Citrus sinensis Peel Oil Extraction and Evaluation as an Antibacterial and Antifungal Agent
Abstract
1. Introduction
2. Materials and Methods
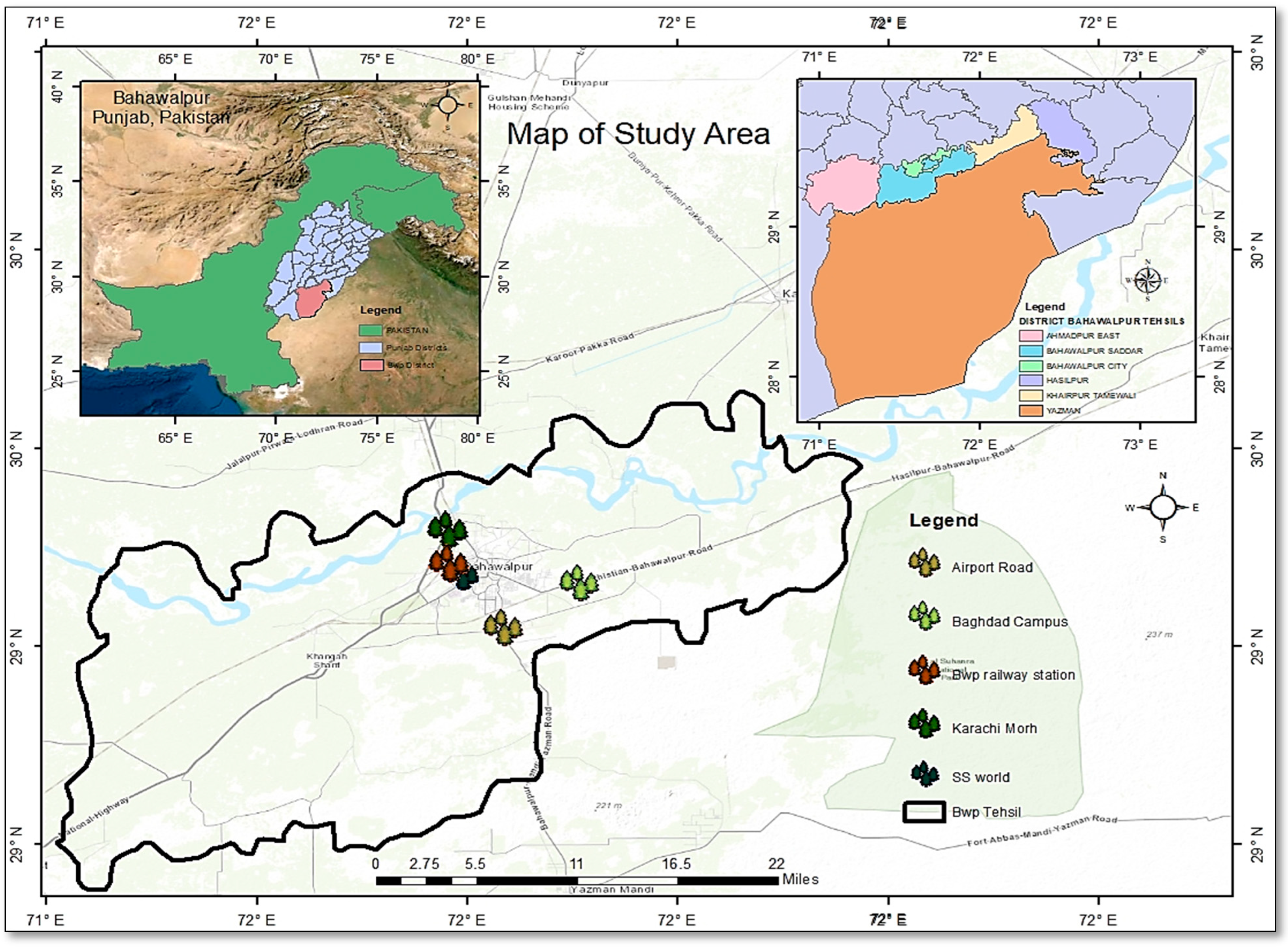
2.1. Study Area
2.2. Collection and Extraction of Oil
2.3. Evaluation of Physical Properties
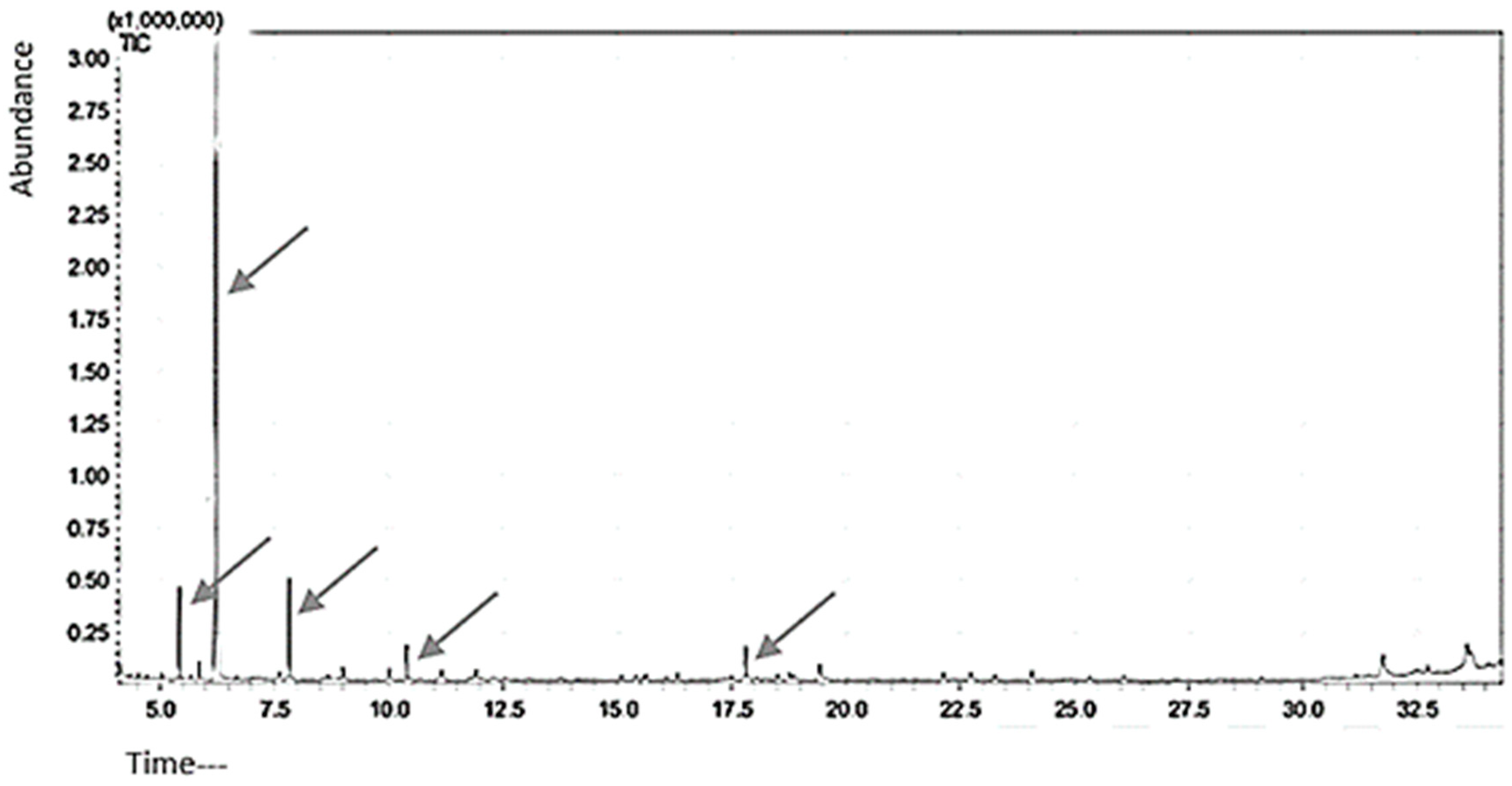
2.4. Qualitative Analysis of Citrus Oil
2.5. Antibacterial Assay
2.5.1. Microbial Organisms
2.5.2. Agar Well Diffusion Method
2.5.3. Minimum Inhibitory Concentration
2.6. Antifungal Assay
2.6.1. Microorganisms and Nutrient Media
2.6.2. Agar Well Diffusion Method
2.6.3. Minimum Inhibitory Concentration (MIC)
2.7. Anti-Parasitic Potential
3. Results and Discussion
3.1. Physical Properties of Essential Oil
3.2. Effect of Temperature on Percentage Yield
3.3. Antibacterial Activity of Essential Oil
3.4. Determination of Minimum Inhibitory Concentration (MIC) against Bacterial Strains
3.5. Antifungal Activity
3.6. Determination of Minimum Inhibitory Concentration against Fungal Strains
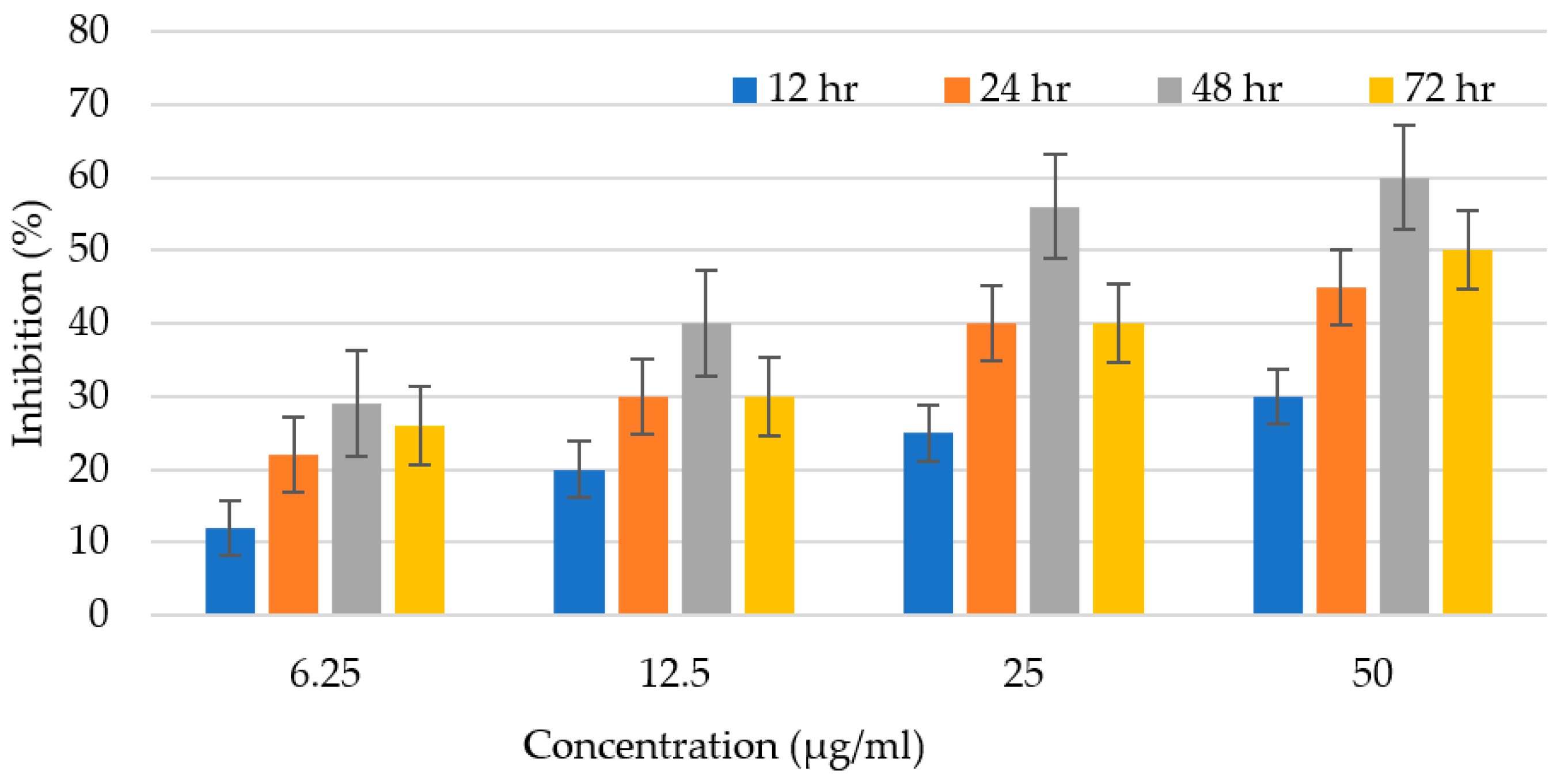
3.7. Antileishmanial Potential
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarmah, N.; Kumari, S. Comparative study of antibacterial activity of ripen and unripen Indigenous Citrus union of Assam, India. Int. J. Adv. Res. Technol. 2013, 2, 25–31. [Google Scholar]
- Khushwaha, A.; Singh, R.P.; Gupta, V.; Singh, M. Antimicrobial properties of peels of citrus fruits. Int. J. Univers Pharm. Life. Sci. 2012, 2, 24–38. [Google Scholar]
- Holmberg, S.D.; Solomon, S.L.; Blake, P.A. Health and economic impacts of antimicrobial resistance. J. Infect. Dis. 1987, 9, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Afroja, S.; Falgunee, F.N.; Jahan, M.M.; Akanda, K.M.; Mehjabin, S.; Parvez, G.M. Antibacterial activity of different citrus fruits. J. Med. Res. Health Sci. 2017, 2, 25–32. [Google Scholar]
- Dhiman, A.; Nanda, A.; Ahmad, S.; Narasimhan, B. In vitro antimicrobial status of methanolic extract of Citrus sinensis Linn. Fruit peel. Chron. Young Sci. 2012, 3, 47–59. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounes, S.A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Elgamal, Z.M.; Yasin, N.M.; Sabri, A.Q.M.; Sihwail, R.; Tubishat, M.; Jarrah, H. Improved equilibrium optimization algorithm using elite opposition-based learning and new local search strategy for feature selection in medical datasets. Computation 2021, 9, 68. [Google Scholar] [CrossRef]
- Beneti, S.C.; Rosset, E.; Corazza, M.L.; Frizzo, C.D.; Di Luccio, M.; Oliveira, J.V. Fractionation of citronella (Cymbopogon winterianus) essential oil and concentrated orange oil phase by batch vacuum distillation. J. Food Eng. 2011, 102, 348–354. [Google Scholar] [CrossRef]
- Mehmood, B.; Dar, K.K.; Ali, S.; Awan, U.A.; Nayyer, A.Q.; Ghous, T.; Andleeb, S. In vitro assessment of antioxidant, antibacterial and phytochemical analysis of peel of Citrus sinensis. Pak. J. Pharm. Sci. 2015, 28, 440–456. [Google Scholar]
- Silvestre, W.P.; Agostini, F.; Muniz, L.A.; Pauletti, G.F. Fractionating of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2016, 178, 90–94. [Google Scholar] [CrossRef]
- Hoshino, M.; Tanaka, M.; Sasaki, M.; Goto, M.; Vinegar, M. Extraction of essential oil from Citrus junos peel using supercritical carbon dioxide. In Proceedings of the 8th International Symposium on Supercritical Fluids, Kyoto, Japan, 5–8 November 2006. [Google Scholar]
- Ahmad, A.; Syed, F.; Imran, M.; Khan, A.U.; Tahir, K.; Khan, Z.U.H.; Yuan, Q. Photosynthesis and antileishmanial activity of gold nanoparticles by Maytenus royleanus. J. Food Bioch. 2016, 40, 420–427. [Google Scholar] [CrossRef]
- Katsuda, M.S.; McClements, D.J.; Miglioranza, L.H.; Decker, E.A. Physical and oxidative stability of fish oil-in-water emulsions stabilized with β-lactoglobulin and pectin. J. Agric. Food Chem. 2008, 56, 5926–5931. [Google Scholar] [CrossRef] [PubMed]
- Samavat, Z.; Mehrgan, M.S.; Jamili, S.; Soltani, M.; Shekarabi, S.P.H. Determination of grapefruit (Citrus paradisi) peel extract bio-active substances and its application in Caspian white fish diet: Growth, haemato-biochemical parameters and intestinal morphology. Aquac. Res. 2019, 50, 2496–2504. [Google Scholar] [CrossRef]
- Bhuyan, N.; Barua, P.C.; Kalita, P.; Saikia, A. Physico-chemical variation in peel oils of Khasi mandarin (Citrus reticulata Blanco) during ripening. Ind. J. Plant Physiol. 2015, 20, 227–231. [Google Scholar] [CrossRef]
- Fekadu, M.; Feleke, S.; Bekele, T. Selection of seed oil biodiesel producing tree species, emission reduction and land suitability. Agric. Eng. Int. J. 2019, 21, 1445–1460. [Google Scholar]
- Sikdar, D.C.; Menon, R.; Duseja, K.; Kumar, P.; Swami, P. Extraction of citrus oil from orange (Citrus sinensis) peels by steam distillation and its characterizations. Int. J. Tech. Res. Appl. 2016, 4, 341–346. [Google Scholar]
- Kamel, F.; Sabir, S.; Mahal, A.; Wei, X. In vitro antibacterial activity of orange peel oil extract from Citrus sinensis fruit in Erbil. Egypt. J. Chem. 2022, 65, 157–160. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Hickey, D.K.; Alonso-Gomez, M.; Wilkinson, M. Evaluation of antimicrobial activities of commercial herb and spice extracts against selected food-borne bacteria. J. Food Resh. 2013, 2, 37–49. [Google Scholar] [CrossRef]
- Obeidat, M. Antimicrobial activities from extracts of seven medicinal plant species against multidrug-resistant bacteria and fungi. J. Pharmacog. Phytother. 2018, 10, 45–55. [Google Scholar]
- Ashok, B.; Umamahesh, M.; Hariram, N.; Siengchin, S.; Rajulu, A.V. Modification of waste leather trimming within situ generated silver nanoparticles by one step method. Appl. Sci. Eng. Prog. 2021, 14, 236–246. [Google Scholar] [CrossRef]
- Rasooli, I.; Abyaneh, M.R. Inhibitory effects of Thyme oils on growth and aflatoxin production by Aspergillus parasiticus. Food Control 2004, 15, 479–483. [Google Scholar] [CrossRef]
- Sreepian, A.; Sreepian, P.M.; Chanthong, C.; Mingkhwancheep, T.; Prathit, P. Antibacterial activity of essential oil extracted from Citrus hystrix (Kaffir Lime) peels: An in vitro study. Trop. Biomed. 2019, 36, 531–541. [Google Scholar] [PubMed]
- Samarakoon, K.; Seneviranthne, M.; Lee, W. Antibacterial effect of Citrus press–cakes dried by high speed and far–infrared radiation drying method. Nutr. Res. Pract. 2012, 6, 187–194. [Google Scholar] [CrossRef]
- Abbasi, N.; Ghaneialvar, H.; Moradi, R.; Zangeneh, M.M.; Zangeneh, A. Formulation and characterization of a novel cutaneous wound healing ointment by silver nanoparticles containing Citrus limon leaf: A chemobiological study. Arab. J. Chem. 2021, 14, 103–112. [Google Scholar] [CrossRef]
- Edogbanya, P.; Suleiman, M.O.; Olorunmola, J.B.; Oijagbe, I.J. Comparative study on the antimicrobial effects of essential oils from peels of three citrus fruits. J. Biomed. Sci. 2019, 4, 49–54. [Google Scholar]
- Peretto, G.; Du, W.X.; Avena, R.J.; Berrios, J.D.J.; Sambo, P.; McHugh, T.H. Optimization of antimicrobial and physical properties of alginate coatings containing carvacrol and methyl cinnamate for strawberry application. J. Agric. Food Chem. 2014, 62, 984–990. [Google Scholar] [CrossRef]
- Kauffmann, C.; Ethur, E.M.; Buhl, B.; Scheibel, T.; Machado, G.M.; Cavalheiro, M.M. Potential antileishmanial activity of essential oils of native species from southern Brazil. ENRR 2016, 6, 18–25. [Google Scholar] [CrossRef]
- de Aquino, T.M.; França, P.H.; Rodrigues, É.E.; Nascimento, I.; Santos-Júnior, P.F.; Aquino, P.G.; de Araújo-Júnior, J.X. Synthesis, Antileishmanial Activity and in silico Studies of Aminoguanidine Hydrazones (AGH) and Thiosemicarbazones (TSC) Against Leishmania chagasi Amastigotes. Med. Chem. 2022, 2, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Meneguetti, D.U.D.O.; Lima, R.A.; Hurtado, F.B.; Passarini, G.M.; Macedo, S.R.A.; Barros, N.B.D.; Facundo, V.A. Screening of the in vitro antileishmanial activities of compounds and secondary metabolites isolated from Maytenus guianensis Klotzsch ex Reissek (Celastraceae) chichuá Amazon. Rev. Soc. Bras. Med. 2016, 49, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.; Li, Y.; Chen, Y.; Angelova, A.; Garamus, V.M.; Li, N.; Drechsler, M.; Angelov, B.; Gong, Y. Self-assembled stable sponge-type nanocarries for Brucea javanica oil delivery. Colloids Surf. B. 2017, 153, 310–319. [Google Scholar] [CrossRef]
- Alharthi, M.N.; Ismail, I.; Bellucci, S.; Jaremko, M.; Abo-Aba, S.E.M.; Abdel Salam, M. Biosynthesized Zinc Oxide nanoparticles using Ziziphus jujube plant extract assisted by ultrasonic irradiation and their biological applications. Separations 2023, 10, 78. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Almahmoud, S.A.; El-Ghaly, E.M.; Khan, F.A.; Emwas, A.; Jaremko, M.; Almulhim, F.; Khan, R.A.; Ragab, E.A. Comparative anticancer potentials of taxifolin and quercetin methylated derivatives against HCT-116 cell lines: Effects of o-methylation on taxifolin and quercetin as preliminary natural leads. ACS Omega 2022, 7, 46629–46639. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Results |
|---|---|
| Odor | Tangy smell |
| Color | Brownish yellow |
| Density | 0.778 g/cm3 |
| Solubility | Insoluble in H2O |
| Specific gravity | 0.843 g/cm3 |
| Solvent | Weight of Peels (g) | Volume of Solvent (mL) | Temperature | Time (min) | Quantity of Oil Extracted (mL) | Percentage Yield of Oil |
|---|---|---|---|---|---|---|
| Petroleum Ether | 50 | 300 | 50 | 240 | 1.2 | 2.4 |
| 50 | 300 | 60 | 240 | 1.6 | 3.2 | |
| 50 | 300 | 80 | 240 | 1.8 | 3.6 |
| Bacterial Strains | Zone of Inhibition (mm) | Minimum | Maximum |
|---|---|---|---|
| Escherichia coli | 14.33 ± 2.08 *a | 12 | 16 |
| Staphylococcus aureus | 11.33 ± 1.16 *b | 10 | 12 |
| Streptococcus agalactiae | 10.67 ± 1.53 *b | 9 | 12 |
| Bacterial Strains | MIC (mg/mL) | Minimum | Maximum |
|---|---|---|---|
| Escherichia coli | 13.02 ± 0.00 *a | 7.810 | 15.620 |
| Staphylococcus aureus | 10.41 ± 4.51 *b | 7.810 | 15.620 |
| Streptococcus agalactiae | 6.51 ± 2.26 *c | 3.900 | 7.810 |
| Fungal Strains | Zone of Inhibition | 95% CI |
|---|---|---|
| Alterneria alternata | 8.667 ± 1.528 *b | (6.828, 10.506) |
| Aspergillus flavus | 12.500 ± 1.323 *a | (10.661, 14.339) |
| Aspergillus niger | 7.000 ± 1.00 *b | (5.161, 8.839) |
| Fungus | MIC (mg/mL) | 95% CI |
|---|---|---|
| Alterneria alternata | 2.500 ± 0.000 a | (−0.830, 5.830) |
| Aspergillus flavus | 8.33 ± 2.89 a | (5.00, 11.66) |
| Aspergillus niger | 6.67 ± 2.89 a | (3.34, 10.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, T.; Qureshi, H.; Fatima, A.; Sattar, K.; Albasher, G.; Kamal, A.; Ayaz, A.; Zaman, W. Citrus sinensis Peel Oil Extraction and Evaluation as an Antibacterial and Antifungal Agent. Microorganisms 2023, 11, 1662. https://doi.org/10.3390/microorganisms11071662
Anwar T, Qureshi H, Fatima A, Sattar K, Albasher G, Kamal A, Ayaz A, Zaman W. Citrus sinensis Peel Oil Extraction and Evaluation as an Antibacterial and Antifungal Agent. Microorganisms. 2023; 11(7):1662. https://doi.org/10.3390/microorganisms11071662
Chicago/Turabian StyleAnwar, Tauseef, Huma Qureshi, Arooj Fatima, Kanwal Sattar, Gadah Albasher, Asif Kamal, Asma Ayaz, and Wajid Zaman. 2023. "Citrus sinensis Peel Oil Extraction and Evaluation as an Antibacterial and Antifungal Agent" Microorganisms 11, no. 7: 1662. https://doi.org/10.3390/microorganisms11071662
APA StyleAnwar, T., Qureshi, H., Fatima, A., Sattar, K., Albasher, G., Kamal, A., Ayaz, A., & Zaman, W. (2023). Citrus sinensis Peel Oil Extraction and Evaluation as an Antibacterial and Antifungal Agent. Microorganisms, 11(7), 1662. https://doi.org/10.3390/microorganisms11071662