Compositional Variations between Adult and Infant Skin Microbiome: An Update
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Participants
2.3. 16S rRNA Gene Sequencing
2.3.1. Microbiome Sample Collection and Processing
2.3.2. DNA Extraction and 16S rRNA Gene Library Preparation and Sequencing
2.3.3. Informatics Processing
2.4. Data Processing
2.4.1. Network Analysis
2.4.2. Statistical Analysis
3. Results
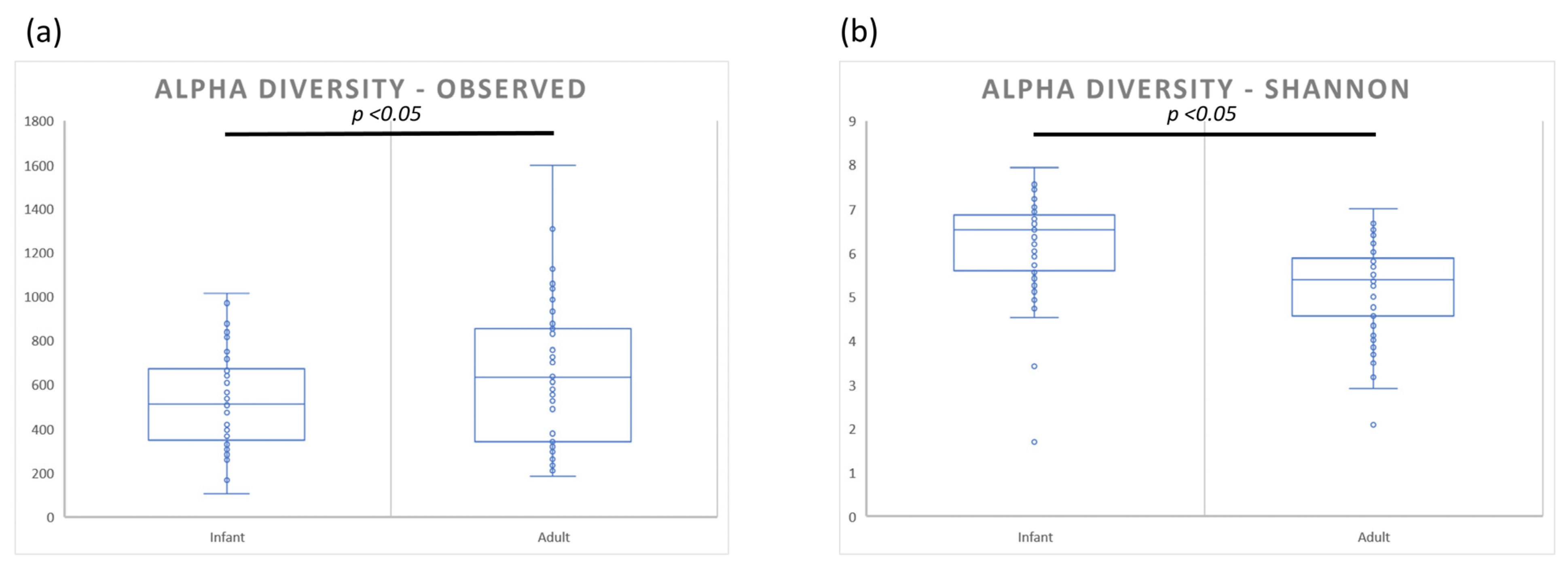
3.1. Bacterial Diversity Analysis
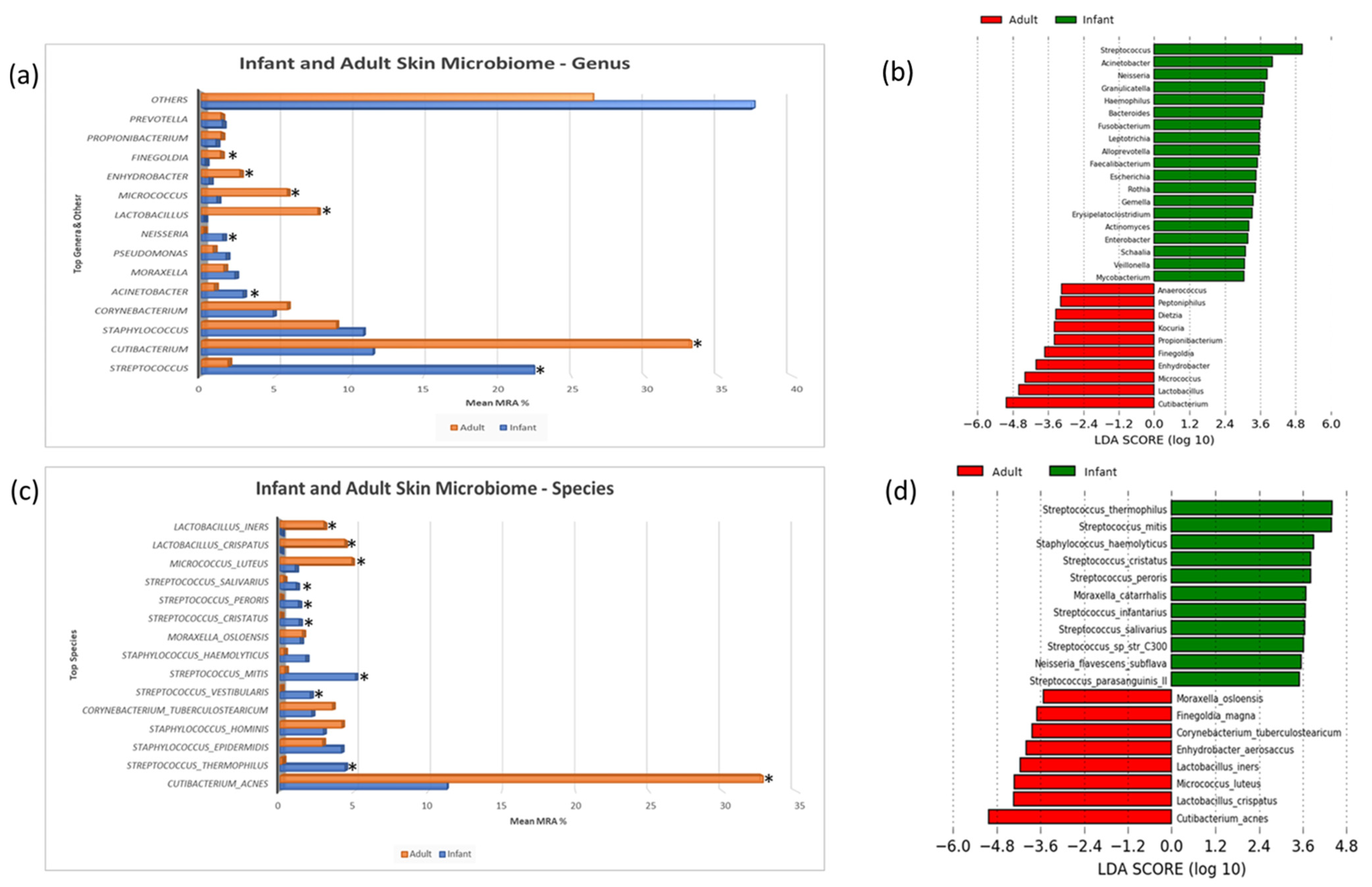
3.2. Taxonomic Composition and Differential Abundance
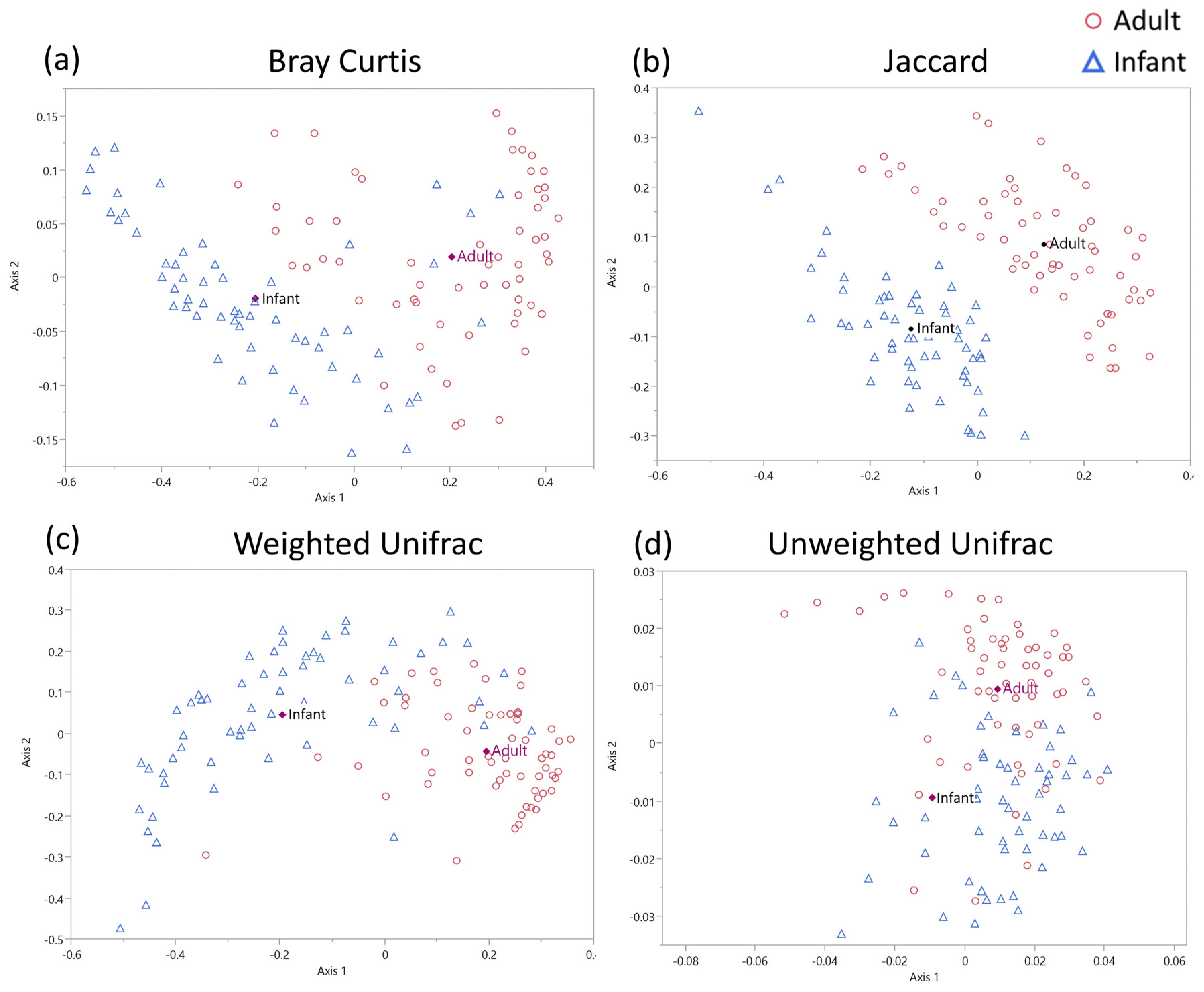
3.3. Beta Diversity Analysis
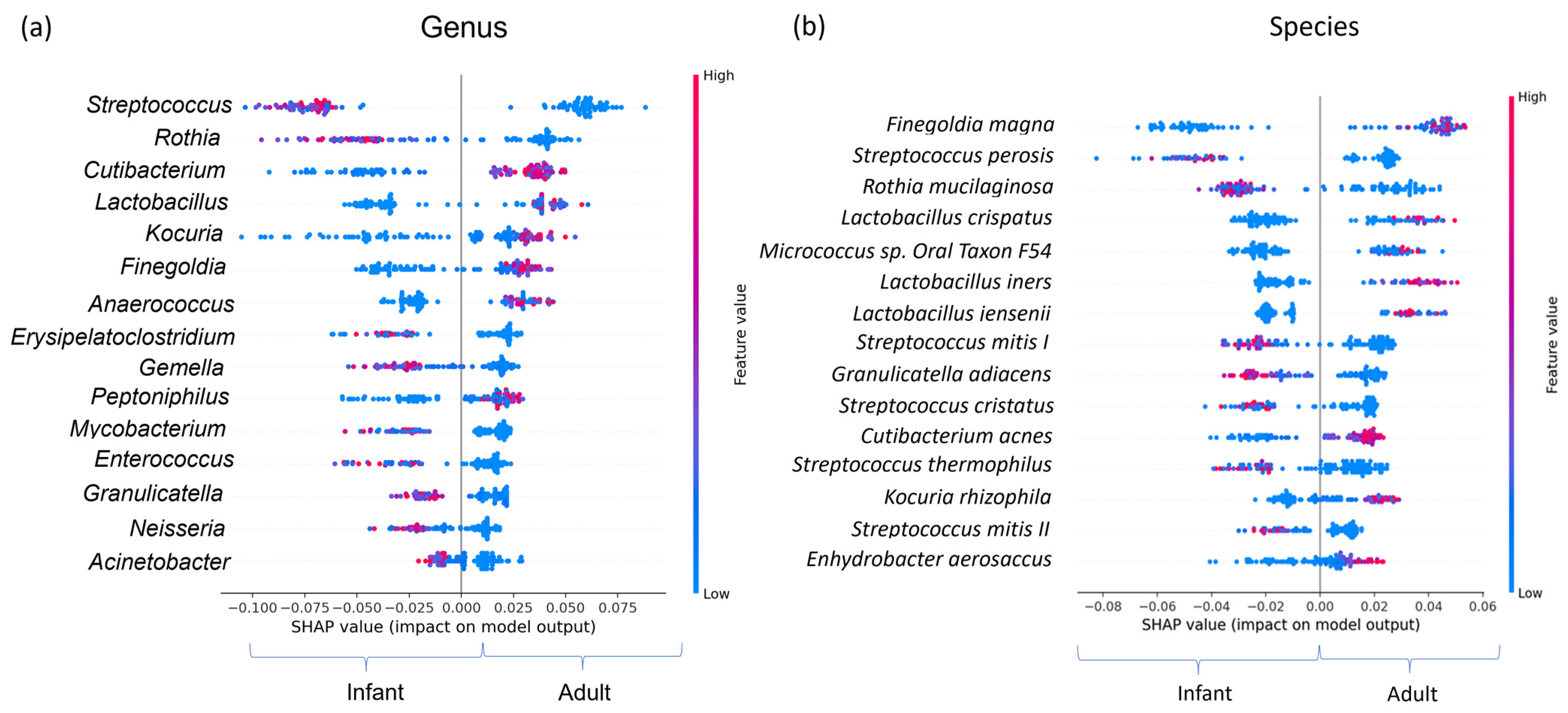
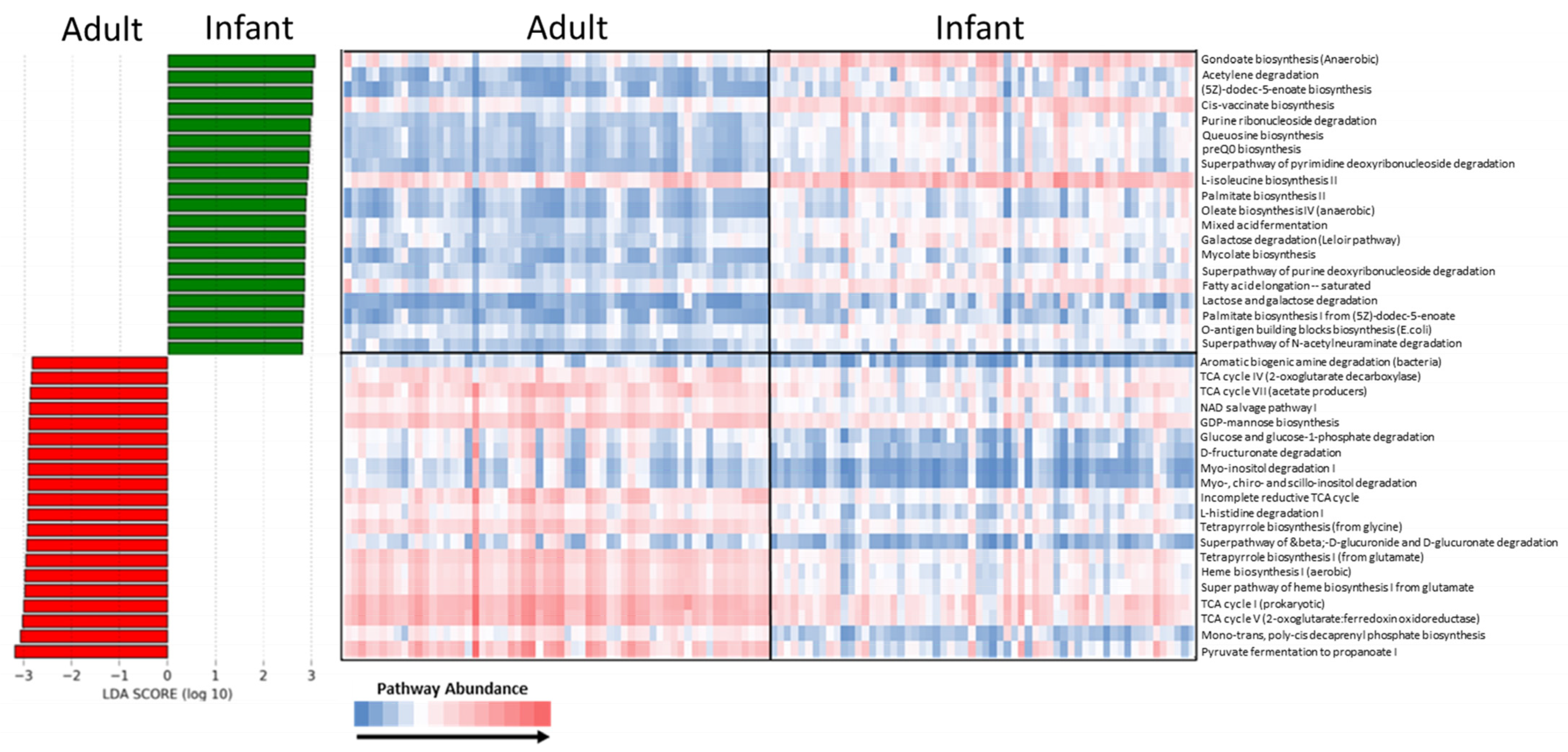
3.4. Functional Analysis
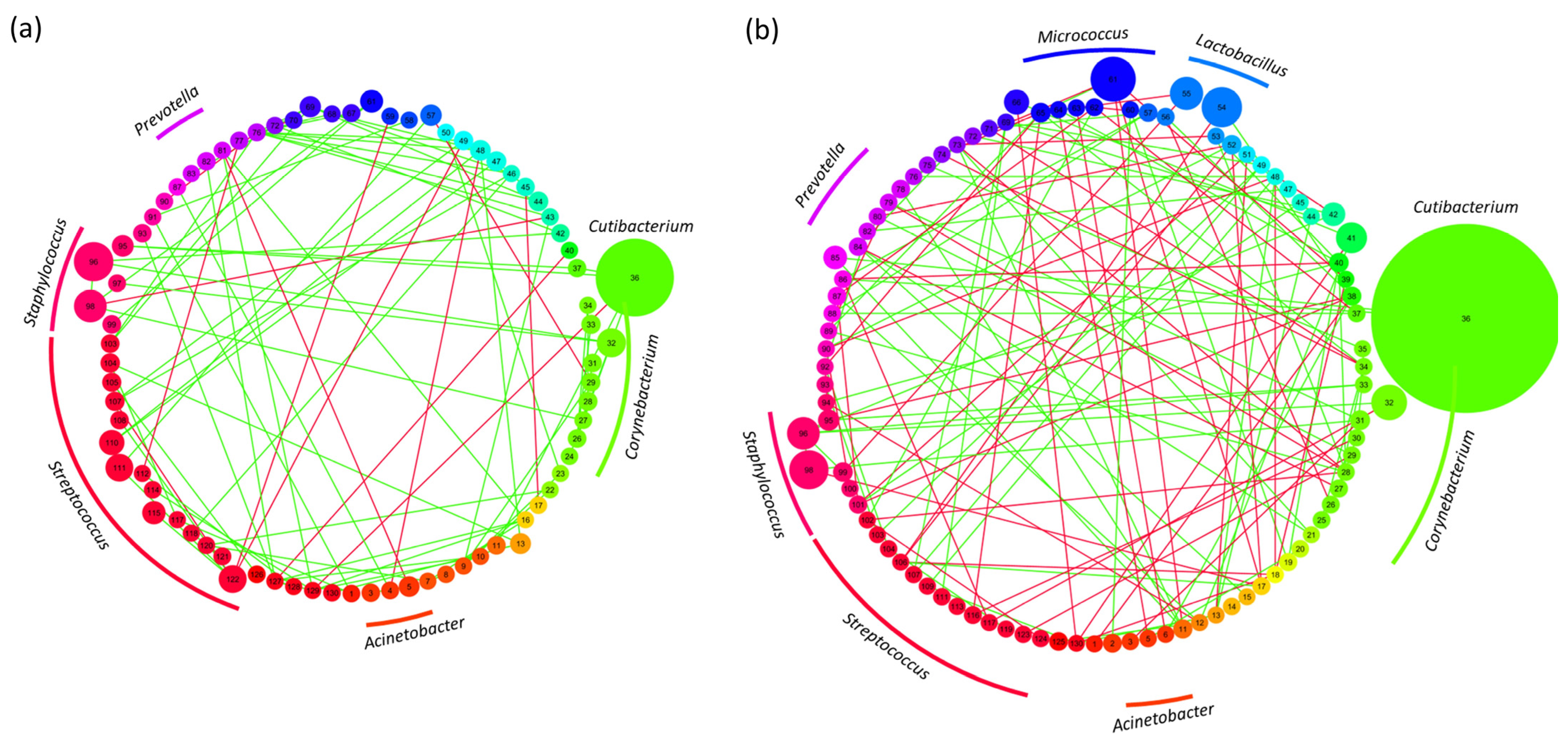
3.5. Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stamatas, G.N.; Nikolovski, J.; Luedtke, M.A.; Kollias, N.; Wiegand, B.C. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr. Dermatol. 2010, 27, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cunico, R.L.; Maibach, H.I.; Khan, H.; Bloom, E. Skin Barrier Properties in the Newborn. Neonatology 1977, 32, 177–182. [Google Scholar] [CrossRef]
- Raone, B.; Raboni, R.; Rizzo, N.; Simonazzi, G.; Patrizi, A. Transepidermal water loss in newborns within the first 24 hours of life: Baseline values and comparison with adults. Pediatr. Dermatol. 2014, 31, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Agache, P.; Blanc, D.; Barrand, C.; Laurent, R. Sebum levels during the first year of life. Br. J. Dermatol. 1980, 103, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Oranges, T.; Dini, V.; Romanelli, M. Skin Physiology of the Neonate and Infant: Clinical Implications. Adv. Wound Care 2015, 4, 587–595. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Maayan-Metzger, A.; Merlob, P.; Sirota, L. Skin barrier properties in different body areas in neonates. Pediatrics 2000, 106, 105–108. [Google Scholar] [CrossRef]
- Maayan-Metzger, A.; Yosipovitch, G.; Hadad, E.; Sirota, L. Transepidermal water loss and skin hydration in preterm infants during phototherapy. Am. J. Perinatol. 2001, 18, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Ski. Pharm. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Lodén, M.; Maibach, H.I. Treatment of Dry Skin Syndrome: The Art and Science of Moisturizers; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, P.H.; Enzmann, C.C. Skin Physiology of the Neonate and Young Infant: A Prospective Study of Functional Skin Parameters During Early Infancy. Pediatr. Dermatol. 2002, 19, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm.-Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef]
- Samaras, S.; Hoptroff, M. Book Chapter—The Microbiome of Healthy Skin in Skin Microbiome Handbook: From Basic Research to Product Development; Dayan, N., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.; Belkaid, Y. Microbial guardians of skin health. Science 2019, 363, 227–228. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Gallo, R.L. The role of the skin microbiome in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 263–269. [Google Scholar] [CrossRef]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Murray, P.R.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef]
- Grimshaw, S.G.; Smith, A.M.; Arnold, D.S.; Xu, E.; Hoptroff, M.; Murphy, B. The diversity and abundance of fungi and bacteria on the healthy and dandruff affected human scalp. PLoS ONE 2019, 14, e0225796. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Yuan, C.; Liu, X.; Yang, F.; Wang, T.; Wang, J.; Manabe, K.; Qin, O.; Wang, X.; et al. Dandruff is associated with the conjoined interactions between host and microorganisms. Sci. Rep. 2016, 6, 24877. [Google Scholar] [CrossRef]
- James, A.G. Book Chapter—The Axillary Microbiome and Its Relationship with Underarm Odor in Skin Microbiome Handbook: From Basic Research to Product Development; Dayan, N., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]
- Bawdon, D.; Cox, D.S.; Ashford, D.; James, A.G.; Thomas, G.H. Identification of axillary Staphylococcus sp. involved in the production of the malodorous thioalcohol 3-methyl-3-sufanylhexan-1-ol. FEMS Microbiol. Lett. 2015, 362, fnv111. [Google Scholar] [CrossRef]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Barnabas, B.; Blakesley, R.; Bouffard, G.; Brooks, S.; Coleman, H.; Dekhtyar, M.; et al. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Gołębiewski, M.; Łoś-Rycharska, E.; Sikora, M.; Grzybowski, T.; Gorzkiewicz, M.; Krogulska, A. Mother’s Milk Microbiome Shaping Fecal and Skin Microbiota in Infants with Food Allergy and Atopic Dermatitis: A Pilot Analysis. Nutrients 2021, 13, 3600. [Google Scholar] [CrossRef]
- Capone, K.A.; Dowd, S.E.; Stamatas, G.N.; Nikolovski, J. Diversity of the human skin microbiome early in life. J. Investig. Derm. 2011, 131, 2026–2032. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, X.; Kong, F.Q.; Duan, Y.Y.; Yee, A.L.; Kim, M.; Galzote, C.; Gilbert, J.A.; Quan, Z.X. Age and Mothers: Potent Influences of Children’s Skin Microbiota. J. Investig. Derm. 2019, 139, 2497–2505.e2496. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef]
- Manus, M.B.; Kuthyar, S.; Perroni-Marañón, A.G.; Mora, A.N.-d.l.; Amato, K.R. Infant Skin Bacterial Communities Vary by Skin Site and Infant Age across Populations in Mexico and the United States. mSystems 2020, 5, e00834-20. [Google Scholar] [CrossRef]
- Telofski, L.; Capone, K.A.; Friscia, D.; Nikolovski, J. Effects of Emollient Use on the Developing Infant Skin Microbiome. Pediatrics 2020, 146, 128. [Google Scholar] [CrossRef]
- Park, J.; Schwardt, N.H.; Jo, J.H.; Zhang, Z.; Pillai, V.; Phang, S.; Brady, S.M.; Portillo, J.A.; MacGibeny, M.A.; Liang, H.; et al. Shifts in the Skin Bacterial and Fungal Communities of Healthy Children Transitioning through Puberty. J. Investig. Derm. 2022, 142, 212–219. [Google Scholar] [CrossRef]
- Luna, P.C. Skin Microbiome as Years Go By. Am. J. Clin. Dermatol. 2020, 21, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Mourelatos, K.; Eady, E.A.; Cunliffe, W.J.; Clark, S.M.; Cove, J.H. Temporal changes in sebum excretion and propionibacterial colonization in preadolescent children with and without acne. Br. J. Dermatol. 2007, 156, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ro, B.I.; Dawson, T.L. The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. J. Investig. Dermatol. Symp. Proc. 2005, 10, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.; Bascom, C.C.; Hu, P.; Binder, R.L.; Fadayel, G.; Huggins, T.G.; Jarrold, B.B.; Osborne, R.; Rocchetta, H.L.; Swift, D.; et al. Aging-Associated Changes in the Adult Human Skin Microbiome and the Host Factors that Affect Skin Microbiome Composition. J. Investig. Dermatol. 2021, 142, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Shibagaki, N.; Suda, W.; Clavaud, C.; Bastien, P.; Takayasu, L.; Iioka, E.; Kurokawa, R.; Yamashita, N.; Hattori, Y.; Shindo, C.; et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci. Rep. 2017, 7, 10567. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, J.J.; Myeong, N.R.; Kim, T.; Kim, D.; An, S.; Kim, H.; Park, T.; Jang, S.I.; Yeon, J.H.; et al. Segregation of age-related skin microbiome characteristics by functionality. Sci. Rep. 2019, 9, 16748. [Google Scholar] [CrossRef]
- Williamson, P.; Kligman, A.M. A new method for the quantitative investigation of cutaneous bacteria. J. Investig. Derm. 1965, 45, 498–503. [Google Scholar] [CrossRef]
- Murphy, B.; Hoptroff, M.; Arnold, D.; Eccles, R.; Campbell-Lee, S. In-vivo impact of common cosmetic preservative systems in full formulation on the skin microbiome. PLoS ONE 2021, 16, e0254172. [Google Scholar] [CrossRef]
- Adams, S.E.; Arnold, D.; Murphy, B.; Carroll, P.; Green, A.K.; Smith, A.M.; Marsh, P.D.; Chen, T.; Marriott, R.E.; Brading, M.G. A randomised clinical study to determine the effect of a toothpaste containing enzymes and proteins on plaque oral microbiome ecology. Sci. Rep. 2017, 7, 43344. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Prepr. 2018, 6, e27295v27292. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Al-Hebshi, N.N.; Nasher, A.T.; Idris, A.M.; Chen, T. Robust species taxonomy assignment algorithm for 16S rRNA NGS reads: Application to oral carcinoma samples. J. Oral Microbiol. 2015, 7, 28934. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yu, W.-H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database J. Biol. Databases Curation 2010, 2010, baq013. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Barbera, P.; Kozlov, A.M.; Czech, L.; Morel, B.; Darriba, D.; Flouri, T.; Stamatakis, A. EPA-ng: Massively Parallel Evolutionary Placement of Genetic Sequences. Syst. Biol. 2018, 68, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Czech, L.; Barbera, P.; Stamatakis, A. Genesis and Gappa: Processing, analyzing and visualizing phylogenetic (placement) data. Bioinformatics 2020, 36, 3263–3265. [Google Scholar] [CrossRef]
- Louca, S.; Doebeli, M. Efficient comparative phylogenetics on large trees. Bioinformatics 2017, 34, 1053–1055. [Google Scholar] [CrossRef]
- Ye, Y.; Doak, T.G. A Parsimony Approach to Biological Pathway Reconstruction/Inference for Genomes and Metagenomes. PLoS Comput. Biol. 2009, 5, e1000465. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef]
- Kurtz, Z.D.; Müller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and Compositionally Robust Inference of Microbial Ecological Networks. PLOS Comput. Biol. 2015, 11, e1004226. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Carrieri, A.P.; Haiminen, N.; Maudsley-Barton, S.; Gardiner, L.J.; Murphy, B.; Mayes, A.E.; Paterson, S.; Grimshaw, S.; Winn, M.; Shand, C.; et al. Explainable AI reveals changes in skin microbiome composition linked to phenotypic differences. Sci. Rep. 2021, 11, 4565. [Google Scholar] [CrossRef]
- Tin Kam, H. Random decision forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995; Volume 271, pp. 278–282. [Google Scholar]
- Matchado, M.S.; Lauber, M.; Reitmeier, S.; Kacprowski, T.; Baumbach, J.; Haller, D.; List, M. Network analysis methods for studying microbial communities: A mini review. Comput. Struct. Biotechnol. J. 2021, 19, 2687–2698. [Google Scholar] [CrossRef]
- Murphy, B.; Grimshaw, S.; Hoptroff, M.; Paterson, S.; Arnold, D.; Cawley, A.; Adams, S.E.; Falciani, F.; Dadd, T.; Eccles, R.; et al. Alteration of barrier properties, stratum corneum ceramides and microbiome composition in response to lotion application on cosmetic dry skin. Sci. Rep. 2022, 12, 5223. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Y.-N.; Chu, C.-C.; Jing, Z.; Chen, Y.; Zhang, J.; Pu, M.; Mi, T.; Du, Y.; Liang, Z.; et al. Facial Skin Microbiota-Mediated Host Response to Pollution Stress Revealed by Microbiome Networks of Individual. mSystems 2021, 6, e00319–e00321. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Nikolovski, J.; Mack, M.C.; Kollias, N. Infant skin physiology and development during the first years of life: A review of recent findings based on in vivo studies. Int. J. Cosmet. Sci. 2011, 33, 17–24. [Google Scholar] [CrossRef]
- Barnard, E.; Shi, B.; Kang, D.; Craft, N.; Li, H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci. Rep. 2016, 6, 39491. [Google Scholar] [CrossRef]
- Kim, M.; Park, T.; Yun, J.I.; Lim, H.W.; Han, N.R.; Lee, S.T. Investigation of Age-Related Changes in the Skin Microbiota of Korean Women. Microorganisms 2020, 8, 1581. [Google Scholar] [CrossRef]
- Zeeuwen, P.L.J.M.; Ederveen, T.H.A.; van der Krieken, D.A.; Niehues, H.; Boekhorst, J.; Kezic, S.; Hanssen, D.A.T.; Otero, M.E.; van Vlijmen-Willems, I.M.J.J.; Rodijk-Olthuis, D.; et al. Gram-positive anaerobe cocci are underrepresented in the microbiome of filaggrin-deficient human skin. J. Allergy Clin. Immunol. 2017, 139, 1368–1371. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.; Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; O’Regan, G.M.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- McAleer, M.A.; Jakasa, I.; Raj, N.; O’Donnell, C.P.F.; Lane, M.E.; Rawlings, A.V.; Voegeli, R.; McLean, W.H.I.; Kezic, S.; Irvine, A.D. Early-life regional and temporal variation in filaggrin-derived natural moisturizing factor, filaggrin-processing enzyme activity, corneocyte phenotypes and plasmin activity: Implications for atopic dermatitis. Br. J. Dermatol. 2018, 179, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Kuo, S.; Wang, Y.; Jiang, Y.; Liu, Y.T.; Gallo, R.L.; Huang, C.M. Porphyrin metabolisms in human skin commensal Propionibacterium acnes bacteria: Potential application to monitor human radiation risk. Curr. Med. Chem. 2013, 20, 562–568. [Google Scholar] [CrossRef]
- Roux, P.F.; Oddos, T.; Stamatas, G. Deciphering the Role of Skin Surface Microbiome in Skin Health: An Integrative Multiomics Approach Reveals Three Distinct Metabolite–Microbe Clusters. J. Investig. Derm. 2022, 142, 469–479.e465. [Google Scholar] [CrossRef] [PubMed]
- Toit, M.d.; Huch, M.; Cho, G.-S.; Franz, C.M.A.P. The genus Streptococcus. In Lactic Acid Bacteria; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014; pp. 457–505. [Google Scholar]
- Rossi, F.; Marzotto, M.; Cremonese, S.; Rizzotti, L.; Torriani, S. Diversity of Streptococcus thermophilus in bacteriocin production; inhibitory spectrum and occurrence of thermophilin genes. Food Microbiol. 2013, 35, 27–33. [Google Scholar] [CrossRef]
- Di Marzio, L.; Cinque, B.; De Simone, C.; Cifone, M.G. Effect of the Lactic Acid BacteriumStreptococcus thermophilus on Ceramide Levels in Human KeratinocytesIn Vitro and Stratum Corneum In Vivo. J. Investig. Dermatol. 1999, 113, 98–106. [Google Scholar] [CrossRef]
- Dimarzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.G.; Giuliani, M. Increase of Skin-Ceramide Levels in Aged Subjects following a Short-Term Topical Application of Bacterial Sphingomyelinase from Streptococcus Thermophilus. Int. J. Immunopathol. Pharmacol. 2008, 21, 137–143. [Google Scholar] [CrossRef]
- Izawa, N.; Hanamizu, T.; Iizuka, R.; Sone, T.; Mizukoshi, H.; Kimura, K.; Chiba, K. Streptococcus thermophilus produces exopolysaccharides including hyaluronic acid. J. Biosci. Bioeng. 2009, 107, 119–123. [Google Scholar] [CrossRef]
- Zhu, T.; Duan, Y.-Y.; Kong, F.-Q.; Galzote, C.; Quan, Z.-X. Dynamics of Skin Mycobiome in Infants. Front. Microbiol. 2020, 11, 1790. [Google Scholar] [CrossRef]
- Ward, T.L.; Knights, D.; Gale, C.A. Infant fungal communities: Current knowledge and research opportunities. BMC Med. 2017, 15, 30. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, B.; Hoptroff, M.; Arnold, D.; Cawley, A.; Smith, E.; Adams, S.E.; Mitchell, A.; Horsburgh, M.J.; Hunt, J.; Dasgupta, B.; et al. Compositional Variations between Adult and Infant Skin Microbiome: An Update. Microorganisms 2023, 11, 1484. https://doi.org/10.3390/microorganisms11061484
Murphy B, Hoptroff M, Arnold D, Cawley A, Smith E, Adams SE, Mitchell A, Horsburgh MJ, Hunt J, Dasgupta B, et al. Compositional Variations between Adult and Infant Skin Microbiome: An Update. Microorganisms. 2023; 11(6):1484. https://doi.org/10.3390/microorganisms11061484
Chicago/Turabian StyleMurphy, Barry, Michael Hoptroff, David Arnold, Andrew Cawley, Emily Smith, Suzanne E. Adams, Alex Mitchell, Malcolm J. Horsburgh, Joanne Hunt, Bivash Dasgupta, and et al. 2023. "Compositional Variations between Adult and Infant Skin Microbiome: An Update" Microorganisms 11, no. 6: 1484. https://doi.org/10.3390/microorganisms11061484
APA StyleMurphy, B., Hoptroff, M., Arnold, D., Cawley, A., Smith, E., Adams, S. E., Mitchell, A., Horsburgh, M. J., Hunt, J., Dasgupta, B., Ghatlia, N., Samaras, S., MacGuire-Flanagan, A., & Sharma, K. (2023). Compositional Variations between Adult and Infant Skin Microbiome: An Update. Microorganisms, 11(6), 1484. https://doi.org/10.3390/microorganisms11061484


_Di_Marco.png)


