The Neonatal Immune System and Respiratory Pathogens
Abstract
1. Introduction
2. The Neonatal Immune System Is Composed of Unique Cells
2.1. Innate Immune System
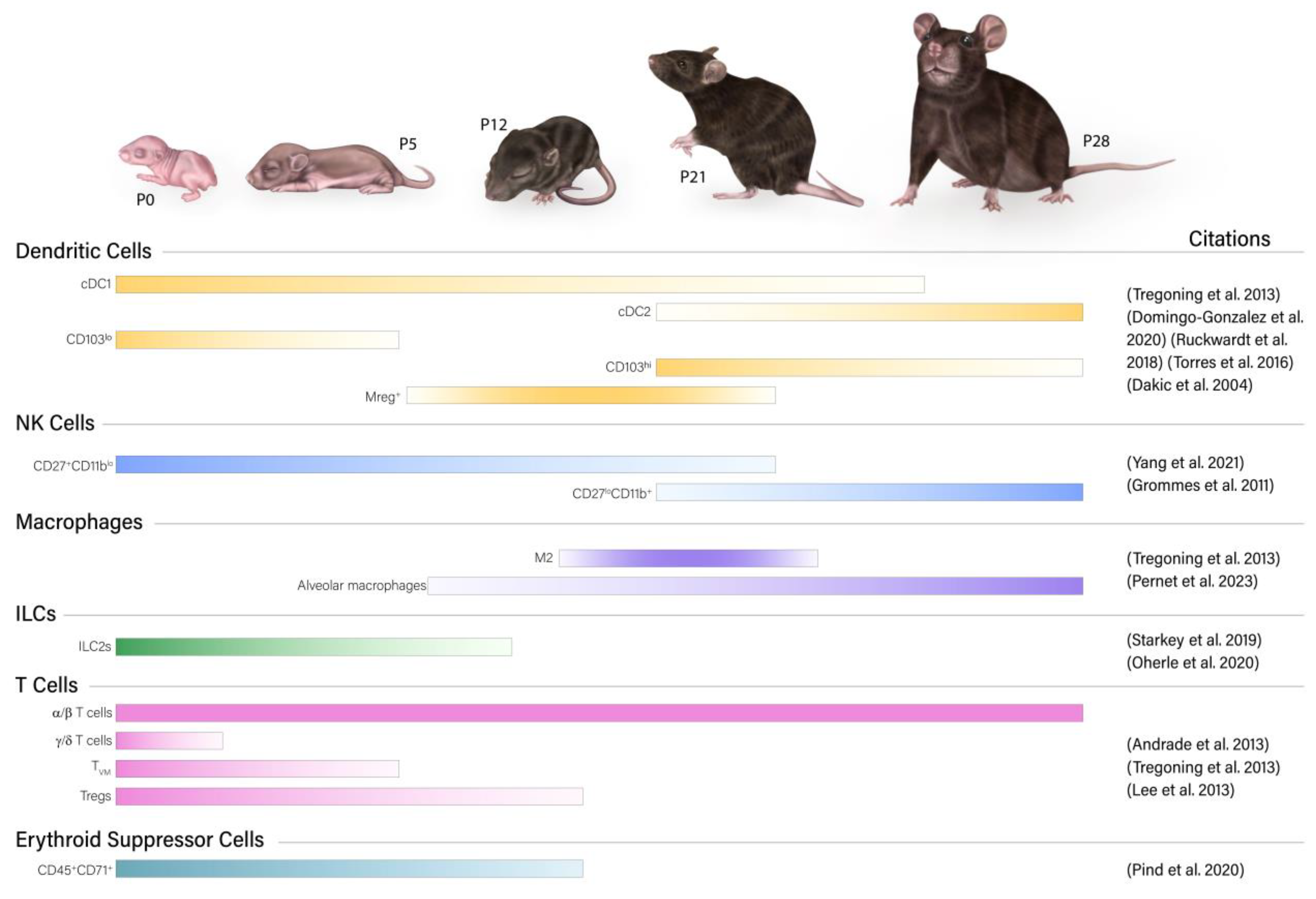
2.1.1. Neutrophils
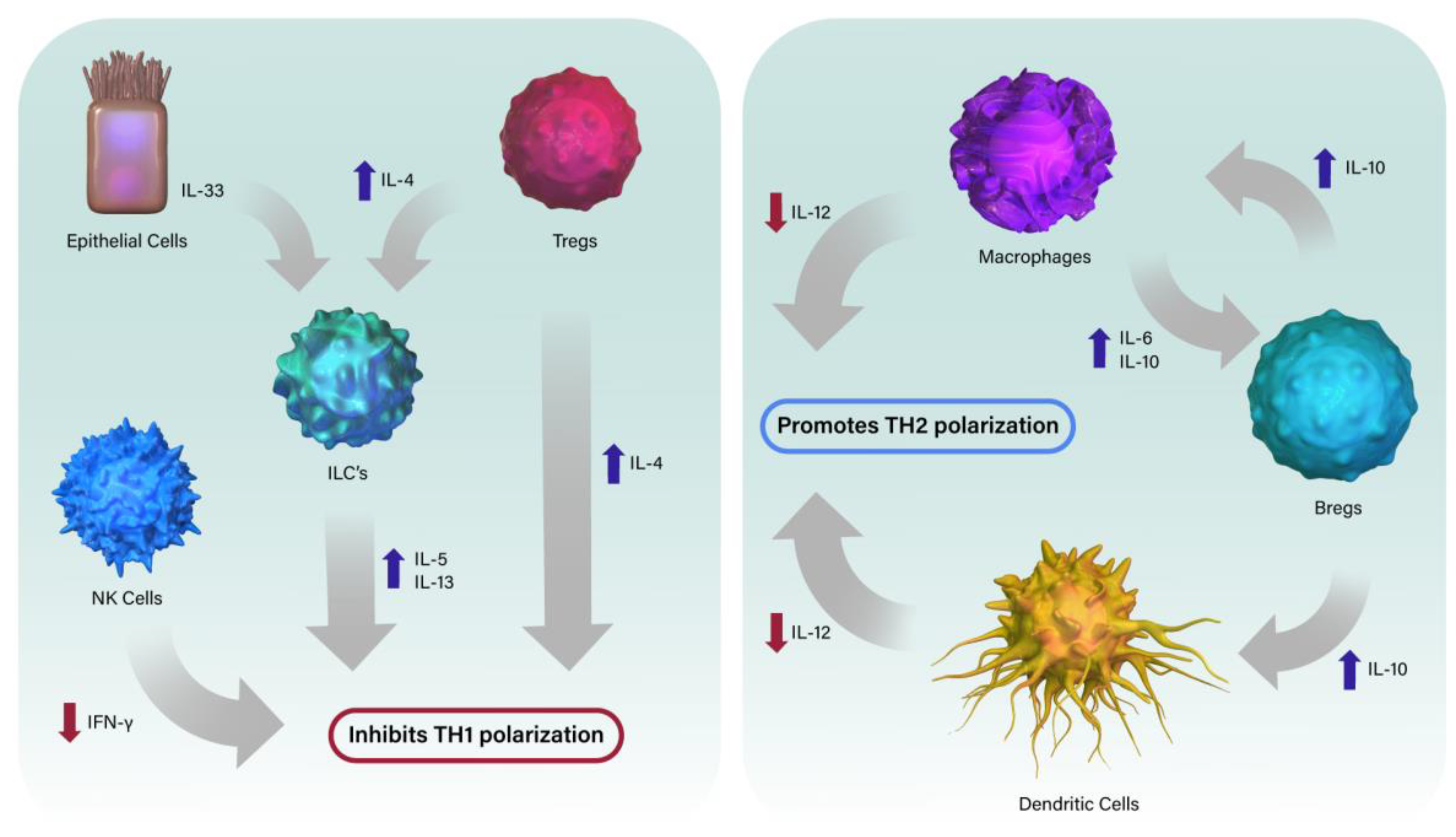
2.1.2. NK Cells
2.1.3. Dendritic Cells
2.1.4. Macrophages
2.1.5. Innate Lymphoid Cells
2.1.6. Myeloid-Derived Suppressor Cells
2.2. Adaptive Immune System
2.2.1. T Cells
2.2.2. B Cells
2.2.3. Erythroid Suppressor Cells
3. Immune Proteins in Neonatal Lungs
3.1. Antibodies
3.2. Complement System
4. The Neonatal Immune System Is Susceptible to Particular Pathogens
4.1. Bordetella Pertussis
4.2. Respiratory Syncytial Virus
4.3. Influenza
5. Conclusions and Future Directions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, B.D. Neonatal T Cells: A Reinterpretation. Annu. Rev. Immunol. 2020, 38, 229–247. [Google Scholar] [CrossRef]
- Tsafaras, G.P.; Ntontsi, P.; Xanthou, G. Advantages and Limitations of the Neonatal Immune System. Front. Pediatr. 2020, 8, 5. [Google Scholar] [CrossRef]
- Forsthuber, T.; Yip, H.C.; Lehmann, P.V. Induction of TH1 and TH2 Immunity in Neonatal Mice. Science 1996, 271, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Adkins, B.; Du, R.-Q. Newborn Mice Develop Balanced Th1/Th2 Primary Effector Responses In Vivo but Are Biased to Th2 Secondary Responses. J. Immunol. 1998, 160, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Harbeson, D.; Ben-Othman, R.; Amenyogbe, N.; Kollmann, T. Outgrowing the Immaturity Myth: The Cost of Defending from Neonatal Infectious Disease. Front. Immunol. 2018, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Hamid, S.; Winn, A.; Parikh, R.; Jones, J.M.; McMorrow, M.; Prill, M.M.; Silk, B.J.; Scobie, H.M.; Hall, A.J. Seasonality of Respiratory Syncytial Virus—United States, 2017–2023. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 355–361. [Google Scholar] [CrossRef]
- Pediatric Flu Deaths Top 100 This Season; Most Unvaccinated|CDC. Available online: https://www.cdc.gov/flu/spotlights/2022-2023/pediatric-flu-deaths.htm (accessed on 23 April 2023).
- Pertussis Surveillance: Cases by Year|CDC. Available online: https://www.cdc.gov/pertussis/surv-reporting/cases-by-year.html (accessed on 23 April 2023).
- Tregoning, J.S.; Wang, B.L.; McDonald, J.U.; Yamaguchi, Y.; Harker, J.A.; Goritzka, M.; Johansson, C.; Bukreyev, A.; Collins, P.L.; Openshaw, P.J. Neonatal antibody responses are attenuated by interferon-γ produced by NK and T cells during RSV infection. Proc. Natl. Acad. Sci. USA 2013, 110, 5576–5581. [Google Scholar] [CrossRef]
- Domingo-Gonzalez, R.; Zanini, F.; Che, X.; Liu, M.; Jones, R.C.; Swift, M.A.; Quake, S.R.; Cornfield, D.N.; Alvira, C.M. Diverse homeostatic and immunomodulatory roles of immune cells in the developing mouse lung at single cell resolution. Elife 2020, 9, e56890. [Google Scholar] [CrossRef]
- Ruckwardt, T.J.; Morabito, K.M.; Bar-Haim, E.; Nair, D.; Graham, B.S. Neonatal mice possess two phenotypically and functionally distinct lung-migratory CD103+ dendritic cell populations following respiratory infection. Mucosal Immunol. 2018, 11, 186–198. [Google Scholar] [CrossRef]
- Torres, D.; Köhler, A.; Delbauve, S.; Caminschi, I.; Lahoud, M.; Shortman, K.; Flamand, V. IL-12p40/IL-10 Producing preCD8α/Clec9A+ Dendritic Cells Are Induced in Neonates upon Listeria monocytogenes Infection. PLoS Pathog. 2016, 12, e1005561. [Google Scholar] [CrossRef]
- Dakic, A.; Shao, Q.-X.; D’amico, A.; O’keeffe, M.; Chen, W.-F.; Shortman, K.; Wu, L. Development of the Dendritic Cell System during Mouse Ontogeny. J. Immunol. 2004, 172, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Tsai, Y.-F.; Pan, Y.-L.; Hwang, T.-L. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed. J. 2021, 44, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Soehnlein, O. Contribution of Neutrophils to Acute Lung Injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Pernet, E.; Sun, S.; Sarden, N.; Gona, S.; Nguyen, A.; Khan, N.; Mawhinney, M.; Tran, K.A.; Chronopoulos, J.; Amberkar, D.; et al. Neonatal imprinting of alveolar macrophages via neutrophil-derived 12-HETE. Nature 2023, 614, 530–538. [Google Scholar] [CrossRef]
- Starkey, M.R.; McKenzie, A.N.; Belz, G.T.; Hansbro, P.M. Pulmonary group 2 innate lymphoid cells: Surprises and challenges. Mucosal Immunol. 2019, 12, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Oherle, K.; Acker, E.; Bonfield, M.; Wang, T.; Gray, J.; Lang, I.; Bridges, J.; Lewkowich, I.; Xu, Y.; Ahlfeld, S.; et al. Insulin-like Growth Factor 1 Supports a Pulmonary Niche that Promotes Type 3 Innate Lymphoid Cell Development in Newborn Lungs. Immunity 2020, 52, 275–294.e9. [Google Scholar] [CrossRef]
- Andrade, E.B.; Alves, J.; Madureira, P.; Oliveira, L.; Ribeiro, A.; Cordeiro-Da-Silva, A.; Correia-Neves, M.; Trieu-Cuot, P.; Ferreira, P. TLR2-Induced IL-10 Production Impairs Neutrophil Recruitment to Infected Tissues during Neonatal Bacterial Sepsis. J. Immunol. 2013, 191, 4759–4768. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Hamilton, S.E.; Akue, A.D.; Hogquist, K.A.; Jameson, S.C. Virtual memory CD8 T cells display unique functional properties. Proc. Natl. Acad. Sci. USA 2013, 110, 13498–13503. [Google Scholar] [CrossRef]
- Pind, A.A.A.; Estupiñan, J.L.M.; Magnusdottir, G.J.; Del Giudice, G.; Jonsdottir, I.; Bjarnarson, S.P. LT-K63 Enhances B Cell Activation and Survival Factors in Neonatal Mice That Translates Into Long-Lived Humoral Immunity. Front. Immunol. 2020, 11, 527310. [Google Scholar] [CrossRef] [PubMed]
- Evrard, M.; Kwok, I.W.H.; Chong, S.Z.; Teng, K.W.W.; Becht, E.; Chen, J.; Sieow, J.L.; Penny, H.L.; Ching, G.C.; Devi, S.; et al. Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity 2018, 48, 364–379.e8. [Google Scholar] [CrossRef]
- Bonadonna, M.; Altamura, S.; Tybl, E.; Palais, G.; Qatato, M.; Polycarpou-Schwarz, M.; Schneider, M.; Kalk, C.; Rüdiger, W.; Ertl, A.; et al. Iron regulatory protein (IRP)–mediated iron homeostasis is critical for neutrophil development and differentiation in the bone marrow. Sci. Adv. 2022, 8, 4469. [Google Scholar] [CrossRef]
- Doyle, N.A.; Bhagwan, S.D.; Meek, B.B.; Kutkoski, G.J.; Steeber, D.A.; Tedder, T.F.; Doerschuk, C.M. Neutrophil margination, sequestration, and emigration in the lungs of L-selectin-deficient mice. J. Clin. Investig. 1997, 99, 526–533. [Google Scholar] [CrossRef]
- Bonville, C.A.; Ptaschinski, C.; Percopo, C.M.; Rosenberg, H.F.; Domachowske, J.B. Inflammatory responses to acute pneumovirus infection in neonatal mice. Virol. J. 2010, 7, 320. [Google Scholar] [CrossRef]
- Liechty, K.W.; Schibler, K.R.; Ohls, R.K.; Perkins, S.L.; Christensen, R.D. The Failure of Newborn Mice Infected with Escherichia coli to Accelerate Neutrophil Production Correlates with Their Failure to Increase Transcripts for Granulocyte Colony-Stimulating Factor and Interleukin-6. Biol. Neonate 1993, 64, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lines, J.L.; Hoskins, S.; Hollifield, M.; Cauley, L.S.; Garvy, B.A. The Migration of T Cells in Response to Influenza Virus Is Altered in Neonatal Mice. J. Immunol. 2010, 185, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-W.; An, M.-X.; Huang, Z.-K.; Ma, L.; Zhao, D.; Yang, Z.; Shi, J.-X.; Liu, D.-X.; Li, Q.; Wu, A.-H.; et al. Lpp of Escherichia coli K1 inhibits host ROS production to counteract neutrophil-mediated elimination. Redox Biol. 2023, 59, 102588. [Google Scholar] [CrossRef]
- Rincon, J.C.; Cuenca, A.L.; Raymond, S.L.; Mathias, B.; Nacionales, D.C.; Ungaro, R.; Efron, P.A.; Wynn, J.L.; Moldawer, L.L.; Larson, S.D. Adjuvant pretreatment with alum protects neonatal mice in sepsis through myeloid cell activation. Clin. Exp. Immunol. 2018, 191, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, T.-S.; Li, Y.-H.; Liu, C.-F.; Yang, S.-J.; Zeng, L.-T.; Huang, S.-H.; Deng, X.-Y.; Peng, L. Memantine Promotes Bactericidal Effect of Neutrophils against Infection with Pseudomonas aeruginosa and Its Drug-Resistant Strain, by Improving Reactive Oxygen Species Generation. Microb. Drug Resist. 2022, 28, 7–17. [Google Scholar] [CrossRef]
- de Araujo, C.V.; Denorme, F.; Stephens, W.Z.; Li, Q.; Cody, M.J.; Crandell, J.L.; Petrey, A.C.; Queisser, K.A.; Rustad, J.L.; Fulcher, J.M.; et al. Neonatal NET-Inhibitory Factor improves survival in the cecal ligation and puncture model of polymicrobial sepsis by inhibiting neutrophil extracellular traps. Front. Immunol. 2023, 13, 8159. [Google Scholar] [CrossRef]
- Denorme, F.; Rustad, J.L.; Portier, I.; Crandell, J.L.; de Araujo, C.V.; Cody, M.J.; Campbell, R.A.; Yost, C.C. Neutrophil extracellular trap inhibition improves survival in neonatal mouse infectious peritonitis. Pediatr. Res. 2022, 93, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Colón, D.F.; Wanderley, C.W.; Franchin, M.; Silva, C.M.; Hiroki, C.H.; Castanheira, F.V.S.; Donate, P.B.; Lopes, A.H.; Volpon, L.C.; Kavaguti, S.K.; et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit. Care 2019, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Sebina, I.; Rashid, R.B.; Sikder, A.A.; Rahman, M.M.; Ahmed, T.; Radford-Smith, D.E.; Kotenko, S.V.; Hill, G.R.; Bald, T.; Phipps, S. IFN-λ Diminishes the Severity of Viral Bronchiolitis in Neonatal Mice by Limiting NADPH Oxidase–Induced PAD4-Independent NETosis. J. Immunol. 2022, 208, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.M.; Smyth, M. A potential role for RAG-1 in NK cell development revealed by analysis of NK cells during ontogeny. Immunol Cell Biol. 2010, 88, 107–116. [Google Scholar] [CrossRef]
- Chiossone, L.; Chaix, N.; Fuseri, N.; Roth, C.; Vivier, E.; Walzer, T. Maturation of mouse NK cells is a 4-stage developmental program. Blood 2009, 113, 588–5496. [Google Scholar] [CrossRef]
- Castaneda, D.C.; Dhommée, C.; Baranek, T.; Dalloneau, E.; Lajoie, L.; Valayer, A.; Arnoult, C.; Demattéi, M.-V.; Fouquenet, D.; Parent, C.; et al. Lack of FcRn Impairs Natural Killer Cell Development and Functions in the Tumor Microenvironment. Front. Immunol. 2018, 9, 2259. [Google Scholar] [CrossRef]
- Beckett, E.L.; Phipps, S.; Starkey, M.R.; Horvat, J.C.; Beagley, K.W.; Foster, P.S.; Hansbro, P.M. TLR2, but Not TLR4, Is Required for Effective Host Defence against Chlamydia Respiratory Tract Infection in Early Life. PLoS ONE 2012, 7, e39460. [Google Scholar] [CrossRef]
- Dunsmore, G.; Bozorgmehr, N.; Delyea, C.; Koleva, P.; Namdar, A.; Elahi, S. Erythroid Suppressor Cells Compromise Neonatal Immune Response against Bordetella pertussis. J. Immunol. 2017, 199, 2081–2095. [Google Scholar] [CrossRef]
- Ganesan, P.; Chandwani, M.N.; Creisher, P.S.; Bohn, L.; O’Donnell, L.A. The neonatal anti-viral response fails to control measles virus spread in neurons despite interferon-gamma expression and a Th1-like cytokine profile. J. Neuroimmunol. 2018, 316, 80–97. [Google Scholar] [CrossRef]
- Ruckwardt, T.J.; Malloy, A.M.M.; Morabito, K.M.; Graham, B.S. Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response. PLoS Pathog. 2014, 10, e1003934. [Google Scholar] [CrossRef]
- Sun, C.-M.; Fiette, L.; Tanguy, M.; Leclerc, C.; Lo-Man, R. Ontogeny and innate properties of neonatal dendritic cells. Blood 2003, 102, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-M.; Deriaud, E.; Leclerc, C.; Lo-Man, R. Upon TLR9 Signaling, CD5+ B Cells Control the IL-12-Dependent Th1-Priming Capacity of Neonatal DCs. Immunity 2005, 22, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Hoeman, C.M.; Hardaway, J.C.; Guloglu, F.B.; Ellis, J.S.; Jain, R.; Divekar, R.; Tartar, D.M.; Haymaker, C.L.; Zaghouani, H. Delayed maturation of an IL-12–producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J. Exp. Med. 2008, 205, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, A.; Coetzee, S.G.; Olsson, A.; Muench, D.E.; Berman, B.P.; Hazelett, D.J.; Salomonis, N.; Grimes, H.L.; Goodridge, H.S. Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity 2017, 47, 890–902.e4. [Google Scholar] [CrossRef]
- de Kleer, I.M.; Kool, M.; de Bruijn, M.J.; Willart, M.; van Moorleghem, J.; Schuijs, M.J.; Plantinga, M.; Beyaert, R.; Hams, E.; Fallon, P.G.; et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity 2016, 45, 1285–1298. [Google Scholar] [CrossRef]
- Pozarska, A.; Rodríguez-Castillo, J.A.; Solaligue, D.E.S.; Ntokou, A.; Rath, P.; Mižíková, I.; Madurga, A.; Mayer, K.; Vadász, I.; Herold, S.; et al. Stereological monitoring of mouse lung alveolarization from the early postnatal period to adulthood. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L882–L895. [Google Scholar] [CrossRef]
- Guilliams, M.; De Kleer, I.; Henri, S.; Post, S.; Vanhoutte, L.; De Prijck, S.; Deswarte, K.; Malissen, B.; Hammad, H.; Lambrecht, B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013, 210, 1977–1992. [Google Scholar] [CrossRef]
- Cohen, M.; Giladi, A.; Gorki, A.-D.; Solodkin, D.G.; Zada, M.; Hladik, A.; Miklosi, A.; Salame, T.-M.; Halpern, K.B.; David, E.; et al. Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell 2018, 175, 1031–1044.e18. [Google Scholar] [CrossRef]
- Jones, C.V.; Williams, T.M.; Walker, K.A.; Dickinson, H.; Sakkal, S.; Rumballe, B.A.; Little, M.H.; Jenkin, G.; Ricardo, S.D. M2 macrophage polarisation is associated with alveolar formation during postnatal lung development. Respir. Res. 2013, 14, 41. [Google Scholar] [CrossRef]
- Hughes, D.A.; Gordon, S. Expression and Function of the Type 3 Complement Receptor in Tissues of the Developing Mouse. J. Immunol. 1998, 160, 4543–4552. [Google Scholar] [CrossRef]
- Chelvarajan, R.L.; Collins, S.M.; Doubinskaia, I.E.; Goes, S.; Van Willigen, J.; Flanagan, D.; de Villiers, W.J.S.; Bryson, J.S.; Bondada, S. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J. Leukoc. Biol. 2004, 75, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.W.; Choudhary, I.; Paudel, K.; Mao, Y.; Sharma, R.; Wang, Y.; Deshane, J.S.; Boucher, R.C.; Patial, S.; Saini, Y. The Innate Lymphoid System Is a Critical Player in the Manifestation of Mucoinflammatory Airway Disease in Mice. J. Immunol. 2020, 205, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Empey, K.M.; Orend, J.G.; Jr, R.S.P.; Egaña, L.; Norris, K.A.; Oury, T.D.; Kolls, J.K. Stimulation of Immature Lung Macrophages with Intranasal Interferon Gamma in a Novel Neonatal Mouse Model of Respiratory Syncytial Virus Infection. PLoS ONE 2012, 7, e40499. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.L.; McKenzie, A.N. Innate Lymphoid Cells of the Lung. Annu. Rev. Physiol. 2019, 81, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.A.; Mathä, L.; Shim, H.; Takei, F. Lung group 2 innate lymphoid cells are trained by endogenous IL-33 in the neonatal period. JCI Insight 2020, 5, e135961. [Google Scholar] [CrossRef]
- Loering, S.; Cameron, G.J.M.; Bhatt, N.P.; Belz, G.T.; Foster, P.S.; Hansbro, P.M.; Starkey, M.R. Differences in pulmonary group 2 innate lymphoid cells are dependent on mouse age, sex and strain. Immunol. Cell Biol. 2021, 99, 542–551. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shen, Z.Y.; Orangi, M.; Martinez-Gonzalez, I.; Wei, L.; Lu, X.; Das, A.; Heravi-Moussavi, A.; Marra, M.A.; Bhandoola, A.; et al. Single-cell analysis of RORα tracer mouse lung reveals ILC progenitors and effector ILC2 subsets. J. Exp. Med. 2019, 217, e20182293. [Google Scholar] [CrossRef]
- Steer, C.A.; Martinez-Gonzalez, I.; Ghaedi, M.; Allinger, P.; Mathä, L.; Takei, F. Group 2 innate lymphoid cell activation in the neonatal lung drives type 2 immunity and allergen sensitization. J. Allergy Clin. Immunol. 2017, 140, 593–595.e3. [Google Scholar] [CrossRef]
- Saluzzo, S.; Gorki, A.-D.; Rana, B.M.; Martins, R.; Scanlon, S.; Starkl, P.; Lakovits, K.; Hladik, A.; Korosec, A.; Sharif, O.; et al. First-Breath-Induced Type 2 Pathways Shape the Lung Immune Environment. Cell Rep. 2017, 18, 1893–1905. [Google Scholar] [CrossRef]
- Saravia, J.; You, D.; Shrestha, B.; Jaligama, S.; Siefker, D.; Lee, G.I.; Harding, J.N.; Jones, T.L.; Rovnaghi, C.; Bagga, B.; et al. Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33. PLoS Pathog. 2015, 11, e1005217. [Google Scholar] [CrossRef]
- Hong, J.Y.; Bentley, J.K.; Chung, Y.; Lei, J.; Steenrod, J.M.; Chen, Q.; Sajjan, U.S.; Hershenson, M.B. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J. Allergy Clin. Immunol. 2014, 134, 429–439.e8. [Google Scholar] [CrossRef] [PubMed]
- Kusmartsev, S.; Gabrilovich, D.I. STAT1 Signaling Regulates Tumor-Associated Macrophage-Mediated T Cell Deletion. J. Immunol. 2005, 174, 4880–4891. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-M.; Li, X.; Perego, M.; Nefedova, Y.; Kossenkov, A.V.; Jensen, E.A.; Kagan, V.; Liu, Y.-F.; Fu, S.-Y.; Ye, Q.-J.; et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat. Med. 2018, 24, 224–231. [Google Scholar] [CrossRef]
- Movahedi, K.; Guilliams, M.; Bossche, J.V.D.; Bergh, R.V.D.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell–suppressive activity. Blood 2008, 111, 4233–4244. [Google Scholar] [CrossRef]
- Guo, X.-Z.J.; Dash, P.; Crawford, J.C.; Allen, E.K.; Zamora, A.E.; Boyd, D.F.; Duan, S.; Bajracharya, R.; Awad, W.A.; Apiwattanakul, N.; et al. Lung γδ T Cells Mediate Protective Responses during Neonatal Influenza Infection that Are Associated with Type 2 Immunity. Immunity 2018, 49, 531–544.e6. [Google Scholar] [CrossRef]
- Scanlon, K.M.; Snyder, Y.G.; Skerry, C.; Carbonetti, N.H. Fatal Pertussis in the Neonatal Mouse Model Is Associated with Pertussis Toxin-Mediated Pathology beyond the Airways. Infect. Immun. 2017, 85, e00355-17. [Google Scholar] [CrossRef]
- Akue, A.D.; Lee, J.-Y.; Jameson, S.C. Derivation and Maintenance of Virtual Memory CD8 T Cells. J. Immunol. 2012, 188, 2516–2523. [Google Scholar] [CrossRef]
- Sedney, C.J.; Caulfield, A.; Dewan, K.K.; Blas-Machado, U.; Callender, M.; Manley, N.R.; Harvill, E.T. Novel murine model reveals an early role for pertussis toxin in disrupting neonatal immunity to Bordetella pertussis. Front. Immunol. 2023, 14, 515. [Google Scholar] [CrossRef]
- Foo, S.Y.; Zhang, V.; Lalwani, A.; Lynch, J.P.; Zhuang, A.; Lam, C.E.; Foster, P.S.; King, C.; Steptoe, R.J.; Mazzone, S.B.; et al. Regulatory T Cells Prevent Inducible BALT Formation by Dampening Neutrophilic Inflammation. J. Immunol. 2015, 194, 4567–4576. [Google Scholar] [CrossRef]
- Kocks, J.R.; Davalos-Misslitz, A.C.M.; Hintzen, G.; Ohl, L.; Förster, R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J. Exp. Med. 2007, 204, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Miyahara, Y.; Guo, Z.; Khattar, M.; Stepkowski, S.M.; Chen, W. “Default” Generation of Neonatal Regulatory T Cells. J. Immunol. 2010, 185, 71–78. [Google Scholar] [CrossRef]
- Oliphant, S.; Lines, J.L.; Hollifield, M.L.; Garvy, B.A. Regulatory T Cells Are Critical for Clearing Influenza A Virus in Neonatal Mice. Viral Immunol. 2015, 28, 580–589. [Google Scholar] [CrossRef]
- McGrath-Morrow, S.A.; Lee, S.; Gibbs, K.; Lopez, A.; Collaco, J.M.; Neptune, E.; Soloski, M.J.; Scott, A.; D’alessio, F. Immune Response to Intrapharyngeal LPS in Neonatal and Juvenile Mice. Am. J. Respir. Cell Mol. Biol. 2015, 52, 323–331. [Google Scholar] [CrossRef]
- Gollwitzer, E.S.; Saglani, S.; Trompette, A.; Yadava, K.; Sherburn, R.; McCoy, K.D.; Nicod, L.P.; Lloyd, C.; Marsland, B.J. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 2014, 20, 642–647. [Google Scholar] [CrossRef]
- Harker, J.A.; Yamaguchi, Y.; Culley, F.J.; Tregoning, J.S.; Openshaw, P.J.M. Delayed Sequelae of Neonatal Respiratory Syncytial Virus Infection Are Dependent on Cells of the Innate Immune System. J. Virol. 2014, 88, 604–611. [Google Scholar] [CrossRef]
- Laubreton, D.; Drajac, C.; Eléouët, J.-F.; Rameix-Welti, M.-A.; Lo-Man, R.; Riffault, S.; Descamps, D. Regulatory B Lymphocytes Colonize the Respiratory Tract of Neonatal Mice and Modulate Immune Responses of Alveolar Macrophages to RSV Infection in IL-10-Dependant Manner. Viruses 2020, 12, 822. [Google Scholar] [CrossRef]
- Elahi, S.; Vega-López, M.A.; Herman-Miguel, V.; Ramírez-Estudillo, C.; Mancilla-Ramírez, J.; Motyka, B.; West, L.; Oyegbami, O. CD71+ Erythroid Cells in Human Neonates Exhibit Immunosuppressive Properties and Compromise Immune Response against Systemic Infection in Neonatal Mice. Front. Immunol. 2020, 11, 597433. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Sosnowska, A.; Rydzynska, Z.; Lazniewski, M.; Plewczynski, D.; Klicka, K.; Malecka-Gieldowska, M.; Rodziewicz-Lurzynska, A.; Ciepiela, O.; Justyniarska, M.; et al. Potent but transient immunosuppression of T-cells is a general feature of CD71+ erythroid cells. Commun. Biol. 2021, 4, 1384. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S.; Ertelt, J.M.; Kinder, J.M.; Jiang, T.T.; Zhang, X.; Xin, L.; Chaturvedi, V.; Strong, B.S.; Qualls, J.E.; Steinbrecher, K.A.; et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013, 504, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Munguía-Fuentes, R.; Yam-Puc, J.C.; Silva-Sánchez, A.; Marcial-Juárez, E.; Gallegos-Hernández, I.A.; Calderón-Amador, J.; Randall, T.D.; Flores-Romo, L. Immunization of newborn mice accelerates the architectural maturation of lymph nodes, but AID-dependent IgG responses are still delayed compared to the adult. Front. Immunol. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Hijano, D.R.; Siefker, D.T.; Shrestha, B.; Jaligama, S.; Vu, L.D.; Tillman, H.; Finkelstein, D.; Saravia, J.; You, D.; Cormier, S.A. Type I Interferon Potentiates IgA Immunity to Respiratory Syncytial Virus Infection During Infancy. Sci. Rep. 2018, 8, 11034. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, W.; Wu, M.; Song, X.; Caro, F.; Sun, X.; Gazzaniga, F.; Stefanetti, G.; Oh, S.; Mekalanos, J.J.; et al. Microbiota-targeted maternal antibodies protect neonates from enteric infection. Nature 2020, 577, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Goymer, J.; Loh, L.N.; Mahant, A.; Aschner, C.B.; Herold, B.C. Murine Model of Maternal Immunization Demonstrates Protective Role for Antibodies That Mediate Antibody-Dependent Cellular Cytotoxicity in Protecting Neonates from Herpes Simplex Virus Type 1 and Type 2. J. Infect. Dis. 2020, 221, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Vono, M.; Eberhardt, C.S.; Auderset, F.; Mastelic-Gavillet, B.; Lemeille, S.; Christensen, D.; Andersen, P.; Lambert, P.-H.; Siegrist, C.-A. Maternal Antibodies Inhibit Neonatal and Infant Responses to Vaccination by Shaping the Early-Life B Cell Repertoire within Germinal Centers. Cell Rep. 2019, 28, 1773–1784.e5. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nabeela, S.; Barbarino, A.; Ibrahim, A.S.; Uppuluri, P. Antibodies targeting Candida albicans Als3 and Hyr1 antigens protect neonatal mice from candidiasis. Front. Immunol. 2022, 13, 925821. [Google Scholar] [CrossRef]
- Aispuro, P.M.; Ambrosis, N.; Zurita, M.E.; Gaillard, M.E.; Bottero, D.; Hozbor, D.F. Use of a Neonatal-Mouse Model to Characterize Vaccines and Strategies for Overcoming the High Susceptibility and Severity of Pertussis in Early Life. Front. Microbiol. 2020, 11, 723. [Google Scholar] [CrossRef]
- Noh, Y.; Shim, B.-S.; Cheon, I.S.; Rho, S.; Kim, H.J.; Choi, Y.; Kang, C.-Y.; Chang, J.; Song, M.K.; Kim, J.-O. Neonatal Immunization with Respiratory Syncytial Virus Glycoprotein Fragment Induces Protective Immunity in the Presence of Maternal Antibodies in Mice. Viral Immunol. 2013, 26, 268–276. [Google Scholar] [CrossRef]
- Astori, M.; Finke, D.; Karapetian, O.; Acha-Orbea, H. Development of T–B cell collaboration in neonatal mice. Int. Immunol. 1999, 11, 445–451. [Google Scholar] [CrossRef]
- Thammavongsa, V.; Rauch, S.; Kim, H.K.; Missiakas, D.M.; Schneewind, O. Protein A-neutralizing monoclonal antibody protects neonatal mice against Staphylococcus aureus. Vaccine 2015, 33, 523–526. [Google Scholar] [CrossRef]
- Amano, S.; Natsuume-Sakai, S.; Hayakawa, J.-I.; Takahashi, M. The Expression of the Allelic Gene of Murine C3 in Fetal and Neonatal Mice. J. Immunol. 1979, 122, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Pihlgren, M.; Fulurija, A.; Villiers, M.-B.; Tougne, C.; Lambert, P.-H.; Villiers, C.L.; Siegrist, C.-A. Influence of complement C3 amount on IgG responses in early life: Immunization with C3b-conjugated antigen increases murine neonatal antibody responses. Vaccine 2004, 23, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.R.; Butko, P.; Ma, M.; Warren, H.B.; Lage, A.L.; Carroll, M.C. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 1995, 92, 11490–11494. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, E.; Edwards, K. Health Burden of Pertussis in Adolescents and Adults. Pediatr. Infect. Dis. J. 2005, 24, S44–S47. [Google Scholar] [CrossRef] [PubMed]
- von König, C.H.W.; Halperin, S.; Riffelmann, M.; Guiso, N. Pertussis of adults and infants. Lancet Infect. Dis. 2002, 2, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Pertussis—United States, 1997–2000. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5104a1.htm (accessed on 22 April 2023).
- Tanaka, M. Trends in Pertussis Among Infants in the United States, 1980–1999. JAMA 2003, 290, 2968–2975. [Google Scholar] [CrossRef]
- Babies Need Whooping Cough Vaccines on Time|CDC. Available online: https://www.cdc.gov/pertussis/pregnant/mom/vaccinate-baby.html (accessed on 22 April 2023).
- Gill, C.J.; Gunning, C.E.; MacLeod, W.B.; Mwananyanda, L.; Thea, D.M.; Pieciak, R.C.; Kwenda, G.; Mupila, Z.; Rohani, P. Asymptomatic Bordetella pertussis infections in a longitudinal cohort of young African infants and their mothers. Elife 2021, 10, e65663. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef]
- Marks, H.M. The Kendrick-Eldering-(Frost) pertussis vaccine field trial. J. R. Soc. Med. 2007, 100, 242. [Google Scholar] [CrossRef]
- Chasaide, C.N.; Mills, K.H. Next-Generation Pertussis Vaccines Based on the Induction of Protective T Cells in the Respiratory Tract. Vaccines 2020, 8, 621. [Google Scholar] [CrossRef]
- Boehm, D.; Hall, J.M.; Wong, T.Y.; DiVenere, A.M.; Sen-Kilic, E.; Bevere, J.R.; Bradford, S.D.; Blackwood, C.; Elkins, C.M.; DeRoos, K.A.; et al. Evaluation of Adenylate Cyclase Toxoid Antigen in Acellular Pertussis Vaccines by Using a Bordetella pertussis Challenge Model in Mice. Infect. Immun. 2018, 86, e00857-17. [Google Scholar] [CrossRef]
- Wilk, M.M.; Borkner, L.; Misiak, A.; Curham, L.; Allen, A.C.; Mills, K.H.G. Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg. Microbes Infect. 2019, 8, 169–185. [Google Scholar] [CrossRef]
- Najminejad, H.; Kalantar, S.M.; Mokarram, A.R.; Dabaghian, M.; Abdollahpour-Alitappeh, M.; Ebrahimi, S.M.; Tebianian, M.; Ramandi, M.F.; Sheikhha, M.H. Bordetella pertussis antigens encapsulated into N-trimethyl chitosan nanoparticulate systems as a novel intranasal pertussis vaccine. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Raguckas, S.E.; Vandenbussche, H.L.; Jacobs, C.; Klepser, M.E. Pertussis Resurgence: Diagnosis, Treatment, Prevention, and Beyond. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2007, 27, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, K.M.; Chen, L.; Carbonetti, N.H. Pertussis Toxin Promotes Pulmonary Hypertension in an Infant Mouse Model of Bordetella pertussis Infection. J. Infect. Dis. 2022, 225, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Zlamy, M. Rediscovering Pertussis. Front. Pediatr. 2016, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Quinn, K.M. Similar but different: Virtual memory CD8 T cells as a memory-like cell population. Immunol. Cell Biol. 2019, 97, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Schneider, O.D.; Weiss, A.A.; Miller, W.E. Pertussis Toxin Signals through the TCR to Initiate Cross-Desensitization of the Chemokine Receptor CXCR4. J. Immunol. 2009, 182, 5730–5739. [Google Scholar] [CrossRef]
- Schneider, O.D.; Weiss, A.A.; Miller, W.E. Pertussis Toxin Utilizes Proximal Components of the T-Cell Receptor Complex To Initiate Signal Transduction Events in T Cells. Infect. Immun. 2007, 75, 4040–4049. [Google Scholar] [CrossRef]
- Campbell, P.; McIntyre, P.; Quinn, H.; Hueston, L.; Gilbert, G.L.; McVernon, J. Increased Population Prevalence of Low Pertussis Toxin Antibody Levels in Young Children Preceding a Record Pertussis Epidemic in Australia. PLoS ONE 2012, 7, e35874. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Chen, Z.; Liu, X.; Peng, X.; He, Q. Determination of serum neutralizing antibodies reveals important difference in quality of antibodies against pertussis toxin in children after infection. Vaccine 2021, 39, 1826–1830. [Google Scholar] [CrossRef]
- Taranger, J.; Trollfors, B.; Lagergård, T.; Sundh, V.; Bryla, D.A.; Schneerson, R.; Robbins, J.B. Correlation between Pertussis Toxin IgG Antibodies in Postvaccination Sera and Subsequent Protection against Pertussis. J. Infect. Dis. 2000, 181, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, K.; Skerry, C.; Carbonetti, N. Association of Pertussis Toxin with Severe Pertussis Disease. Toxins 2019, 11, 373. [Google Scholar] [CrossRef]
- Connelly, C.E.; Sun, Y.; Carbonetti, N.H. Pertussis Toxin Exacerbates and Prolongs Airway Inflammatory Responses during Bordetella pertussis Infection. Infect. Immun. 2012, 80, 4317–4332. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, N.H.; Artamonova, G.V.; Mays, R.M.; Worthington, Z.E.V. Pertussis Toxin Plays an Early Role in Respiratory Tract Colonization by Bordetella pertussis. Infect. Immun. 2003, 71, 6358–6366. [Google Scholar] [CrossRef]
- Kirimanjeswara, G.S.; Agosto, L.M.; Kennett, M.J.; Bjornstad, O.N.; Harvill, E.T. Pertussis toxin inhibits neutrophil recruitment to delay antibody-mediated clearance of Bordetella pertussis. J. Clin. Investig. 2005, 115, 3594–3601. [Google Scholar] [CrossRef]
- Andreasen, C.; Carbonetti, N.H. Pertussis Toxin Inhibits Early Chemokine Production To Delay Neutrophil Recruitment in Response to Bordetella pertussis Respiratory Tract Infection in Mice. Infect. Immun. 2008, 76, 5139–5148. [Google Scholar] [CrossRef]
- Carbonetti, N.H.; Artamonova, G.V.; Andreasen, C.; Dudley, E.; Mays, R.M.; Worthington, Z.E.V. Suppression of Serum Antibody Responses by Pertussis Toxin after Respiratory Tract Colonization by Bordetella pertussis and Identification of an Immunodominant Lipoprotein. Infect. Immun. 2004, 72, 3350–3358. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Tripp, R.A. Immunopathology of RSV: An Updated Review. Viruses 2021, 13, 2478. [Google Scholar] [CrossRef]
- Baker, K.; Ryan, M. RSV infection in infants and young children: What’s new in diagnosis, treatment, and prevention? Postgrad. Med. 1999, 106, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Killikelly, A.; Tunis, M.; House, A.; Quach, C.; Vaudry, W.; Moore, D. Overview of the respiratory syncytial virus vaccine candidate pipeline in Canada. Can. Commun. Dis. Rep. 2020, 46, 56–61. [Google Scholar] [CrossRef] [PubMed]
- AREXVY|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/arexvy (accessed on 30 May 2023).
- Yamaguchi, Y.; Harker, J.A.; Wang, B.; Openshaw, P.J.; Tregoning, J.S.; Culley, F.J. Preexposure to CpG Protects against the Delayed Effects of Neonatal Respiratory Syncytial Virus Infection. J. Virol. 2012, 86, 10456–10461. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Bolder, R.; der Meer, M.H.-V.; Drijver, J.; van Polanen, Y.; Serroyen, J.; Langedijk, J.P.M.; Schuitemaker, H.; Saeland, E.; Zahn, R. Adenovector 26 encoded prefusion conformation stabilized RSV-F protein induces long-lasting Th1-biased immunity in neonatal mice. NPJ Vaccines 2020, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Tasker, L.; Lindsay, R.W.B.; Clarke, B.T.; Cochrane, D.W.R.; Hou, S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin. Exp. Immunol. 2008, 153, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Jacek, C.; Karolina, S.; Orzeł, A.; Frączek, M.; Tomasz, Z. Comparison of the clinical differences between COVID-19, SARS, influenza, and the common cold: A systematic literature review. Adv. Clin. Exp. Med. 2021, 30, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ruf, B.R.; Knuf, M. The burden of seasonal and pandemic influenza in infants and children. Eur. J. Pediatr. 2014, 173, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Harvill, E.T. Cultivating our ‘Frienemies’: Viewing immunity as microbiome management. mBio 2013, 4, e00027-13. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedney, C.J.; Harvill, E.T. The Neonatal Immune System and Respiratory Pathogens. Microorganisms 2023, 11, 1597. https://doi.org/10.3390/microorganisms11061597
Sedney CJ, Harvill ET. The Neonatal Immune System and Respiratory Pathogens. Microorganisms. 2023; 11(6):1597. https://doi.org/10.3390/microorganisms11061597
Chicago/Turabian StyleSedney, Colleen J., and Eric T. Harvill. 2023. "The Neonatal Immune System and Respiratory Pathogens" Microorganisms 11, no. 6: 1597. https://doi.org/10.3390/microorganisms11061597
APA StyleSedney, C. J., & Harvill, E. T. (2023). The Neonatal Immune System and Respiratory Pathogens. Microorganisms, 11(6), 1597. https://doi.org/10.3390/microorganisms11061597






