Age-Related Subgingival Colonization of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Parvimonas micra—A Pragmatic Microbiological Retrospective Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Collections
2.2. Cultivation and Quantification of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Parvimonas micra
2.3. Comparison of Proportions and Concentrations of A. actinomycetemcomitans in Periodontal Pockets Samples
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of Virulence Factors for the Persistence of Oral Bacteria in the Inflamed Gingival Crevice and in the Pathogenesis of Periodontal Disease. J. Clin. Med. 2019, 8, 1339. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Belstrøm, D.; Eick, S.; Gursoy, U.K.; Johansson, A.; Könönen, E. Periodontal microbiology and microbial etiology of periodontal diseases: Historical concepts and contemporary perspectives. Periodontology 2000, 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Caroline daSilva, F.; Piazzetta, C.M.; Torres-Pereira, C.C.; Schussel, J.L.; Amenábar, J.M. Gingival proliferative lesions in children and adolescents in Brazil: A 15-year-period cross-sectional study. J. Indian Soc. Periodontol. 2016, 20, 63–66. [Google Scholar]
- van der Velden, U.; Amaliya, A.; Loos, B.G.; Timmerman, M.F.; van der Weijden, F.A.; Winkel, E.G.; Abbas, F. Java project on periodontal diseases: Causes of tooth loss in a cohort of untreated individuals. J. Clin. Periodontol. 2015, 42, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Claesson, R.; Johansson, A.; Belibasakis, G.N. Clinical laboratory diagnostics in dentistry: Application of microbiological methods. Front. Oral Health 2022, 3, 983991. [Google Scholar] [CrossRef]
- Bostanci, N.; Bao, K.; Greenwood, D.; Silbereisen, A.; Belibasakis, G.N. Periodontal disease: From the lenses of light microscopy to the specs of proteomics and next-generation sequencing. Adv. Clin. Chem. 2019, 93, 263–290. [Google Scholar]
- Hbibi, A.; Bouziane, A.; Lyoussi, B.; Zouhdi, M.; Benazza, D. Aggregatibacter actinomycetemcomitans: From Basic to Advanced Research. Adv. Exp. Med. Biol. 2022, 1373, 45–67. [Google Scholar]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef]
- Dahlén, G. Role of suspected periodontopathogens in microbiological monitoring of periodontitis. Adv. Dent. Res. 1993, 7, 163–174. [Google Scholar] [CrossRef]
- Öztürk, V.Ö.; Belibasakis, G.N.; Emingil, G.; Bostanci, N. Impact of aging on TREM-1 responses in the periodontium: A cross-sectional study in an elderly population. BMC Infect. Dis. 2016, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 2011, 3, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Haubek, D.; Ennibi, O.K.; Poulsen, K.; Væth, M.; Poulsen, S.; Kilian, M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Höglund Åberg, C.; Kwamin, F.; Claesson, R.; Dahlén, G.; Johansson, A.; Haubek, D. Progression of attachment loss is strongly associated with presence of the JP2 genotype of Aggregatibacter actinomycetemcomitans: A prospective cohort study of a young adolescent population. J. Clin. Periodontol. 2014, 41, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Claesson, R.; Höglund Åberg, C.; Haubek, D.; Lindholm, M.; Jasim, S.; Oscarsson, J. Genetic Profiling of Aggregatibacter actinomycetemcomitans Serotype B Isolated from Periodontitis Patients Living in Sweden. Pathogens 2019, 8, 153. [Google Scholar] [CrossRef]
- Hočevar, K.; Potempa, J.; Turk, B. Host cell-surface proteins as substrates of gingipains, the main proteases of Porphyromonas gingivalis. Biol. Chem. 2018, 399, 1353–1361. [Google Scholar] [CrossRef]
- Neilands, J.; Davies, J.R.; Bikker, F.J.; Svensäter, G. Parvimonas micra stimulates expression of gingipains from Porphyromonas gingivalis in multi-species communities. Anaerobe 2019, 55, 54–60. [Google Scholar] [CrossRef]
- Persson, S.; Claesson, R.; Carlsson, J. The capacity of subgingival microbiotas to produce volatile sulfur compounds in human serum. Oral Microbiol. Immunol. 1989, 4, 169–172. [Google Scholar] [CrossRef]
- Proctor, G.B.; Shaalan, A.M. Disease-Induced Changes in Salivary Gland Function and the Composition of Saliva. J. Dent. Res. 2021, 100, 1201–1209. [Google Scholar] [CrossRef]
- Feres, M.; Teles, F.; Teles, R.; Figueiredo, L.C.; Faveri, M. The subgingival periodontal microbiota of the aging mouth. Periodontol. 2000 2016, 72, 30–53. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N. Microbiological changes of the ageing oral cavity. Arch. Oral Biol. 2018, 96, 230–232. [Google Scholar] [CrossRef]
- Loesche, W.J.; Grossman, N.S. Periodontal disease as a specific, albeit chronic, infection: Diagnosis and treatment. Clin. Microbiol. Rev. 2001, 14, 727–752. [Google Scholar] [CrossRef] [PubMed]
- Claesson, R.; Höglund-Åberg, C.; Haubek, D.; Johansson, A. Age-related prevalence and characteristics of Aggregatibacter actinomycetemcomitans in periodontitis patients living in Sweden. J. Oral Microbiol. 2017, 9, 1334504. [Google Scholar] [CrossRef]
- Kaman, W.E.; Galassi, F.; de Soet, J.J.; Bizzarro, S.; Loos, B.G.; Veerman, E.C.; van Belkum, A.; Hays, J.P.; Bikker, F.J. Highly specific protease-based approach for detection of porphyromonas gingivalis in diagnosis of periodontitis. J. Clin. Microbiol. 2012, 50, 104–112. [Google Scholar] [CrossRef]
- Nørskov-Lauritsen, N.; Claesson, R.; Jensen, A.B.; Åberg, C.H.; Haubek, D. Aggregatibacter Actinomycetemcomitans: Clinical Significance of a Pathobiont Subjected to Ample Changes in Classification and Nomenclature. Pathogens 2019, 8, 243. [Google Scholar] [CrossRef]
- Savitt, E.D.; Kent, R.L. Distribution of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis by subject age. J. Periodontol. 1991, 62, 490–494. [Google Scholar] [CrossRef]
- Rodenburg, J.P.; Van Winkelhoff, A.J.; Winkel, E.G.; Goene, R.J.; Abbas, F.; De Graaff, J. Occurrence of Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in severe periodontitis in relation to age and treatment history. J. Clin. Periodontol. 1990, 17, 392–399. [Google Scholar] [CrossRef]
- Jepsen, K.; Falk, W.; Brune, F.; Fimmers, R.; Jepsen, S.; Bekeredjian-Ding, I. Prevalence and antibiotic susceptibility trends of periodontal pathogens in the subgingival microbiota of German periodontitis patients: A retrospective surveillance study. J. Clin. Periodontol. 2021, 48, 1216–1227. [Google Scholar] [CrossRef]
- Razooqi, Z.; Höglund Åberg, C.; Kwamin, F.; Claesson, R.; Haubek, D.; Oscarsson, J.; Johansson, A. Aggregatibacter actinomycetemcomitans and Filifactor alocis as Associated with Periodontal Attachment Loss in a Cohort of Ghanaian Adolescents. Microorganisms 2022, 10, 2511. [Google Scholar] [CrossRef] [PubMed]
- Ennibi, O.K.; Claesson, R.; Akkaoui, S.; Reddahi, S.; Kwamin, F.; Haubek, D.; Johansson, A. High salivary levels of JP2 genotype of Aggregatibacter actinomycetemcomitans is associated with clinical attachment loss in Moroccan adolescents. Clin. Exp. Dent. Res. 2019, 5, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Alazemi, A.M.; Jamal, W.; Al Khabbaz, A.; Rotimi, V.O. Prevalence of target anaerobes associated with chronic periodontitis. Access Microbiol. 2020, 2, acmi000177. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, J.R.; Kiyak, H.A.; Darveau, R.; Persson, G.R. Correlates of periodontal decline and biologic markers in older adults. J. Periodontol. 2008, 79, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef] [PubMed]
- López, R.; Dahlén, G.; Retamales, C.; Baelum, V. Clustering of subgingival microbial species in adolescents with periodontitis. Eur. J. Oral. Sci. 2011, 119, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.W.; Barlow, R.M.; Pow, I. Isolation of louping-ill virus from red deer (Cervus elaphus). Vet. Rec. 1978, 102, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Bostanci, N.; Thurnheer, T.; Grossmann, J.; Wolski, W.E.; Thay, B.; Belibasakis, G.N.; Oscarsson, J. Aggregatibacter actinomycetemcomitans H-NS promotes biofilm formation and alters protein dynamics of other species within a polymicrobial oral biofilm. NPJ Biofilms Microbiomes 2018, 4, 12. [Google Scholar] [CrossRef]
- Bao, K.; Bostanci, N.; Selevsek, N.; Thurnheer, T.; Belibasakis, G.N. Quantitative proteomics reveal distinct protein regulations caused by Aggregatibacter actinomycetemcomitans within subgingival biofilms. PLoS ONE 2015, 10, e0119222. [Google Scholar] [CrossRef]
- Větrovský, T.; Baldrian, P. The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PLoS ONE 2013, 8, e57923. [Google Scholar] [CrossRef]
- Thorbert-Mros, S.; Ali, N.; Ali, M.; Ayas, M.; Trullenque-Eriksson, A.; Dahlén, G. A comparative study on periodontitis and periodontitis-associated bacteria in Somali and non-Somali children and adolescents living in Trollhättan, Sweden. Eur. J. Oral. Sci. 2022, 130, e12843. [Google Scholar] [CrossRef]
- Johansson, A.; Claesson, R.; Höglund Åberg, C.; Haubek, D.; Oscarsson, J. The cagE gene sequence as a diagnostic marker to identify JP2 and non-JP2 highly leukotoxic Aggregatibacter actinomycetemcomitans serotype b strains. J. Periodontal. Res. 2017, 52, 903–912. [Google Scholar] [CrossRef]
- Haubek, D.; Ennibi, O.K.; Poulsen, K.; Poulsen, S.; Benzarti, N.; Kilian, M. Early-onset periodontitis in Morocco is associated with the highly leukotoxic clone of Actinobacillus actinomycetemcomitans. J. Dent. Res. 2001, 80, 1580–1583. [Google Scholar] [CrossRef] [PubMed]

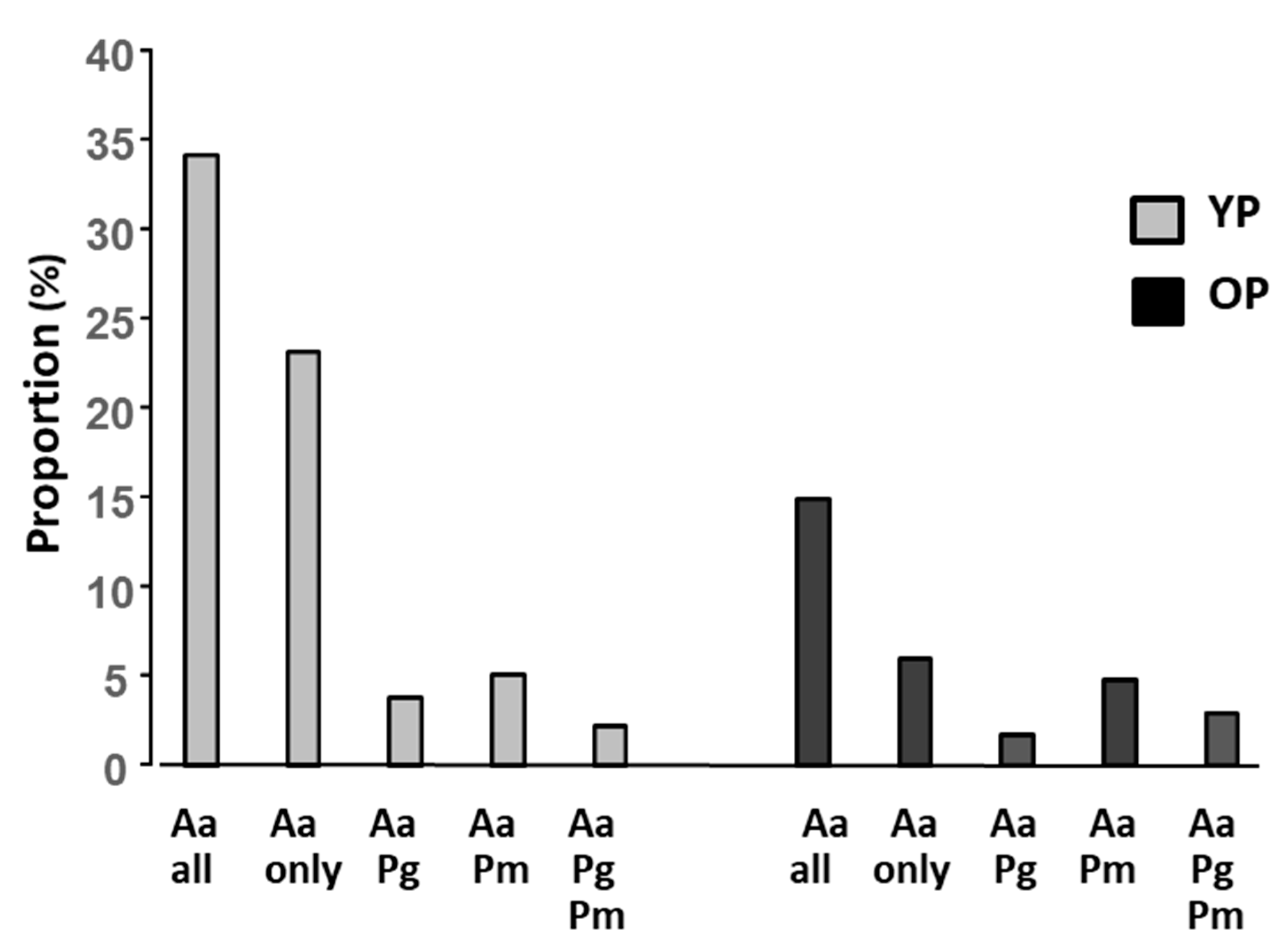
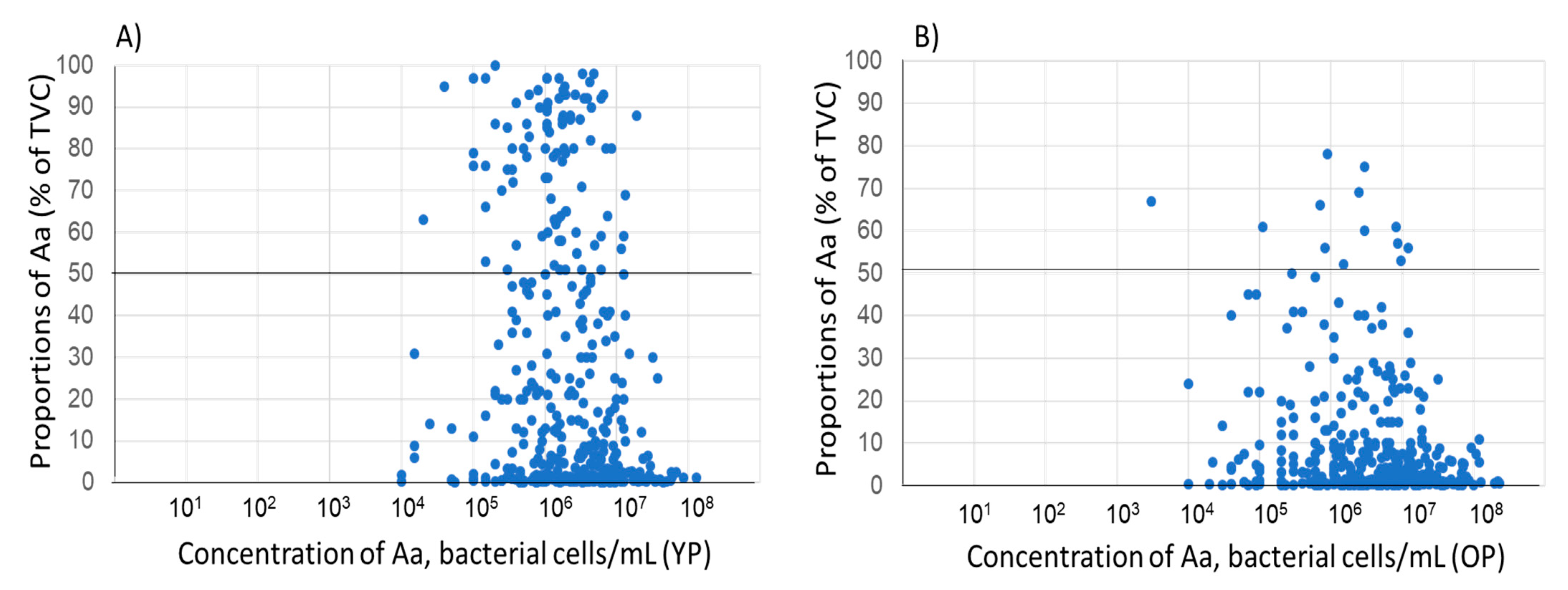
| (A) | |||||
|---|---|---|---|---|---|
| Age | n | Aa, n (%) | Pg, n (%) | Pm, n (%) | negative |
| YP | 424 | 179 (42.2) | 99 (23.3) | 156 (36.8) | 122 (28.8) |
| OP | 1081 | 221 (20.4) | 307 (28.4) | 582 (53.8) | 286 (26.4) |
| All patients | 1505 | 400 (26.6) | 406 (27.0) | 738 (49.0) | 408 (27.1) |
| (B) | |||||
| Age | n | Aa, n (%) | Pg, n (%) | Pm, n (%) | negative |
| YP | 1087 | 371 (34.1) | 203 (18.7) | 295 (27.1) | 426 (39.2) |
| OP | 2360 | 352 (14.9) | 539 (22.8) | 1095 (46.4) | 907 (38.4) |
| All patients | 3447 | 723 (21.0) | 742 (22.1) | 1390 (40.3) | 1333 (38.7) |
| ≥0.1–1% | >1–5% | >5–25% | >25–50% | >50% | ||
|---|---|---|---|---|---|---|
| Aa | ||||||
| YP | 78 (21.0) | 66 (17.8) | 87 (23.5) | 45 (12.1) | 95 (25.6) | 371 |
| OP | 116 (33.0) | 84 (23.9) | 112 (31.8) | 27 (7.6) | 13 (3.7) | 352 |
| YP + OP | 194 (26.9) | 150 (20.7) | 199 (27.5) | 72 (10.0) | 108 (14.9) | 723 |
| Pg | ||||||
| YP | 9 (4.4) | 26 (12,8) | 49 (24.1) | 44 (21.7) | 75 (36.9) | 203 |
| OP | 16 (3.03) | 59 (10.9) | 142 (26.3) | 116 (21.5) | 206 (38.2) | 539 |
| YP + OP | 25 (3.4) | 85 (11.4) | 191 (25.7) | 160 (21.6) | 281 (37.9) | 742 |
| Pm | ||||||
| YP | 37 (12.5) | 102 (34.6) | 106 (35.9) | 37(12.5) | 13 (4.4) | 295 |
| OP | 91 (8.3) | 297 (27.1) | 495 (45.2) | 150 (13.7) | 62 (5.7) | 1095 |
| YP + OP | 128 (9.2) | 399 (28.7) | 601 (43.2) | 187 (13.5) | 75 (5.4) | 1390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claesson, R.; Johansson, A.; Belibasakis, G.N. Age-Related Subgingival Colonization of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Parvimonas micra—A Pragmatic Microbiological Retrospective Report. Microorganisms 2023, 11, 1434. https://doi.org/10.3390/microorganisms11061434
Claesson R, Johansson A, Belibasakis GN. Age-Related Subgingival Colonization of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Parvimonas micra—A Pragmatic Microbiological Retrospective Report. Microorganisms. 2023; 11(6):1434. https://doi.org/10.3390/microorganisms11061434
Chicago/Turabian StyleClaesson, Rolf, Anders Johansson, and Georgios N. Belibasakis. 2023. "Age-Related Subgingival Colonization of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Parvimonas micra—A Pragmatic Microbiological Retrospective Report" Microorganisms 11, no. 6: 1434. https://doi.org/10.3390/microorganisms11061434
APA StyleClaesson, R., Johansson, A., & Belibasakis, G. N. (2023). Age-Related Subgingival Colonization of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Parvimonas micra—A Pragmatic Microbiological Retrospective Report. Microorganisms, 11(6), 1434. https://doi.org/10.3390/microorganisms11061434








