Abstract
Background: Mounting evidence suggests a pivotal role for the gut microbiome in energy disequilibrium characteristic of obesity. The clinical utility of microbial profiling for the distinction between metabolically healthy obesity (MHO) and metabolically unhealthy obesity (MUO) remains ill-defined. We aim to probe microbial composition and diversity in young adult Saudi females with MHO and MUO. This observational study included anthropometric and biochemical measurements and shotgun sequencing of stool DNA for 92 subjects. α- and β-diversity metrics were calculated to determine the richness and variability in microbial communities, respectively. Results showed that Bacteroides and Bifidobacterium merycicum were less abundant in MUO compared to healthy and MHO groups. BMI was negatively correlated with B. adolescentis, B. longum, and Actinobacteria in MHO, while being positively correlated with Bacteroides thetaiotaomicron in both MHO and MUO. Positive correlations between waist circumference and B. merycicum and B. thetaiotaomicron were observed in MHO and MUO, respectively. Compared to MHO and MUO groups, higher α-diversity was detected in healthy individuals who also had higher β-diversity compared to those with MHO. We conclude that modulation of the gut microbiome cohorts through prebiotics, probiotics, and fecal microbiota transplantation may be a promising preventive and therapeutic approach to obesity-associated disease.
1. Introduction
Obesity is defined as the presence of high body fat that may be related to adverse health conditions. Body mass index (BMI), which is determined by a simple calculation of weight and height (weight (kg)/height (m2)), is used to define overweight (≥25) and obesity (≥30) [1].
Obesity has been shown to increase the risk of type 2 diabetes, dyslipidemia, hypertension, cardiovascular diseases, and cancers, with these factors often occurring in tandem [2]. However, at an individual level, the limitations inherent in using BMI as a tool, such as an inability to distinguish between fat and lean tissue and between fat storage sites, means it is not a diagnostic tool for assessing health status [3]. In fact, obesity can and does exist in metabolically healthy individuals, called metabolically healthy obesity (MHO) [4,5]. MHO can be defined as obesity without metabolic abnormalities [4]. People with MHO tend to have less systemic inflammation despite having a similar fat mass, and some studies have shown a decreased risk of mortality and disease among people with MHO compared to those with metabolically unhealthy obesity (MUO) [5,6].
The possible role of gut microbiota in determining why some people with obesity are metabolically healthy and others are not is an emergent area of study [7]. The gut microbiota, the collective term for the microbial community inhabiting the gastrointestinal tract, has been recognized for its role in maintaining metabolic health [8,9,10]. It performs crucial functions in the body, including the maintenance of metabolic homeostasis [8], the regulation of immunity [11], the strengthening of the intestinal epithelium [12], and protection against pathogens [13]. Maintaining homeostasis in the gut microbiota is critical, as altered bacterial composition, or dysbiosis, is an acknowledged factor in developing infections and inflammatory diseases [14], insulin resistance [15,16], and obesity [17,18]. While certain groups of microorganisms dominate the gut, amounts and species vary greatly [19]. Identifying microbial composition can help in efforts to modulate the gut microbiota to manage and prevent adverse health outcomes.
In 2020, Kim et al. carried out one of the first studies that investigated the differences in gut microbiota between metabolically healthy and unhealthy adults with overweight and obesity and found significant differences in bacterial composition and alpha diversity [7]. While these findings may help develop strategies to prevent the progression of metabolic abnormalities among those with obesity, the generalizability may be limited [7]. Differences in gut microbiota composition can be ascribed to the heterogeneity of groups [20], lifestyle habits [21], cultural and dietary traditions [22], genetic factors [20], or study techniques [21,23]. Extrapolating the results from one cohort or to generalized biological relevance may be inappropriate [20]. Thus, it is necessary to perform population and geography-specific studies to enhance our understanding of the relationship between gut microbiota and health [20,24]. This study aims to explore whether there are differences in gut microbiota in young adult females with MHO and MUO in Saudi Arabia.
2. Materials and Methods
2.1. Study Design
The present study is an analytical, case-control investigation that builds upon previously published methods [25]. Female college students over 18 years of age were recruited between January 2019 and March 2020. We used various recruitment methods such as emails, social media networks, etc. Those included were females with a BMI of ≥30 kg/m2 (obese) or BMI = 18.5–24.9 kg/m2 (normal weight).
Out of the initial 400 women who expressed interest, 290 met the eligibility criteria. Of those, we excluded 198 individuals for various reasons, e.g., those who had chronic illnesses such as acute/chronic diarrhea in the past two months, pregnant females, those on special diets (e.g., weight-reduction), those using multi-vitamins or antibiotics, or those who did not hand in stool samples. Ultimately, 92 females were part of the final sample, and they signed a written consent to participate in the study. The Institutional Ethics Committee at King Saud University (KSU-IRB #E-19-3625) reviewed and approved the study protocol.
2.2. Biochemical Data
Participants fasted for more than 10 h overnight before two 5-mL tubes of blood were collected at the study clinic. Serum was removed from the blood and centrifuged within minutes of collection. The serum was then sent to the study laboratory for analysis. A biochemical analyzer (Konelab, Espoo, Finland) measured fasting blood glucose (FBG) levels (mmol/L), and a LIAISON XL analyzer (DiaSorin, Saluggia, Italy) measured insulin levels (mU/L). The homeostasis model assessment of insulin resistance (HOMA-IR) index was determined using the following calculation: [fasting serum insulin × (fasting glucose)/22.5]. Lipid profiles were measured with a biochemical analyzer (Konelab, Espoo, Finland). Previously published equations were used to determine the levels of low- and high-density lipoprotein cholesterol (LDL-C and HDL-C, respectively) [26]. High-sensitivity C-reactive protein (hs-CRP) was assessed using commercial enzyme-linked immunosorbent assay kits.
2.3. Anthropometric Indices
Weight and height measurements to the nearest 0.1 kg and 0.1 cm, respectively, were taken twice, with participants wearing light clothing and no shoes. BMI was calculated as kilograms divided by the square of height in meters. Waist circumference was measured according to the World Health Organization standard at the lowest rib, umbilicus, and narrowest waist area [27]. Hip circumference was measured twice to the nearest 0.5 cm with an inelastic tape around the great trochanter with legs close together. A third measurement was taken if the first two differed by more than 2 cm, and then an average of the two most similar measurements was used. Body composition data were identified with bioelectrical impedance analysis (BIA) using an InBody 770 machine (Seoul, Republic of Korea) [28].
2.4. Obesity Phenotypes
Participants were categorized based on their BMI into the following groups: normal weight (BMI 18.5–24.9 kg/m2) and obesity (BMI ≥ 30 kg/m2). A waist circumference of ≥85 cm was classified as abdominal obesity [29]. Participants with HOMA-IR levels in the top quartile were considered insulin resistant [30].
MHO and MUO phenotypes were defined as per National Cholesterol Education Program-Adult Treatment Panel III (ATPIII) [31] and Karelis [32], based on obesity markers (BMI or waist circumference) and specific cardiometabolic abnormalities. Participants were classified as MHO if they presented with one of the obesity markers but did not have any metabolic abnormalities. Participants were categorized as MUO if they presented with obesity (either BMI- or waist circumference-defined) and three or more metabolic abnormalities [33], including: FBG, ≥5.6 mmol/L or on treatment, triglyceride ≥ 1.7 mmol/L or on treatment, HDL-C < 1.29 mmol/L, LDL-C ≤ 2.6 mmol/L, HOMA-IR level in the top quartile. Finally, participants were classified as ‘healthy’ if they had normal weight and none of the metabolic abnormalities.
2.5. Stool Collection Characterization of Gut Composition
Stool samples were gathered under sterile conditions using dry, screw-cap containers. The samples were then placed in a large container with dried ice and transported to the study lab. There, samples were promptly frozen at −80 °C until further processing. DNA extraction was executed using 0.25 g aliquots of frozen stool and the QIAamp Power Fecal DNA Isolation Kit (Qiagen, Hilden, Germany) in line with the manufacturer’s instructions. The DNA was eluted in a 100 μL C6 elution buffer. Its purity and concentration were assessed with a Nano Drop spectrophotometer (Nano Drop Technologies, Wilmington, DE, USA). The Illumina Nextera XT Library Preparation Kit (Illumina, Inc., San Diego, CA, USA) was used for DNA library preparation. Sequencing was conducted on an Illumina sequencer. For accurate quantification, a Qubit® fluorometer was utilized.
Gut microbiota composition was determined with genomic sequencing using the WGS analysis method [34]. This approach enabled the identification of major phyla in the gut. The CosmosID bioinformatics platform (CosmosID, Inc., Germantown, MD, USA) was utilized to analyze the sequencing data. With this method, we were able to profile antibiotic resistance and virulence genes and quantify the relative abundance of organisms in the multi-kingdom microbiome [35,36,37,38]. High-performance data mining and the curated genome databases enabled the rapid identification of distinct sequences that produced microbes. In addition, the CosmosID antibiotic resistance and virulence-associated gene databases were used to search the unassembled sequence reads for the identification of the community resistome.
2.6. Dietary Data
We collected information on food and drink intake using the Saudi Food and Drug Authority Food Frequency Questionnaire (FFQ) [39], consisting of 133 food and beverage items. Dietitians who were experts in the field asked participants about their habitual food and drink intake, while estimating portion sizes using food modules. For nutrition analysis, a food processor software, ESHA version 11.1 (ESHA Research, Salem, OR, USA), was used.
2.7. Statistical Analyses
IBM SPSS Statistics for Windows (version 24; IBM Corp, Armonk, NY, USA) was used to conduct statistical analyses. Normality tests for quantitative variables were conducted prior to analysis. Means with standard deviations were used for continuous variables and frequencies were used for categorical variables. Means and standard deviations of anthropometric indices, biochemical data, and gut flora according to obesity phenotype; healthy, MHO, MUO, were compared with one-way analysis of variance (ANOVA). The correlation between metabolic markers and gut flora for each obesity phenotype was assessed with Pearson’s correlation coefficient. Statistical significance was set at a p-value of ≤0.05.
The α-diversity, a measure of gut microbiota richness, was determined using the CosmosID taxonomic analysis (R software Vegan package, version 2.5-6). The gut composition at the species-level relative abundance matrices was determined using β-diversity. Wilcoxon Rank-Sum tests and the ggsignif package for R were used to calculate the statistical difference between phenotypes for α-diversity by Shannon index. The nonparametric PERMANOVA analysis was conducted using Vegan’s function adonis2 based on the Jaccard index for β-diversity analysis.
3. Results
3.1. Characteristics of Participants
The current study included 92 females with a (mean ± SD) age of (21.1 ± 1.5 years) Table S1. Anthropometric data were as follows; BMI (28.56 ± 8.0 kg/m2), waist-to-hip ratio (0.7 ± 0.1), fat percentage (42.5 ± 9.4%), and muscle percentage (28.2 ± 7.0%). Total cholesterol was (4.1 ± 1.5 mmol/L), HDL-C (1.0 ± 0.3 mmol/L), LDL-C (2.9 ± 1.3), triglycerides [0.7 (0.5–1.0) mmol/L], FBG (4.6 ± 0.7 mmol/L), insulin (9.9 ± 11.2 µIU/mL), HOMA-IR (2.0 ± 2.8) and hs-CRP (1.5 ± 1.0 mg/L).
3.2. Anthropometric Indices, Biochemical Data, and Gut Flora Stratified by Obesity Phenotype
When participants were divided according to obesity phenotype, markers of obesity such as BMI and waist were higher in MHO (n = 19) and MUO (n = 19) compared to healthy individuals (n = 48) (Table 1). Total cholesterol, LDL, triglycerides, fasting blood sugar, insulin, HOMA-IR, and hs-CRP were higher in MUO compared to those with normal weight (p < 0.01). Those with MUO had lower abundance of Bacteroides (unidentified species) (p < 0.06), Bacteroides uniformis and Bifidobacterium merycicum (p < 0.03) compared to healthy individuals and MHO. For dietary intake, total calorie intake was higher in MUO compared to MHO and healthy individuals, however the difference was not significant. Total fat intake (%) was higher in MUO than MHO and healthy individuals (p < 0.05) (Table 1).

Table 1.
Anthropometric indices, biochemical data, and gut flora by obesity phenotype, n = 92 1.
3.3. Correlations of Metabolic Markers with Gut Flora for Each Obesity Phenotype
Among healthy individuals, there was a positive correlation between BMI and Bifidobacterium kashiwanohense (r = 0.24) (Table 2). Flavonifractor plautii was positively correlated with two markers; HDL-C and (r = 0.49) and triglycerides (r = 0.39).

Table 2.
Correlation coefficients between metabolic markers and gut flora in healthy individuals, n = 48.
Bifidobacterium longum was inversely correlated with waist (r = −0.25) and hs-CRP (r = −0.24). Proteobacteria was also inversely correlated with several metabolic markers; triglycerides (r = −0.27), insulin (r = −0.30), and HOMA-IR (r = −0.30) (Table 2).
Table 3 presents the correlation between metabolic markers and gut flora in the MHO phenotype. Bacteria_u_p was inversely correlated with both BMI (r = −0.45) and insulin (r = −0.48). Similarly, Bifidobacterium adolescentis was inversely correlated with a number of metabolic markers; BMI (r = −0.54), HDL-C (r = −0.46), FBG (r = −0.55), insulin (r = −0.61), and HOMA-IR (r = −0.66). Bifidobacterium longum was also inversely correlated with BMI (r = −0.43), FBG (r = −0.54), and HOMA-IR (r = −0.42).

Table 3.
Correlation coefficients between metabolic markers and gut flora in individuals with metabolically healthy obesity, n = 21.
Bifidobacterium merycicum was positively correlated with waist (r = 0.47) and inversely with a number of metabolic markers including BMI (r = −0.26), triglycerides (r = −41), insulin (r = −0.46), and HOMA-IR (r = −0.43).
Actinobacteria was inversely correlated with several markers; BMI (r = −0.49), FBG (r = −0.51), insulin (r = −0.46), and HOMA-IR (r = −0.54).
Positive correlations were observed between Akkermansia muciniphila and both FBG (r = 0.45) and HOMA-IR (r = 0.51). Similarly, Verrucomicrobia showed positive correlations with insulin (r = 0.42) and HOMA-IR (0.52). Further, Bacteroides faecichinchillae was positively correlated with BMI and hs-CRP (r = 0.44, 0.46, respectively) (Table 3).
In the MUO phenotype, Clostridium difficile was positively correlated with several metabolic markers; BMI and waist (r = 0.42, 0.47, respectively) and inversely with HDL-C (r = −0.50). Bacteroides faecichinchillae was positively correlated a number of markers; BMI (r = 0.71), waist (r = 0.68), and HOMA-IR (r = 0.40).
HDL-C was positively correlated with Akkermansia muciniphila (r = 0.44), Verrucomicrobia (r = 0.44), Bacteroides faecichinchillae (r = 0.50) and inversely with Clostridium bolteae (r = −0.50) (Table 4).

Table 4.
Correlation coefficients between metabolic markers and gut flora in individuals with metabolically unhealthy obesity, n = 23.
3.4. α- and β-Diversity in Each Obesity Phenotype
Healthy individuals had higher microbial α-diversity based on the Shannon index compared to the MHO and MUO phenotypes, and the difference was marginally significant (p = 0.06) (Figure 1).
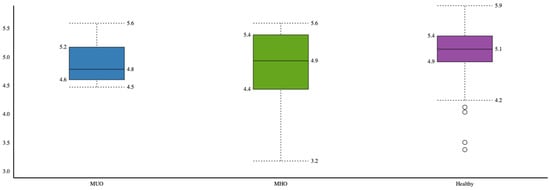
Figure 1.
Comparison of microbial α-diversity between obesity phenotypes (healthy, MHO and MUO).
The overall microbial β-diversity was significant between the three groups MHO, MUO and the healthy group (p = 0.01) (Figure 2). Among obesity phenotypes, healthy individuals had higher β-diversity compared to MHO (p = 0.005).
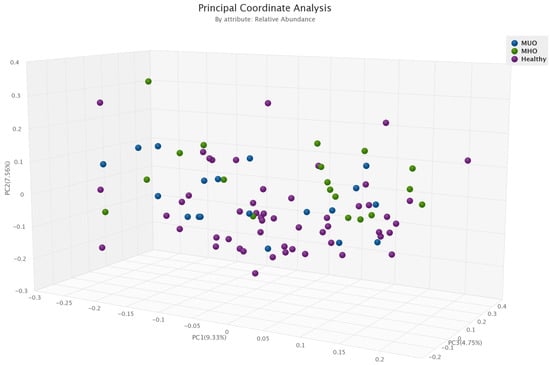
Figure 2.
Comparison of microbial β-diversity between obesity phenotypes (healthy, MHO and MUO).
4. Discussion
Outcomes from this study add to the mounting body of literature demonstrating differences in the gut microbiota between people with MUO and MHO. This work investigated the variations in gut microbiota among metabolically healthy and metabolically unhealthy young females with obesity in Saudi Arabia.
The main findings reveal that those with MUO have a lower abundance of Bacteroides, Bacteroides uniformis, and Bifidobacterium merycicum compared to both healthy individuals without obesity and compared to those with MHO. These bacteria have essential functions in metabolic health, which may explain these distinctions. Bacteroides is a genus of anaerobic, non-spore-forming, bile-resistant, gram-negative rods and includes over 30 species of bacteria [40]. Of all gram-negative bacteria, it is the most abundant one colonizing the human gastrointestinal tract [40]. Bacteroides has been demonstrated to enhance host immunity, conserve microecological balance in the gut, and speed up angiogenesis in the intestinal mucosa [40]. Moreover, some research has shown that more Bacteroides in the gastrointestinal tract could reduce obesity risk [41,42]. Bacteroides uniformis, a type of Bacteroides, exhibits significant glycolytic capability, and it can adapt to various gut environments [43]. Low levels of Bacteroides uniformis have been associated with obesity risk [44] and, by potentially limiting the biosynthesis of lipopolysaccharides, which promote pro-inflammatory cytokines, Bacteroides uniformis could help alleviate inflammation [43]. Bifidobacterium merycicum belongs to the Bifidobacterium genus, which has been positively associated with host health, including demonstrations of preventing and/or treating colorectal cancer [45], diarrhea [46], inflammatory bowel disease [47] and obesity [44,48,49].
We also found that the Shannon index, which indicated microbial alpha diversity (richness), was lower in the MUO group than in the MHO group. The microbial β-diversity significantly differed between all three groups (MUO, MHO, and healthy without obesity). Our prior research in this same cohort investigating the difference in gut microbiota between participants with and without obesity (regardless of phenotype) likewise found that alpha diversity was not associated with BMI [25]. However, those with obesity had a distinct gut microbiota profile compared to those without obesity [25]. The literature has also previously established the relationship between various metabolic factors and microbial diversity. For instance, lower microbial richness has been associated with unfavorable lipid profiles (i.e., high triglycerides and low HDL) [50], and a higher Shannon index and richness have been associated with lower rates of insulin resistance [51].
Limited research has investigated the difference in gut microbiota between MHO and MUO groups. Much of the work that has been done has found similar results to the present study. Kim et al. found that alpha diversity was lower among the MUO than both the MHO and the healthy non-obesity group in their study of 747 adults with overweight or obesity [7]. The microbial composition also differed between the three study groups, and they noted differences in bacterial composition between those with MUO and MHO [7]. A comparable research question has also been investigated among children and older adults with obesity [52,53]. Children with MUO had a decreased alpha diversity and richness compared to those with MHO; however, microbial β-diversity did not differ [52]. On the other hand, there was neither alpha nor β-diversity differed between the MHO and MUO groups among older adults [53]. The present study and the previous studies have all emerged with variations in the bacterial species that stood out as significantly different between the groups [7,52,53,54]. Such an outcome was anticipated considering different cohorts, age groups, and methods were used for each study, and these are key aspects impacting the gut microbiota or the measurement of it [7,52,53,54,55,56].
Another area of study that future efforts can focus on is microbial metabolic potential. Gut microbes consume, exchange, and secrete metabolites, which are small molecules created from metabolic activity [57]. Metabolites enter the human gut through the intake of nutrients and are consumed by microbes which then convert some of the metabolites into their biomass and secrete the rest for consumption by other microbes [57]. Eventually, the remaining metabolites will exit the gastrointestinal tract [57]. Microbes interact through these metabolites to maintain homeostasis in the gastrointestinal tract, but metabolites can also impact the host [58,59,60]. In fact, some research indicates that host health is more related to metabolic pathways than microbe taxonomy [61,62]. Visconti et al. found that metabolic pathways are more likely to be shared between people than species are, sharing an average of 82% compared to just 43%, respectively [61]. Specifically, metabolic pathways were associated with 95% of fecal and 34% of blood metabolites compared to the microbiome, which was associated with 71% and 15%, respectively [61]. Thus, some of the discrepancies in research focusing solely on the taxonomy of the gut microbiota may be further explained by exploring the metabolome as well. Metabolites have also been studied in terms of MUO and MHO, demonstrating key differences. A recent systematic review of studies investigating the difference in metabolomic signature between MUO and MHO found that the overall signature for MHO trended more favorably [63]. Specifically, amino acids (branched-chain and aromatic), lipids, and acylcarnitines may be elevated in MUO [63].
One consideration when interpreting these results is the theory that MHO is a steppingstone to MUO. Previous work has shown that between 33% and 48% of people with MHO end up with MUO within 5 to 10 years, which may explain why MHO is less common in older age groups [64]. As such, longitudinal studies examining how the gut microbiota changes over time and the potential for modulating gut microbiota to avoid or delay such a progression may be useful.
The present study also demonstrated a higher hs-CRP level among those with MUO than those with MHO, both of which had higher levels than participants that were metabolically healthy without obesity. Previous work has come to conflicting conclusions in this regard, with some studies reporting higher hs-CRP levels among MUO compared to MHO [65,66], some finding similar levels among obesity phenotypes [67,68,69], and others finding higher levels among those with obesity in general compared to metabolically healthy individuals without obesity [66,67,68,69] or metabolically unhealthy individuals without obesity [69]. Variations in findings may result from the cohorts used as well as the statistical treatments (e.g., adjusting for abdominal obesity or body fat percentage) [67].
The present study had several strengths. Data collection accuracy was enhanced through the collection of information through several channels, including in-person interviews, in-depth health history questionnaires, and repeated measures of anthropometric data. This study used whole-genome sequencing, which has heightened accuracy, can detect more microbial species, and has better microbial resolution than the commonly used 16S rRNA sequencing [70]. In terms of limitations, the cohort in this study was exclusive of people with overweight and included only those with normal weight or obesity. While this limits the interpretation that can be made with regard to the gut microbiota of those with overweight, it more distinctly categorizes individuals into the extreme BMI groups for comparisons. As an observational study, causality cannot be confirmed, and confounding cannot be rejected. Lastly, while the selection of a specific cohort of young females in Saudi Arabia addresses the lack of research previously conducted in this population, the generalizability to other groups is limited, which is a drawback common to gut microbiota studies [20,24].
5. Conclusions
In conclusion, this study was the first to examine differences in gut microbiota among young females with MHO and MUO in Saudi Arabia. The findings support work demonstrating differences in diversity and microbial composition between phenotypes of obesity. Further investigations are required to identify the mechanisms underlying the relationship between the microbiome and metabolism in MHO and MUO, which may play a key part in preventing metabolic disease. Modulation of the gut microbiome cohorts through prebiotics, probiotics, and fecal microbiota transplantation may be a promising preventive and therapeutic approach to obesity-associated disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11061430/s1, Table S1: General characteristics of total participants.
Author Contributions
Conceptualization, S.A.-M., G.S.A., M.A.A. and M.M.A.; methodology, E.A.A. and G.S.A.; software, E.A.A.; validation, M.A.A., G.S.A. and S.A.-M.; formal analysis, G.S.A. and E.A.A.; investigation, E.A.A.; resources, S.A.-M.; data curation, E.A.A. and S.A.-M.; writing—original draft preparation, G.S.A.; writing—review and editing, S.A.-M., M.A.A. and M.M.A.; visualization, G.S.A. and E.A.A.; supervision, S.A.-M.; funding acquisition, S.A.-M. and G.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research and Innovation, ‘Ministry of Education’ in Saudi Arabia for funding this research work through the Directed Research Projects Fund number IFKSUDR_H137.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board Committee of the Deanship of Scientific Research at King Saud University (IRB #E-19-3625).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Datasets generated for this study can be found in the Figshare repository: 10.6084/m9.figshare.20106176.
Conflicts of Interest
The authors declare no conflict of interest. The sponsor had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
References
- World Health Organization (WHO). Obesity. 2016. Available online: https://www.who.int/topics/obesity/en/ (accessed on 25 February 2023).
- Dixon, J.B. The effect of obesity on health outcomes. Mol. Cell. Endocrinol. 2010, 316, 104–108. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Pugliese, G.; Fantauzzi, C.B.; Federici, M.; Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 2019, 92, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M. Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev. Endocr. Metab. Disord. 2013, 14, 219–227. [Google Scholar] [CrossRef]
- Hinnouho, G.-M.; Czernichow, S.; Dugravot, A.; Nabi, H.; Brunner, E.J.; Kivimaki, M.; Singh-Manoux, A. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: The Whitehall II cohort study. Eur. Heart J. 2015, 36, 551–559. [Google Scholar] [CrossRef]
- Kim, M.-H.; Yun, K.E.; Kim, J.; Park, E.; Chang, Y.; Ryu, S.; Kim, H.-L.; Kim, H.-N. Gut microbiota and metabolic health among overweight and obese individuals. Sci. Rep. 2020, 10, 19417. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Bäckhed, F. The gut microbiota and metabolic disease: Current understanding and future perspectives. J. Intern. Med. 2016, 280, 339–349. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Natividad, J.M.; Verdu, E.F. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol. Res. 2013, 69, 42–51. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; García-Fuentes, E.; Cardona, F.; Queipo-Ortuño, M.I.; Tinahones, F.J. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am. J. Transl. Res. 2016, 8, 5672. [Google Scholar] [PubMed]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Stachowicz, N.; Kiersztan, A. The role of gut microbiota in the pathogenesis of obesity and diabetes. Postep. Hig. Med. Dosw. (Online) 2013, 67, 288–303. [Google Scholar] [CrossRef]
- Tagliabue, A.; Elli, M. The role of gut microbiota in human obesity: Recent findings and future perspectives. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 160–168. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Hermes, G.D.; Reijnders, D.; Kootte, R.S.; Goossens, G.H.; Smidt, H.; Nieuwdorp, M.; Blaak, E.E.; Zoetendal, E.G. Individual and cohort-specific gut microbiota patterns associated with tissue-specific insulin sensitivity in overweight and obese males. Sci. Rep. 2020, 10, 7523. [Google Scholar] [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef]
- Yasir, M.; Angelakis, E.; Bibi, F.; Azhar, E.I.; Bachar, D.; Lagier, J.C.; Gaborit, B.; Hassan, A.M.; Jiman-Fatani, A.A.; Alshali, K.Z.; et al. Comparison of the gut microbiota of people in France and Saudi Arabia. Nutr. Diabetes 2015, 5, e153. [Google Scholar] [CrossRef]
- Hermes, G.D.; Zoetendal, E.G.; Smidt, H. Molecular ecological tools to decipher the role of our microbial mass in obesity. Benef. Microbes 2015, 6, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Aljazairy, E.A.A.; Al-Musharaf, S.; Abudawood, M.; Almaarik, B.; Hussain, S.D.; Alnaami, A.M.; Sabico, S.; Al-Daghri, N.M.; Clerici, M.; Aljuraiban, G.S. Influence of Adiposity on the Gut Microbiota Composition of Arab Women: A Case-Control Study. Biology 2022, 11, 1586. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 February 2023).
- So, W.-Y.; Swearingin, B.; Crooms, B.; Lee, R.; Choi, Y.; Dail, T.K.; Ha, C. Body composition measurements determined by air displacement plethysmography and eight-polar bioelectrical impedance analysis are equivalent in African American college students. HealthMED 2012, 6, 1896–1899. [Google Scholar]
- Al-Lawati, J.A.; Barakat, N.M.; Al-Lawati, A.M.; Mohammed, A.J. Optimal cut-points for body mass index, waist circumference and waist-to-hip ratio using the Framingham coronary heart disease risk score in an Arab population of the Middle East. Diabetes Vasc. Dis. Res. 2008, 5, 304–309. [Google Scholar] [CrossRef]
- Balkau, B.; Charles, M.A. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 1999, 16, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef] [PubMed]
- Karelis, A.D.; Brochu, M.; Rabasa-Lhoret, R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004, 30, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Rey-López, J.P.; de Rezende, L.F.; Pastor-Valero, M.; Tess, B.H. The prevalence of metabolically healthy obesity: A systematic review and critical evaluation of the definitions used. Obes. Rev. 2014, 15, 781–790. [Google Scholar] [CrossRef]
- Mas-Lloret, J.; Obón-Santacana, M.; Ibáñez-Sanz, G.; Guinó, E.; Pato, M.L.; Rodriguez-Moranta, F.; Mata, A.; García-Rodríguez, A.; Moreno, V.; Pimenoff, V.N. Gut microbiome diversity detected by high-coverage 16S and shotgun sequencing of paired stool and colon sample. Sci. Data 2020, 7, 92. [Google Scholar] [CrossRef]
- Ottesen, A.; Ramachandran, P.; Reed, E.; White, J.R.; Hasan, N.; Subramanian, P.; Ryan, G.; Jarvis, K.; Grim, C.; Daquiqan, N.; et al. Enrichment dynamics of Listeria monocytogenes and the associated microbiome from naturally contaminated ice cream linked to a listeriosis outbreak. BMC Microbiol. 2016, 16, 275. [Google Scholar] [CrossRef]
- Ponnusamy, D.; Kozlova, E.V.; Sha, J.; Erova, T.E.; Azar, S.R.; Fitts, E.C.; Kirtley, M.L.; Tiner, B.L.; Andersson, J.A.; Grim, C.J.; et al. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc. Natl. Acad. Sci. USA 2016, 113, 722. [Google Scholar] [CrossRef]
- Hasan, N.A.; Young, B.A.; Minard-Smith, A.T.; Saeed, K.; Li, H.; Heizer, E.M.; McMillan, N.J.; Isom, R.; Abdullah, A.S.; Bornman, D.M.; et al. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS ONE 2014, 9, e97699. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.; Smith, D.P.; Hampton-Marcell, J.; Owens, S.M.; Handley, K.M.; Scott, N.M.; Gibbons, S.M.; Larsen, P.; Shogan, B.D.; Weiss, S.; et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 2014, 345, 1048. [Google Scholar] [CrossRef]
- Alkhalaf, M.; Edwards, C.; Combet, E. Validation of a food frequency questionnaire specific for salt intake in Saudi Arabian adults using urinary biomarker and repeated multiple pass 24-hour dietary recall. Proc. Nutr. Soc. 2015, 74, e337. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Zhang, H.; Lee, Y.-K.; Zhai, Q.; Chen, W. Roles of intestinal bacteroides in human health and diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 3518–3536. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Jang, S.; Kim, M.J.; Kim, J.H.; Chung, M.J.; Kim, K.J.; Ha, N.J. Anti-proliferative effects of Bifidobacterium adolescentis SPM0212 extract on human colon cancer cell lines. BMC Cancer 2008, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Rerksuppaphol, S.; Rerksuppaphol, L. Lactobacillus acidophilus and Bifidobacterium bifidum stored at ambient temperature are effective in the treatment of acute diarrhoea. Ann. Trop. Paediatr. 2010, 30, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Guan, X.-X.; Tang, Y.-J.; Sun, J.-F.; Wang, X.-K.; Wang, W.-D.; Fan, J.-M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef]
- Solito, A.; Bozzi Cionci, N.; Calgaro, M.; Caputo, M.; Vannini, L.; Hasballa, I.; Archero, F.; Giglione, E.; Ricotti, R.; Walker, G.E.; et al. Supplementation with Bifidobacterium breve BR03 and B632 strains improved insulin sensitivity in children and adolescents with obesity in a cross-over, randomized double-blind placebo-controlled trial. Clin. Nutr. 2021, 40, 4585–4594. [Google Scholar] [CrossRef]
- Schellekens, H.; Torres-Fuentes, C.; van de Wouw, M.; Long-Smith, C.M.; Mitchell, A.; Strain, C.; Berding, K.; Bastiaanssen, T.F.S.; Rea, K.; Golubeva, A.V.; et al. Bifidobacterium longum counters the effects of obesity: Partial successful translation from rodent to human. EBioMedicine 2021, 63, 103176. [Google Scholar] [CrossRef]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J. Association of insulin resistance and type 2 diabetes with gut microbial diversity: A microbiome-wide analysis from population studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef]
- Alcazar, M.; Escribano, J.; Ferré, N.; Closa-Monasterolo, R.; Selma-Royo, M.; Feliu, A.; Castillejo, G.; Luque, V.; Feliu-Rovira, A.; Muñoz-Hernando, J. Gut microbiota is associated with metabolic health in children with obesity. Clin. Nutr. 2022, 41, 1680–1688. [Google Scholar] [CrossRef]
- Zhong, X.; Harrington, J.M.; Millar, S.R.; Perry, I.J.; O’Toole, P.W.; Phillips, C.M. Gut microbiota associations with metabolic health and obesity status in older adults. Nutrients 2020, 12, 2364. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut microbiota and predicted metabolic pathways in a sample of Mexican women affected by obesity and obesity plus metabolic syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Di Cagno, R.; De Angelis, M.; Turroni, S.; Vannini, L.; Bancalari, E.; Rantsiou, K.; Cardinali, G.; Neviani, E.; Cocolin, L. Fecal microbiota in healthy subjects following omnivore, vegetarian and vegan diets: Culturable populations and rRNA DGGE profiling. PLoS ONE 2015, 10, e0128669. [Google Scholar] [CrossRef]
- Salazar, N.; Arboleya, S.; Fernández-Navarro, T.; de Los Reyes-Gavilán, C.G.; Gonzalez, S.; Gueimonde, M. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: A cross-sectional study. Nutrients 2019, 11, 1765. [Google Scholar] [CrossRef]
- Wang, T.; Goyal, A.; Dubinkina, V.; Maslov, S. Evidence for a multi-level trophic organization of the human gut microbiome. PLoS Comput. Biol. 2019, 15, e1007524. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Wang, T.; Dubinkina, V.; Maslov, S. Ecology-guided prediction of cross-feeding interactions in the human gut microbiome. Nat. Commun. 2021, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Loo, R.L.; Stamler, J.; Bictash, M.; Yap, I.K.; Chan, Q.; Ebbels, T.; De Iorio, M.; Brown, I.J.; Veselkov, K.A. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008, 453, 396–400. [Google Scholar] [CrossRef]
- Sung, J.; Kim, S.; Cabatbat, J.J.T.; Jang, S.; Jin, Y.-S.; Jung, G.Y.; Chia, N.; Kim, P.-J. Global metabolic interaction network of the human gut microbiota for context-specific community-scale analysis. Nat. Commun. 2017, 8, 15393. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, X.; Yang, S.; Cui, H.; Wang, G. Metabolomic signature between metabolically healthy overweight/obese and metabolically unhealthy overweight/obese: A systematic review. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 991–1010. [Google Scholar] [CrossRef]
- Phillips, C.M. Metabolically healthy obesity across the life course: Epidemiology, determinants, and implications. Ann. N. Y. Acad. Sci. J. 2017, 1391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Faraj, M.; Bastard, J.-P.; St-Pierre, D.H.; Brochu, M.; Prud’homme, D.; Rabasa-Lhoret, R. The metabolically healthy but obese individual presents a favorable inflammation profile. J. Clin. Endocrinol. Metab. 2005, 90, 4145–4150. [Google Scholar] [CrossRef]
- Shaharyar, S.; Roberson, L.L.; Jamal, O.; Younus, A.; Blaha, M.J.; Ali, S.S.; Zide, K.; Agatston, A.A.; Blumenthal, R.S.; Conceição, R.D. Obesity and metabolic phenotypes (metabolically healthy and unhealthy variants) are significantly associated with prevalence of elevated C-reactive protein and hepatic steatosis in a large healthy Brazilian population. J. Obes. 2015, 2015, 178526. [Google Scholar] [CrossRef]
- Liao, C.; Gao, W.; Cao, W.; Lv, J.; Yu, C.; Wang, S.; Pang, Z.; Cong, L.; Wang, H.; Wu, X. Associations of metabolic/obesity phenotypes with insulin resistance and C-reactive protein: Results from the cntr study. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Iglesias Molli, A.E.; Penas Steinhardt, A.; López, A.P.; González, C.D.; Vilarino, J.; Frechtel, G.D.; Cerrone, G.E. Metabolically healthy obese individuals present similar chronic inflammation level but less insulin-resistance than obese individuals with metabolic syndrome. PLoS ONE 2017, 12, e0190528. [Google Scholar] [CrossRef]
- Oguoma, V.M.; Abu-Farha, M.; Coffee, N.T.; Alsharrah, S.; Al-Refaei, F.H.; Abubaker, J.; Daniel, M.; Al-Mulla, F. Metabolically healthy and unhealthy obese phenotypes among Arabs and South Asians: Prevalence and relationship with cardiometabolic indicators. Nutrients 2022, 14, 915. [Google Scholar] [CrossRef] [PubMed]
- Brumfield, K.D.; Huq, A.; Colwell, R.R.; Olds, J.L.; Leddy, M.B. Microbial resolution of whole genome shotgun and 16S amplicon metagenomic sequencing using publicly available NEON data. PLoS ONE 2020, 15, e0228899. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).