Hospital-Acquired Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Observational Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Settings
2.2. Protocol and Registration
2.3. Search Strategy
2.4. Eligibility Criteria
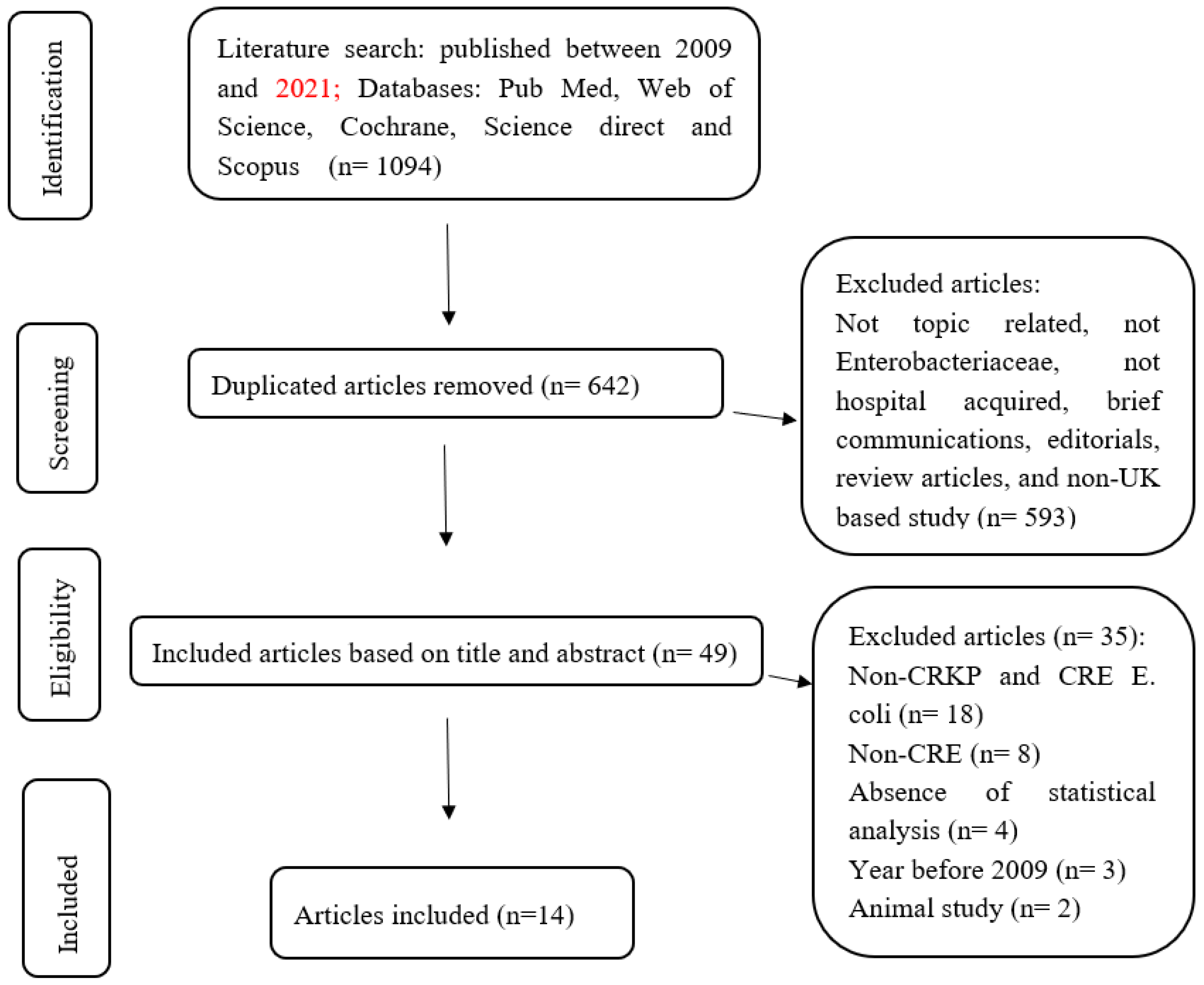
2.5. Study Selection
2.6. Data Recording
2.7. Data Analysis and Ethical Approval
3. Results
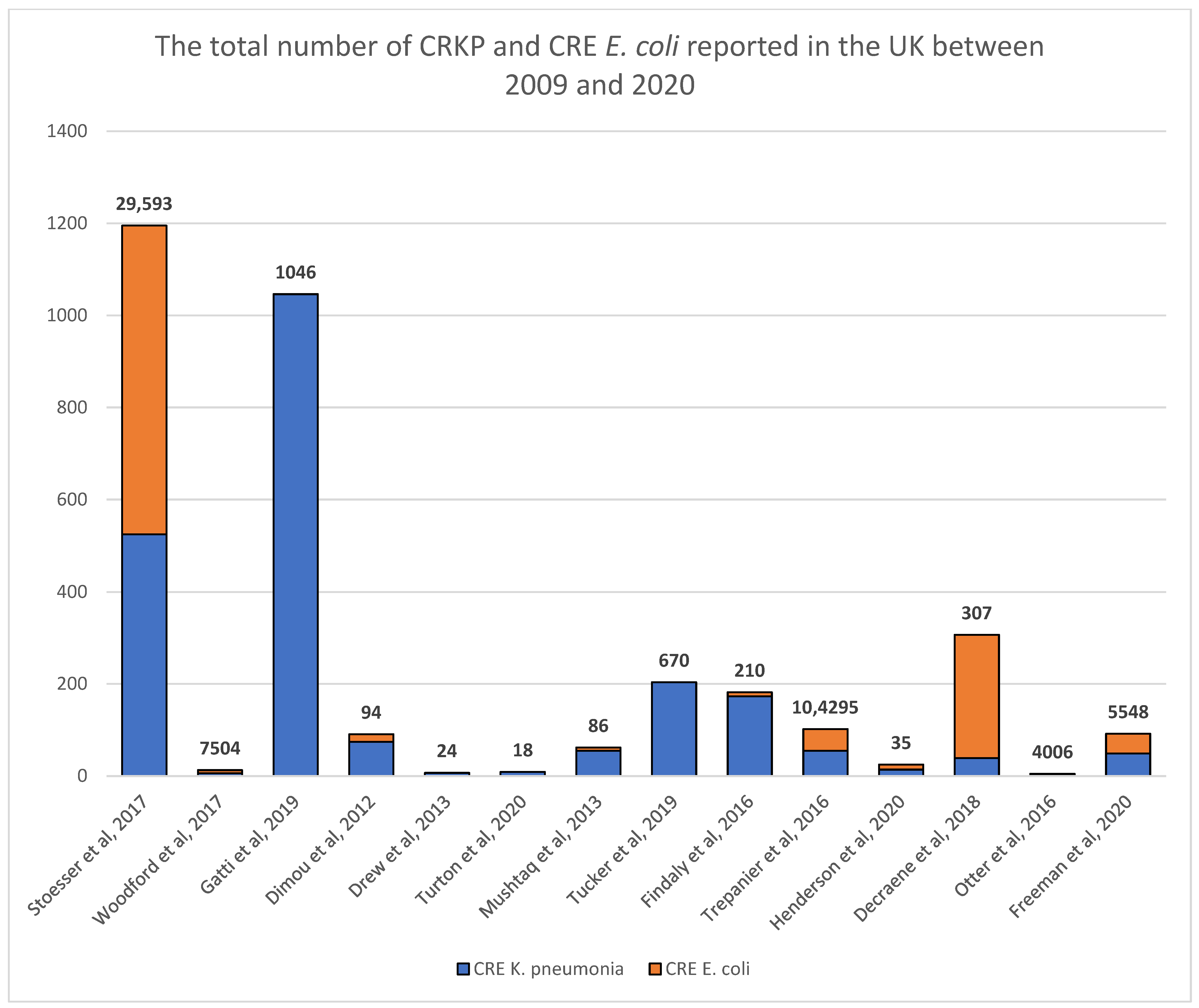
3.1. Spread of CRE from Reported Hospitals
3.2. Major Carbapenemase Plasmids
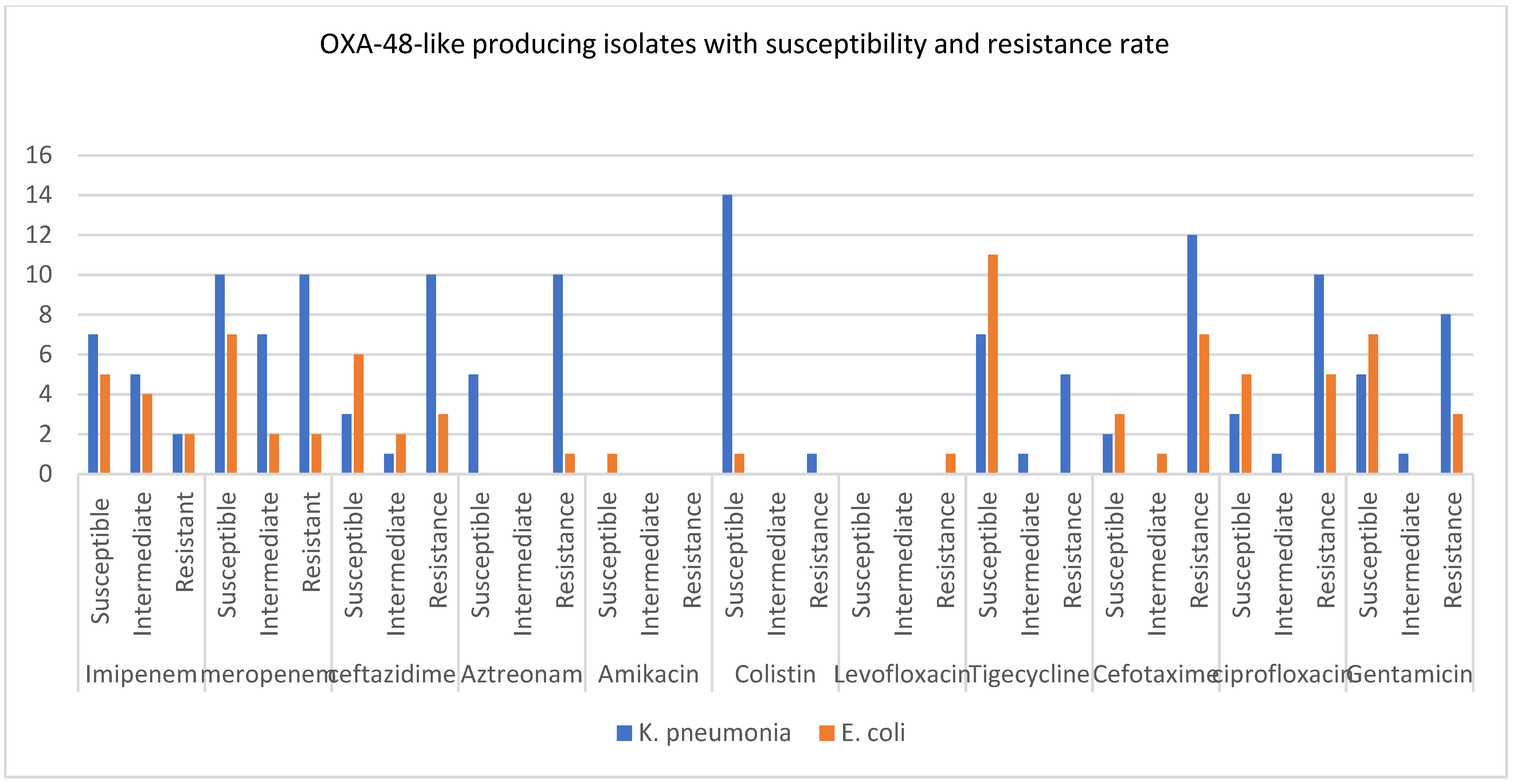
3.3. Minimum Inhibitory Concentration Calculations
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tassew, S.; Alebachew Woldu, M.; Amogne Degu, W.; Shibeshi, W. Management of hospital-acquired infections among patients hospitalized at Zewditu memorial hospital, Addis Ababa, Ethiopia: A prospective cross-sectional study. PLoS ONE 2020, 15, e0231949. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A. Control of hospital-acquired infections and antimicrobial resistance in Europe: The way to go. Wien. Med. Wochenschr. 2019, 169, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Richet, H. Seasonality in Gram-negative and healthcare-associated infections. Clin. Microbiol. Infect. 2012, 18, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Carlet, J.; Jarlier, V.; Harbarth, S.; Voss, A.; Goossens, H.; Pittet, D. Ready for a world without antibiotics? The Pensières Antibiotic Resistance Call to Action. Antimicrob. Resist. Infect. Control. 2012, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Walsh, F. The multiple roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 255. [Google Scholar] [CrossRef]
- Habte-Gabr, E. Antimicrobial Resistance: A Global Public Health Threat. J. Eritrean Med. Assoc. 2010, 3, 36–40. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Andini, R.; Zampino, R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin. Microbiol. Infect. 2019, 25, 943–950. [Google Scholar] [CrossRef]
- Iovleva, A.; Doi, Y. Carbapenem-Resistant Enterobacteriaceae. Clin. Lab. Med. 2017, 37, 303–315. [Google Scholar] [CrossRef]
- Sheu, C.-C.; Chang, Y.-T.; Lin, S.-Y.; Chen, Y.-H.; Hsueh, P.-R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Livermore, D.M.; Warner, M.; Mushtaq, S.; Doumith, M.; Zhang, J.; Woodford, N. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 2011, 37, 415–419. [Google Scholar] [CrossRef]
- Spagnolo, A.M.; Orlando, P.; Panatto, D.; Perdelli, F.; Cristina, M.L. An overview of carbapenem-resistant Klebsiella pneumoniae. Rev. Med. Microbiol. 2014, 25, 7–14. [Google Scholar] [CrossRef]
- Weisenberg, S.A.; Morgan, D.J.; Espinal-Witter, R.; Larone, D.H. Clinical outcomes of patients with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae after treatment with imipenem or meropenem. Diagn. Microbiol. Infect. Dis. 2009, 64, 233–235. [Google Scholar] [CrossRef]
- Liang, W.-J.; Liu, H.-Y.; Duan, G.-C.; Zhao, Y.-X.; Chen, S.-Y.; Yang, H.-Y.; Xi, Y.-L. Emergence and mechanism of carbapenem-resistant Escherichia coli in Henan, China, 2014. J. Infect. Public Health 2018, 11, 347–351. [Google Scholar] [CrossRef]
- Mahmoud, N.; Altayb, H.; Gurashi, R. Detection of Carbapenem-Resistant Genes in Escherichia coli Isolated from Drinking Water in Khartoum, Sudan. J. Environ. Public Health 2020, 2020, 2571293. [Google Scholar] [CrossRef]
- Huang, S.-R.; Liu, M.-F.; Lin, C.-F.; Shi, Z.-Y. Molecular surveillance and clinical outcomes of carbapenem-resistant Escherichia coli and Klebsiella pneumoniae infections. J. Microbiol. Immunol. Infect. 2014, 47, 187–196. [Google Scholar] [CrossRef]
- Potron, A.; Poirel, L.; Rondinaud, E.; Nordmann, P. Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over an 11-year period, 2001 to 2011. Eurosurveillance 2013, 18, 20549. [Google Scholar] [CrossRef]
- Drew, R.; Turton, J.; Hill, R.; Livermore, D.; Woodford, N.; Paulus, S.; Cunliffe, N. The emergence of carbapenem-resistant Enterobacteriaceae in a UK paediatric hospital. J. Hosp. Infect. 2013, 84, 300–304. [Google Scholar] [CrossRef]
- Gatti, M.; Raschi, E.; De Ponti, F. Relationship between adverse drug reactions to antibacterial agents and the Klebsiella pneumoniae carbapenemase-producing (KPC) Klebsiella pneumoniae outbreak: Insight from a pharmacovigilance study. BMC Pharmacol. Toxicol. 2019, 20, 65. [Google Scholar] [CrossRef]
- Tucker, A.; George, R.; Welfare, W.; Cleary, P.; Cawthorne, J.; Dodgson, A. Screening for carbapenemase-producing Enterobacteriaceae in previous carriers readmitted to hospital: Evaluation of a change in screening policy. J. Hosp. Infect. 2019, 103, 156–159. [Google Scholar] [CrossRef]
- Mushtaq, S.; Woodford, N.; Hope, R.; Adkin, R.; Livermore, D.M. Activity of BAL30072 alone or combined with β-lactamase inhibitors or with meropenem against carbapenem-resistant Enterobacteriaceae and non-fermenters. J. Antimicrob. Chemother. 2013, 68, 1601–1608. [Google Scholar] [CrossRef]
- Trepanier, P.; Mallard, K.; Meunier, D.; Pike, R.; Brown, D.; Ashby, J.P.; Donaldson, H.; Awad-El-Kariem, F.M.; Balakrishnan, I.; Cubbon, M.; et al. Carbapenemase-producing Enterobacteriaceae in the UK: A national study (EuSCAPE-UK) on prevalence, incidence, laboratory detection methods and infection control measures. J. Antimicrob. Chemother. 2016, 72, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.F.; Davies, F.; Taori, S.K.; Turton, J.A.; Smith, S.L.; Sajedi, N.; Wootton, M. IncN3 and IncHI2 plasmids with an In1763 integron carrying bla IMP-1 in carbapenem-resistant Enterobacterales clinical isolates from the UK. J. Med. Microbiol. 2020, 69, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Stoesser, N.; Sheppard, A.E.; Peirano, G.; Anson, L.W.; Pankhurst, L.; Sebra, R.; Phan, H.T.T.; Kasarskis, A.; Mathers, A.J.; Peto, T.E.A.; et al. Genomic epidemiology of global Klebsiella pneumoniae carbapenemase (KPC)-producing Escherichia coli. Sci. Rep. 2017, 7, 5917. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.; Hopkins, K.L.; Doumith, M.; Meunier, D.; Wiuff, C.; Hill, R.; Pike, R.; Loy, R.; Mustafa, N.; Livermore, D.M.; et al. KPC enzymes in the UK: An analysis of the first 160 cases outside the North-West region. J. Antimicrob. Chemother. 2016, 71, 1199–1206. [Google Scholar] [CrossRef]
- Decraene, V.; Phan, H.; George, R.; Wyllie, D.H.; Akinremi, O.; Aiken, Z.; Cleary, P.; Dodgson, A.; Pankhurst, L.; Crook, D.W.; et al. A Large, Refractory Nosocomial Outbreak of Klebsiella pneumoniae Carbapenemase-Producing Escherichia coli Demonstrates Carbapenemase Gene Outbreaks Involving Sink Sites Require Novel Approaches to Infection Control. Antimicrob. Agents Chemother. 2018, 62, e01689-18. [Google Scholar] [CrossRef]
- Dimou, V.; Dhanji, H.; Pike, R.; Livermore, D.M.; Woodford, N. Characterization of Enterobacteriaceae producing OXA-48-like carbapenemases in the UK. J. Antimicrob. Chemother. 2012, 67, 1660–1665. [Google Scholar] [CrossRef]
- Henderson, J.; Ciesielczuk, H.; Nelson, S.M.; Wilks, M.; Cummins, M.N. A point prevalence study to determine the inpatient rate of carbapenemase-producing organisms at a large London NHS Trust. J. Hosp. Infect. 2020, 104, 12–19. [Google Scholar] [CrossRef]
- Woodford, N.; Xu-McCrae, L.; Mushtaq, S.; Wu, H.H.T.; Ellington, M.J.; Lancaster, O.; Davies, F.; Donaldson, H.; Rao, G.G.; Verma, A.; et al. Prevalence of carbapenem resistance and carbapenemase production among Enterobacteriaceae isolated from urine in the UK: Results of the UK infection-Carbapenem Resistance Evaluation Surveillance Trial (iCREST-UK). J. Antimicrob. Chemother. 2017, 73, 698–702. [Google Scholar] [CrossRef]
- Otter, J.A.; Dyakova, E.; Bisnauthsing, K.N.; Querol-Rubiera, A.; Patel, A.; Ahanonu, C.; Auguet, O.T.; Edgeworth, J.D.; Goldenberg, S.D. Universal hospital admission screening for carbapenemase-producing organisms in a low-prevalence setting. J. Antimicrob. Chemother. 2016, 71, 3556–3561. [Google Scholar] [CrossRef]
- Freeman, R.; Ironmonger, D.; Hopkins, K.L.; Puleston, R.; Staves, P.; Hope, R.; Muller-Pebody, B.; Brown, C.S.; Hopkins, S.; Johnson, A.P.; et al. Epidemiology of carbapenemase-producing Enterobacterales in England, May 2015–March 2019: National enhanced surveillance findings and approach. Infect. Prev. Pract. 2020, 2, 100051. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Data from the ECDC Surveillance Atlas Antimicrobial Resistance. Available online: https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/data-ecdc (accessed on 2 February 2023).
- Public Health England. Carbapenemase-Producing Enterobacteriaceae: Laboratory Confirmed Cases, 2003 to 2015. 2016. Available online: https://www.gov.uk/government/publications/carbapenemase-producing-enterobacteriaceae-laboratory-confirmed-cases/carbapenemase-producing-enterobacteriaceae-laboratory-confirmed-cases-2003-to-2013 (accessed on 2 February 2023).
- Hughes, J.; Goldenberg, S.D.; Tosas, O.; Edgeworth, J.D.; Otter, J.A. Recent emergence of carbapenem-resistant organisms in a low prevalence UK setting in London. J. Infect. Prev. 2015, 17, 130–134. [Google Scholar] [CrossRef]
- Ellaby, N.; Doumith, M.; Hopkins, K.L.; Woodford, N.; Ellington, M.J. Emergence of diversity in carbapenemase-producing Escherichia coli ST131, England, January 2014 to June 2016. Eurosurveillance 2019, 24, 1800627. [Google Scholar] [CrossRef]
- Doumith, M.; Godbole, G.; Ashton, P.; Larkin, L.; Dallman, T.; Day, M.; Day, M.; Muller-Pebody, B.; Ellington, M.J.; de Pinna, E.; et al. Detection of the plasmid-mediatedmcr-1gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J. Antimicrob. Chemother. 2016, 71, 2300–2305. [Google Scholar] [CrossRef]
- Donker, T.; Henderson, K.L.; Hopkins, K.L.; Dodgson, A.R.; Thomas, S.; Crook, D.W.; Peto, T.E.A.; Johnson, A.P.; Woodford, N.; Walker, A.S.; et al. The relative importance of large problems far away versus small problems closer to home: Insights into limiting the spread of antimicrobial resistance in England. BMC Med. 2017, 15, 86. [Google Scholar] [CrossRef]
- Bedenić, B.; Plečko, V.; Sardelić, S.; Uzunović, S.; Godič Torkar, K. Carbapenemases in Gram-Negative Bacteria: Laboratory Detection and Clinical Significance. BioMed Res. Int. 2014, 2014, 841951. [Google Scholar] [CrossRef]
- Woodford, N.; Zhang, J.; Warner, M.; Kaufmann, M.E.; Matos, J.; MacDonald, A.; Brudney, D.; Sompolinsky, D.; Navon-Venezia, S.; Livermore, D.M. Arrival of Klebsiella pneumoniae producing KPC carbapenemase in the United Kingdom. J. Antimicrob. Chemother. 2008, 62, 1261–1264. [Google Scholar] [CrossRef]
- Thomas, C.P.; Moore, L.S.; Elamin, N.; Doumith, M.; Zhang, J.; Maharjan, S.; Warner, M.; Perry, C.; Turton, J.F.; Johnstone, C.; et al. Early (2008–2010) hospital outbreak of Klebsiella pneumoniae producing OXA-48 carbapenemase in the UK. Int. J. Antimicrob. Agents 2013, 42, 531–536. [Google Scholar] [CrossRef]
- Falagas, M.E.; Lourida, P.; Poulikakos, P.; Rafailidis, P.I.; Tansarli, G.S. Antibiotic Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae: Systematic Evaluation of the Available Evidence. Antimicrob. Agents Chemother. 2013, 58, 654–663. [Google Scholar] [CrossRef]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef]
- Stewart, A.; Harris, P.; Henderson, A.; Paterson, D. Treatment of Infections by OXA-48-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e01195-18. [Google Scholar] [CrossRef]
- Banach, D.; Seville, M.; Kusne, S. Infection Prevention and Control Issues After Solid Organ Transplantation. In Transplant Infections; Springer: Cham, Switzerland, 2016; pp. 843–867. [Google Scholar]
- Glasner, C.; Albiger, B.; Buist, G.; Andrašević, A.T.; Cantón, R.; Carmeli, Y.; Friedrich, A.W.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; et al. Carbapenemase-producing Enterobacteriaceae in Europe: A survey among national experts from 39 countries, February 2013. Eurosurveillance 2013, 18, 20525. [Google Scholar] [CrossRef] [PubMed]
- Njuangang, S.; Liyanage, C.; Akintoye, A. The history of healthcare facilities management services: A UK perspective on infection control. Facilities 2018, 36, 369–385. [Google Scholar] [CrossRef]



| Observed N | Expected N | Residual | Chi-Square | df | p-Value | |
|---|---|---|---|---|---|---|
| E. coli | 972 | 1378.0 | -406.0 | 239.24 | 1 | <0.001 |
| Carbapenem resistant K. pneumoniae (CRKP) | 1784 | 1378.0 | 406.0 | |||
| Total | 2756 |
| KPC | OXA-48-Like | NDM | Total | |
|---|---|---|---|---|
| E. coli | 314 | 648 | 10 | 972 |
| CRKP | 1729 | 39 | 16 | 1784 |
| Total column | 2043 | 687 | 26 | 2756 |
| Observed N | Expected N | Residual | Chi-Square | df | p-Value | |
|---|---|---|---|---|---|---|
| KPC | 2043 | 918.7 | 1124.3 | 2301.87 | 2 | <0.0001 |
| OXA-48-Like | 687 | 918.7 | -231.7 | |||
| NDM | 26 | 918.7 | -892.7 | |||
| Total | 2756 |
| Type | Total | Pearson Chi-Square | df | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| KPC | OXA-48-Like | NDM | |||||||
| Category | E. coli | Count | 314 | 648 | 10 | 972 | 1403.91 | 2 | <0.001 |
| % within Type | 15.4% | 94.3% | 38.5% | 35.3% | |||||
| CRKP | Count | 1729 | 39 | 16 | 1784 | ||||
| % within Type | 84.6% | 5.7% | 61.5% | 64.7% | |||||
| Total | Count | 2043 | 687 | 26 | 2756 | ||||
| % within Type | 100.0% | 100.0% | 100.0% | 100.0% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldali, H.J.; Khan, A.; Alshehri, A.A.; Aldali, J.A.; Meo, S.A.; Hindi, A.; Elsokkary, E.M. Hospital-Acquired Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Observational Study. Microorganisms 2023, 11, 1595. https://doi.org/10.3390/microorganisms11061595
Aldali HJ, Khan A, Alshehri AA, Aldali JA, Meo SA, Hindi A, Elsokkary EM. Hospital-Acquired Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Observational Study. Microorganisms. 2023; 11(6):1595. https://doi.org/10.3390/microorganisms11061595
Chicago/Turabian StyleAldali, Hamzah J., Azra Khan, Abdullah A. Alshehri, Jehad A. Aldali, Sultan Ayoub Meo, Ali Hindi, and Emadeldin M. Elsokkary. 2023. "Hospital-Acquired Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Observational Study" Microorganisms 11, no. 6: 1595. https://doi.org/10.3390/microorganisms11061595
APA StyleAldali, H. J., Khan, A., Alshehri, A. A., Aldali, J. A., Meo, S. A., Hindi, A., & Elsokkary, E. M. (2023). Hospital-Acquired Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Observational Study. Microorganisms, 11(6), 1595. https://doi.org/10.3390/microorganisms11061595






