1512–1520. [Google Scholar] [CrossRef]
- Roy, P.; Orecchioni, M.; Ley, K. How the Immune System Shapes Atherosclerosis: Roles of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Zanet, D.L.; Thorne, A.; Singer, J.; Maan, E.J.; Sattha, B.; Le Campion, A.; Soudeyns, H.; Pick, N.; Murray, M.; Money, D.M.; et al. Association between Short Leukocyte Telomere Length and HIV Infection in a Cohort Study: No Evidence of a Relationship with Antiretroviral Therapy. Clin. Infect. Dis. 2014, 58, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, S.; Meyer, T.D.; Rietzschel, E.R.; Buyzere, M.L.D.; Bacquer, D.D.; Langlois, M.; Segers, P.; Cooman, L.; Damme, P.V.; Cassiman, P.; et al. Telomere Length and Cardiovascular Risk Factors in a Middle-aged Population Free of Overt Cardiovascular Disease. Aging Cell 2007, 6, 639–647. [Google Scholar] [CrossRef]
- D’Mello, M.J.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between Shortened Leukocyte Telomere Length and Cardiometabolic Outcomes. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef]
- Li, X.; Ploner, A.; Wang, Y.; Magnusson, P.K.; Reynolds, C.; Finkel, D.; Pedersen, N.L.; Jylhävä, J.; Hägg, S. Longitudinal Trajectories, Correlations and Mortality Associations of Nine Biological Ages across 20-Years Follow-Up. eLife 2020, 9, e51507. [Google Scholar] [CrossRef] [PubMed]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-Wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.N.; Hui, Q.; Rimland, D.; Xu, K.; Freiberg, M.S.; Justice, A.C.; Marconi, V.C.; Sun, Y.V. Identification of HIV Infection-Related DNA Methylation Sites and Advanced Epigenetic Aging in HIV-Positive, Treatment-Naive U.S. Veterans. AIDS 2017, 31, 571–575. [Google Scholar] [CrossRef]
- Horvath, S.; Levine, A.J. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. J. Infect. Dis. 2015, 212, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.M.; Jaeger, P.A.; Kreisberg, J.F.; Licon, K.; Jepsen, K.L.; Khosroheidari, M.; Morsey, B.M.; Swindells, S.; Shen, H.; Ng, C.T.; et al. Methylome-Wide Analysis of Chronic HIV Infection Reveals Five-Year Increase in Biological Age and Epigenetic Targeting of HLA. Mol. Cell 2016, 62, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Rickabaugh, T.M.; Baxter, R.M.; Sehl, M.; Sinsheimer, J.S.; Hultin, P.M.; Hultin, L.E.; Quach, A.; Martínez-Maza, O.; Horvath, S.; Vilain, E.; et al. Acceleration of Age-Associated Methylation Patterns in HIV-1-Infected Adults. PLoS ONE 2015, 10, e0119201. [Google Scholar] [CrossRef]
- Yang, C.X.; Schon, E.; Obeidat, M.; Kobor, M.S.; McEwen, L.; MacIsaac, J.; Lin, D.; Novak, R.M.; Hudson, F.; Klinker, H.; et al. Occurrence of Accelerated Epigenetic Aging and Methylation Disruptions in Human Immunodeficiency Virus Infection Before Antiretroviral Therapy. J. Infect. Dis. 2020, 223, 1681–1689. [Google Scholar] [CrossRef]
- Caro-Murillo, A.M.; Castilla, J.; Pérez-Hoyos, S.; Miró, J.M.; Podzamczer, D.; Rubio, R.; Riera, M.; Viciana, P.; Aldeguer, J.L.; Iribarren, J.A.; et al. Cohorte RIS de Pacientes Con Infección Por VIH Sin Tratamiento Antirretroviral Previo (CoRIS): Metodología y Primeros Resultados. Enfermedades Infecc. Microbiol Clín. 2007, 25, 23–31. [Google Scholar] [CrossRef]
- Masiá, M.; Padilla, S.; Álvarez, D.; López, J.C.; Santos, I.; Soriano, V.; Hernández-Quero, J.; Santos, J.; Tural, C.; del Amo, J.; et al. Risk, Predictors, and Mortality Associated with Non-AIDS Events in Newly Diagnosed HIV-Infected Patients. AIDS 2013, 27, 181–189. [Google Scholar] [CrossRef] [PubMed]
- García-Merino, I.; de las Cuevas, N.; Jiménez, J.L.; Gallego, J.; Gómez, C.; Prieto, C.; Serramía, M.J.; Lorente, R.; Muñoz-Fernández, M.Á.; BioBank, S.H. The Spanish HIV BioBank: A Model of Cooperative HIV Research. Retrovirology 2009, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Montejano, R.; Stella-Ascariz, N.; Monge, S.; Bernardino, J.I.; Pérez-Valero, I.; Montes, M.L.; Valencia, E.; Martín-Carbonero, L.; Moreno, V.; González-García, J.; et al. Impact of Antiretroviral Treatment Containing Tenofovir Difumarate on the Telomere Length of Aviremic HIV-Infected Patients. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 76, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Li, J.; Nakanishi, M.; Asada, T.; Ikesue, M.; Goto, Y.; Fukushima, Y.; Iwai, N. Establishment of the MethyLight Assay for Assessing Aging, Cigarette Smoking, and Alcohol Consumption. Biomed Res. Int. 2015, 2015, 451981. [Google Scholar] [CrossRef]
- Weidner, C.I.; Lin, Q.; Koch, C.M.; Eisele, L.; Beier, F.; Ziegler, P.; Bauerschlag, D.O.; Jöckel, K.-H.; Erbel, R.; Mühleisen, T.W.; et al. Aging of Blood Can Be Tracked by DNA Methylation Changes at Just Three CpG Sites. Genome Biol. 2014, 15, R24. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kronmal, R.A.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Walston, J.; Kimura, M.; Aviv, A. Leukocyte Telomere Length and Cardiovascular Disease in the Cardiovascular Health Study. Am. J. Epidemiol. 2007, 165, 14–21. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, M.J.J.; Ross, S.A.; Anand, S.S.; Gerstein, H.; McQueen, M.; Yusuf, S.; Paré, G. Telomere Length and Risk of Myocardial Infarction in a MultiEthnic Population the INTERHEART Study. J. Am. Coll. Cardiol. 2016, 67, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Merkin, S.S.; Cawthon, R.; Blackburn, E.H.; Adler, N.E.; Pletcher, M.J.; Seeman, T.E. The Rate of Leukocyte Telomere Shortening Predicts Mortality from Cardiovascular Disease in Elderly Men: A Novel Demonstration. Aging 2008, 1, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Toupance, S.; Labat, C.; Temmar, M.; Rossignol, P.; Kimura, M.; Aviv, A.; Benetos, A. Short Telomeres, but Not Telomere Attrition Rates, Are Associated with Carotid Atherosclerosis. Hypertension 2018, 70, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.D.; Nawrot, T.; Bekaert, S.; Buyzere, M.L.D.; Rietzschel, E.R.; Andrés, V. Telomere Length as Cardiovascular Aging Biomarker JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 805–813. [Google Scholar] [CrossRef]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte Telomere Length and Risk of Cardiovascular Disease: Systematic Review and Meta-Analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Raffenberg, M.; Schoepf, I.C.; Kootstra, N.A.; Reiss, P.; Thorball, C.W.; Hasse, B.; Hirzel, C.; Wissel, K.; Roth, J.A.; et al. Telomere Length, Traditional Risk Factors, Factors Related to Human Immunodeficiency Virus (HIV) and Coronary Artery Disease Events in Swiss Persons Living with HIV. Clin. Infect. Dis. 2020, 73, e2070–e2076. [Google Scholar] [CrossRef] [PubMed]
- Stella-Ascariz, N.; Montejano, R.; Rodriguez-Centeno, J.; Alejos, B.; Schwimmer, C.; Bernardino, J.I.; Rodes, B.; Allavena, C.; Hoffmann, C.; Gisslén, M.; et al. Blood Telomere Length Changes After Ritonavir-Boosted Darunavir Combined with Raltegravir or Tenofovir-Emtricitabine in Antiretroviral-Naive Adults Infected with HIV-1. J. Infect. Dis. 2018, 218, 1523–1530. [Google Scholar] [CrossRef]
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. A Systematic Review of Leukocyte Telomere Length and Age in Adults. Ageing Res. Rev. 2013, 12, 509–519. [Google Scholar] [CrossRef]
- Montejano, R.; Stella-Ascariz, N.; Monge, S.; Bernardino, J.I.; Pérez-Valero, I.; Montes, M.L.; Valencia, E.; Martín-Carbonero, L.; Moreno, V.; González-Garcia, J.; et al. Impact of Nucleos(t)Ide Reverse Transcriptase Inhibitors on Blood Telomere Length Changes in a Prospective Cohort of Aviremic HIV–Infected Adults. J. Infect. Dis. 2018, 218, 1531–1540. [Google Scholar] [CrossRef]
- Fernández-Sanlés, A.; Sayols-Baixeras, S.; Subirana, I.; Sentí, M.; Pérez-Fernández, S.; Moura, M.d.C.; Esteller, M.; Marrugat, J.; Elosua, R. DNA Methylation Biomarkers of Myocardial Infarction and Cardiovascular Disease. Clin. Epigenet. 2021, 13, 86. [Google Scholar] [CrossRef]
- Agha, G.; Mendelson, M.M.; Ward-Caviness, C.K.; Joehanes, R.; Huan, T.; Gondalia, R.; Salfati, E.; Brody, J.A.; Fiorito, G.; Bressler, J.; et al. Blood Leukocyte DNA Methylation Predicts Risk of Future Myocardial Infarction and Coronary Heart Disease. Circulation 2019, 140, 645–657. [Google Scholar] [CrossRef]
- Marioni, R.E.; Shah, S.; McRae, A.F.; Chen, B.H.; Colicino, E.; Harris, S.E.; Gibson, J.; Henders, A.K.; Redmond, P.; Cox, S.R.; et al. DNA Methylation Age of Blood Predicts All-Cause Mortality in Later Life. Genome Biol. 2015, 16, 25. [Google Scholar] [CrossRef]
- Chen, B.H.; Marioni, R.E.; Colicino, E.; Peters, M.J.; Ward-Caviness, C.K.; Tsai, P.-C.; Roetker, N.S.; Just, A.C.; Demerath, E.W.; Guan, W.; et al. DNA Methylation-Based Measures of Biological Age: Meta-Analysis Predicting Time to Death. Aging 2016, 8, 1844–1859. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An Epigenetic Biomarker of Aging for Lifespan and Healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA Methylation GrimAge Strongly Predicts Lifespan and Healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef]
- Sehl, M.E.; Rickabaugh, T.M.; Shih, R.; Martinez-Maza, O.; Horvath, S.; Ramirez, C.M.; Jamieson, B.D. The Effects of Anti-Retroviral Therapy on Epigenetic Age Acceleration Observed in HIV-1-Infected Adults. Pathog. Immun. 2020, 5, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Cantos, A.; Rodríguez-Centeno, J.; Barruz, P.; Alejos, B.; Saiz-Medrano, G.; Nevado, J.; Martin, A.; Gayá, F.; Miguel, R.D.; Bernardino, J.I.; et al. Epigenetic Age Acceleration Changes 2 Years after Antiretroviral Therapy Initiation in Adults with HIV: A Substudy of the NEAT001/ANRS143 Randomised Trial. Lancet HIV 2021, 8, e197–e205. [Google Scholar] [CrossRef] [PubMed]
- Angelidou, K.; Hunt, P.W.; Landay, A.L.; Wilson, C.C.; Rodriguez, B.; Deeks, S.G.; Bosch, R.J.; Lederman, M.M. Changes in Inflammation but Not in T-Cell Activation Precede Non-AIDS-Defining Events in a Case-Control Study of Patients on Long-Term Antiretroviral Therapy. J. Infect. Dis. 2018, 218, 239–248. [Google Scholar] [CrossRef]
- Esteban-Cantos, A.; Montejano, R.; Rodríguez-Centeno, J.; Saiz-Medrano, G.; Miguel, R.D.; Barruz, P.; Bernardino, J.I.; Mena-Garay, B.; Cadiñanos, J.; Jiménez-González, M.; et al. Longitudinal Changes in Epigenetic Age Acceleration in Aviremic Human Immunodeficiency Virus–Infected Recipients of Long-Term Antiretroviral Treatment. J. Infect. Dis. 2021, 225, 287–294. [Google Scholar] [CrossRef]
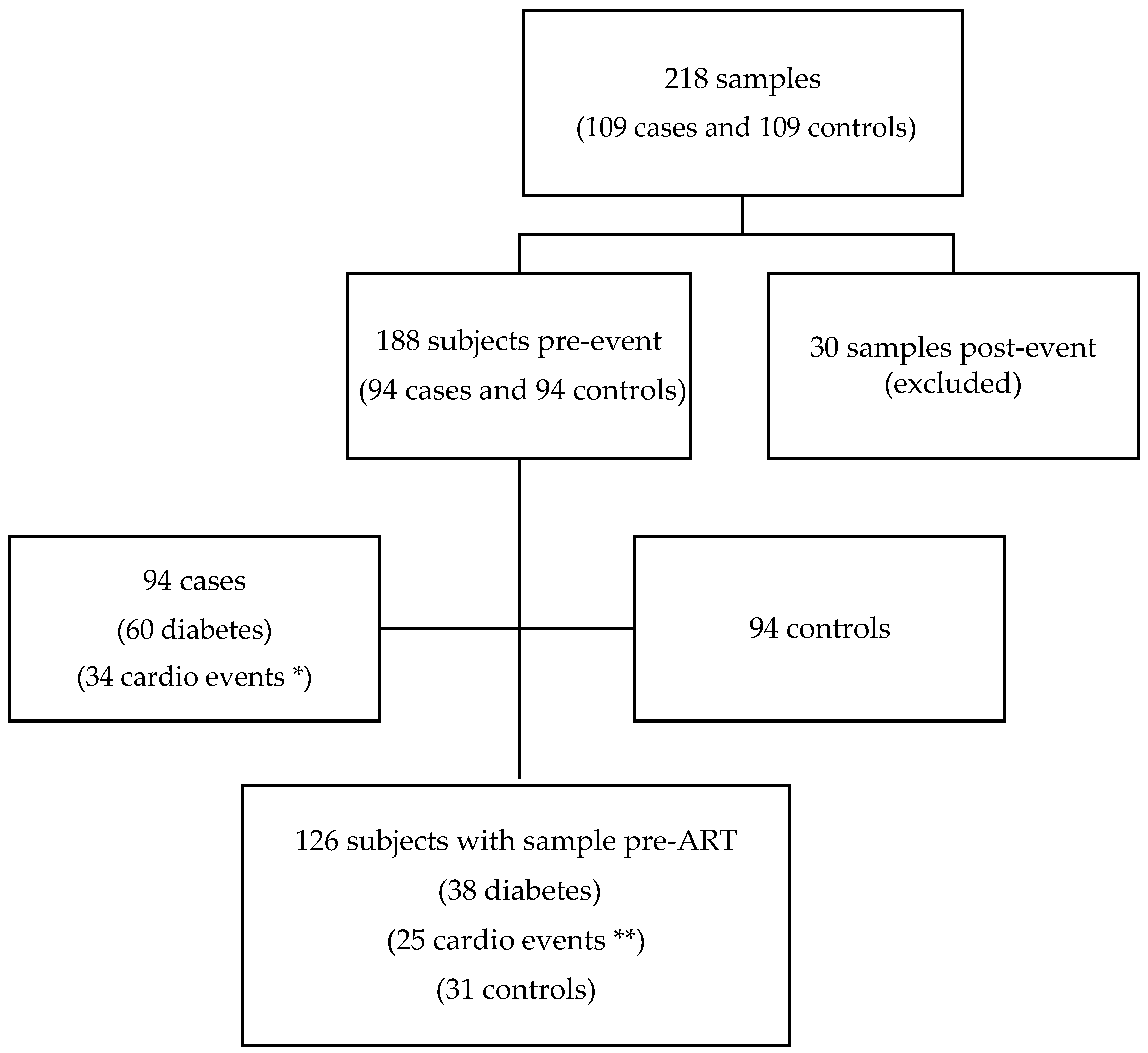
| Cases n = 94 | Controls n = 94 | p-Value | |
|---|---|---|---|
| Age (years) | 46 (39–56) | 46 (40–55) | 0.763 |
| Female sex | 15 (16.0%) | 15 (16.0%) | |
| MSM | 57 (60.6%) | 45 (47.9%) | 0.079 |
| Caucasic | 72 (76.6%) | 84 (89.36%) | 0.106 |
| HIV duration | 8.23 (1.51–26.16) | 3.59 (1.25–18.30) | 0.846 |
| Never smoked | 29 (30.9%) | 29 (30.9%) | |
| CD4 (cells/mm3) | 384 (234–574) | 442 (226–692) | 0.375 |
| CD4 < 200 (%) | 21 (22.3%) | 21 (22.3%) | |
| Viral load (log) | 4.53 (3.65–5.07) | 4.49 (3.85–5.13) | 0.847 |
| CD4:CD8 ratio | 0.33 (0.20–0.62) | 0.44 (0.26–0.63) | 0.292 |
| Time to event (years) | 2.39 (0.78–4.50) | ------ | |
| TL (T/S ratio) | 1.09 (0.94–1.23) | 1.10 (0.98–1.22) | 0.965 |
| Epigenetic age (years) | 50.1 (43.8–57.2) | 49 (42.6–56.6) | 0.400 |
| EAA (years) | 0.10 (−2.7; 3.98) | −0.45 (−3.86; 3.26) | 0.827 |
| sCD14 (pg/mL) | 1771.9 (1437.1–2105.5) | 1751.5 (1497–2081.7) | 0.591 |
| sCD163 (pg/mL) | 1192.4 (648.9–1903.1) | 1022.4 (696.5–1586.61) | 0.782 |
| CCL2/MCP-1 (ng/mL) | 260.2 (178.6–373.2) | 261.4 (153.63–414.92) | 0.520 |
| As Continuous Variable | As Binary Variable | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| TL (T/S ratio) | 0.96 | 0.16–5.66 | 0.965 | 1.09 | 0.52–2.28 | 0.827 |
| EAA (Years) | 1.01 | 0.96–1.06 | 0.668 | 1.35 | 0.71–2.58 | 0.365 |
| sCD14 | 1.0 | 1.0–1.0 | 0.591 | 1.29 | 0.66–2.54 | 0.456 |
| sCD163 | 1.0 | 1.0–1.0 | 0.782 | 1.86 | 0.89–3.87 | 0.097 |
| CCL2/MCP1 | 1.0 | 1.0–1.0 | 0.520 | 0.98 | 0.46–2.06 | 0.951 |
| As Continuous Variable | As Binary Variable | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| TL (T/S ratio) | 0.21 | 0.01–3.39 | 0.268 | 0.63 | 0.18–2.21 | 0.471 |
| EAA (Years) | 0.98 | 0.90–1.06 | 0.594 | 0.98 | 0.90–1.06 | 0.594 |
| sCD14 | 1.0 | 1.0–1.0 | 0.149 | 1.45 | 0.47–4.45 | 0.512 |
| sCD163 | 1.0 | 1.0–1.0 | 0.990 | 1.75 | 0.43–7.17 | 0.436 |
| CCL2/MCP1 | 1.0 | 0.99–1.0 | 0.409 | 1.20 | 0.35–4.07 | 0.772 |
| As Continuous Variable | As Binary Variable | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| TL (T/S ratio) | 4.23 | 0.25–70.64 | 0.315 | 1.43 | 0.50–4.10 | 0.500 |
| EAA (Years) | 1.01 | 0.96–1.07 | 0.610 | 1.01 | 0.96–1.07 | 0.610 |
| sCD14 | 1.0 | 1.0–1.0 | 0.578 | 1.07 | 0.44–2.59 | 0.878 |
| sCD163 | 1.0 | 1.0–1.0 | 0.939 | 1.62 | 0.67–3.92 | 0.282 |
| CCL2/MCP1 | 1.0 | 1.0–1.0 | 0.265 | 0.89 | 0.34–2.32 | 0.813 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardino, J.I.; Alejos, B.; Rodriguez-Centeno, J.; Esteban-Cantos, A.; Mora-Rojas, B.; Montejano, R.; De Miguel, R.; Montero-Alonso, M.; Ayerdi, O.; Hernández-Gutierrez, C.; et al. Monocyte Activation and Ageing Biomarkers in the Development of Cardiovascular Ischaemic Events or Diabetes in People with HIV. Microorganisms 2023, 11, 1818. https://doi.org/10.3390/microorganisms11071818
Bernardino JI, Alejos B, Rodriguez-Centeno J, Esteban-Cantos A, Mora-Rojas B, Montejano R, De Miguel R, Montero-Alonso M, Ayerdi O, Hernández-Gutierrez C, et al. Monocyte Activation and Ageing Biomarkers in the Development of Cardiovascular Ischaemic Events or Diabetes in People with HIV. Microorganisms. 2023; 11(7):1818. https://doi.org/10.3390/microorganisms11071818
Chicago/Turabian StyleBernardino, Jose I., Belen Alejos, Javier Rodriguez-Centeno, Andrés Esteban-Cantos, Beatriz Mora-Rojas, Rocío Montejano, Rosa De Miguel, Marta Montero-Alonso, Oskar Ayerdi, Cristina Hernández-Gutierrez, and et al. 2023. "Monocyte Activation and Ageing Biomarkers in the Development of Cardiovascular Ischaemic Events or Diabetes in People with HIV" Microorganisms 11, no. 7: 1818. https://doi.org/10.3390/microorganisms11071818
APA StyleBernardino, J. I., Alejos, B., Rodriguez-Centeno, J., Esteban-Cantos, A., Mora-Rojas, B., Montejano, R., De Miguel, R., Montero-Alonso, M., Ayerdi, O., Hernández-Gutierrez, C., Curran, A., Arribas, J. R., & Rodés, B., on behalf of the Cohort of the Spanish National AIDS Network (CoRIS). (2023). Monocyte Activation and Ageing Biomarkers in the Development of Cardiovascular Ischaemic Events or Diabetes in People with HIV. Microorganisms, 11(7), 1818. https://doi.org/10.3390/microorganisms11071818






