Efficacy of Streptomyces murinus JKTJ-3 in Suppression of Pythium Damping-Off of Watermelon
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Screening of Biocontrol Agents
2.3. 16S rDNA Sequencing
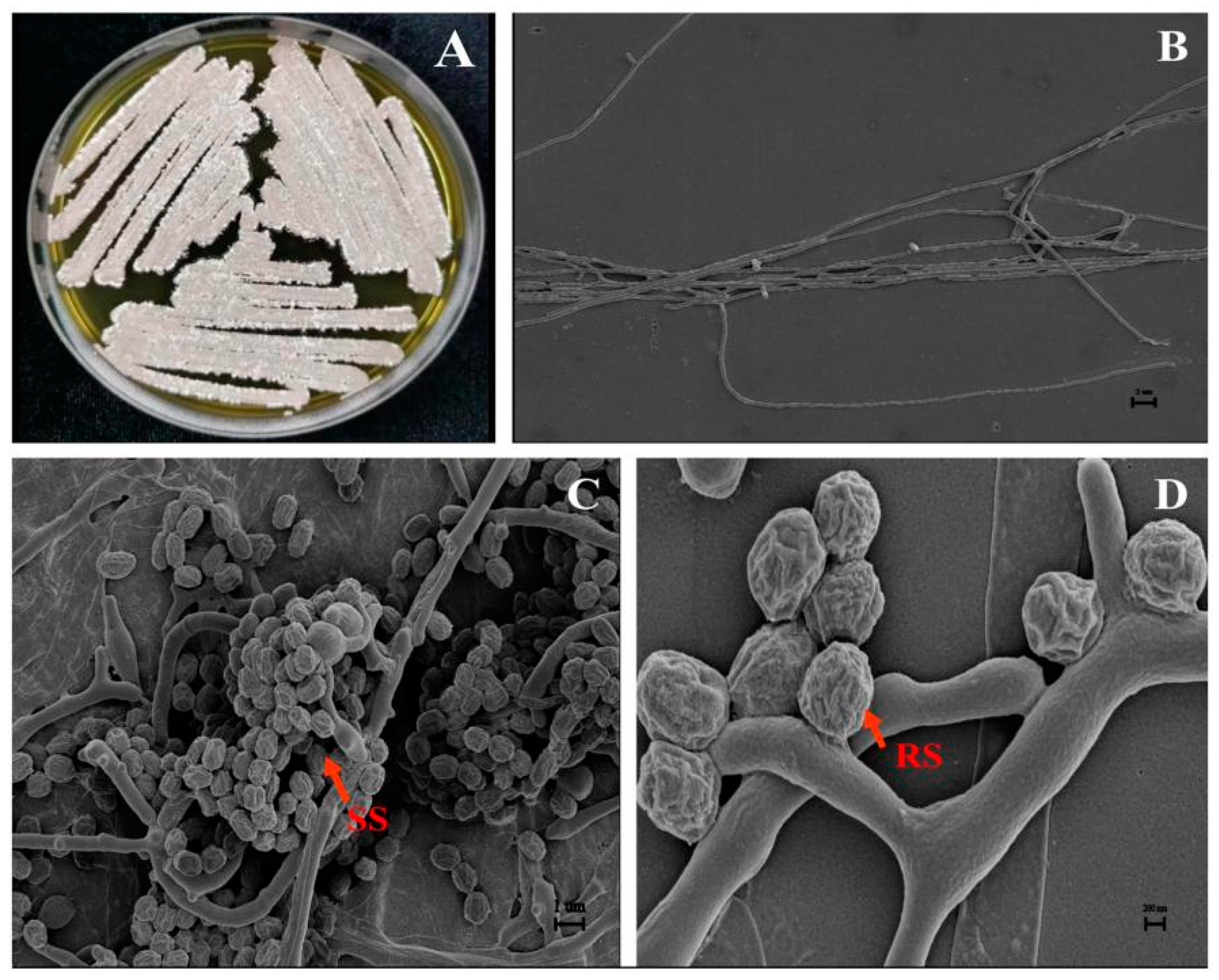
2.4. Determination of Morphological, Cultural, and Physiological Features
2.5. Determination of Biocontrol Efficacy of Isolate JKTJ-3
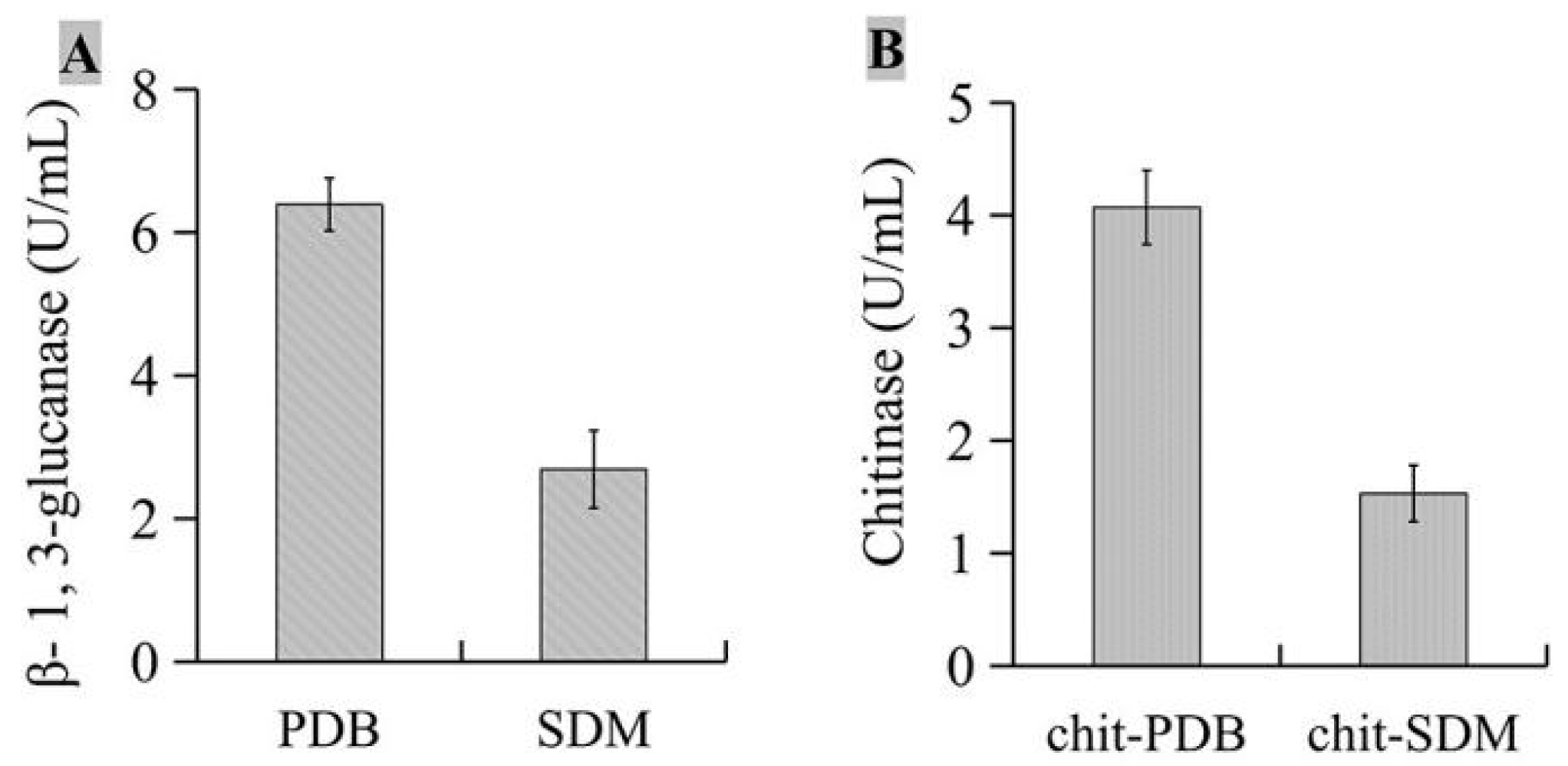
2.6. Determination of β-1,3-Glucanase and Chitosanase Activities Produced by JKTJ-3
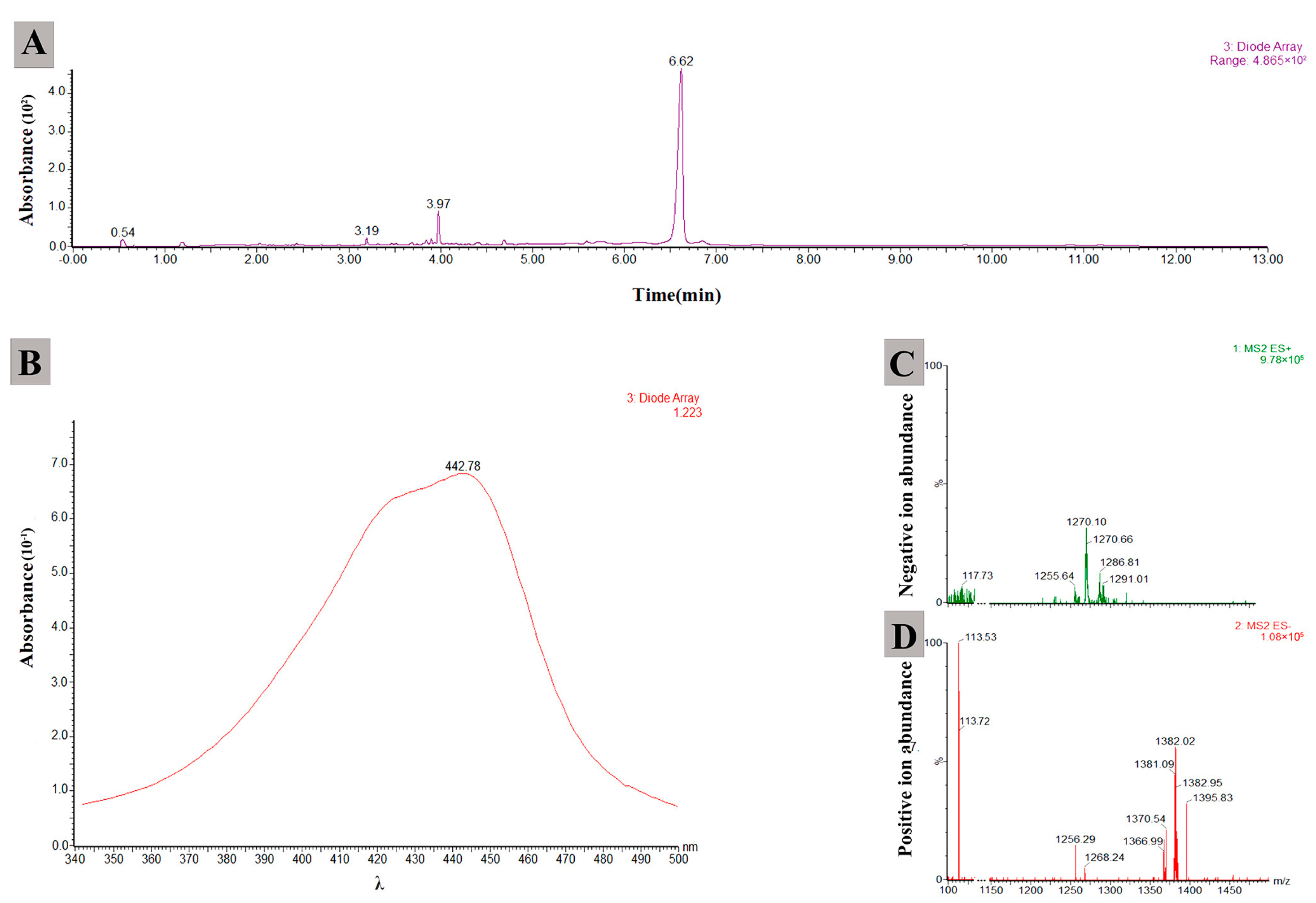
2.7. Determination of Antifungal Metabolites Produced by JKTJ-3
2.8. Statistical Analysis
3. Results
3.1. In Vitro Screening of Antagonists
3.2. In Vivo Screening of Biocontrol Agents
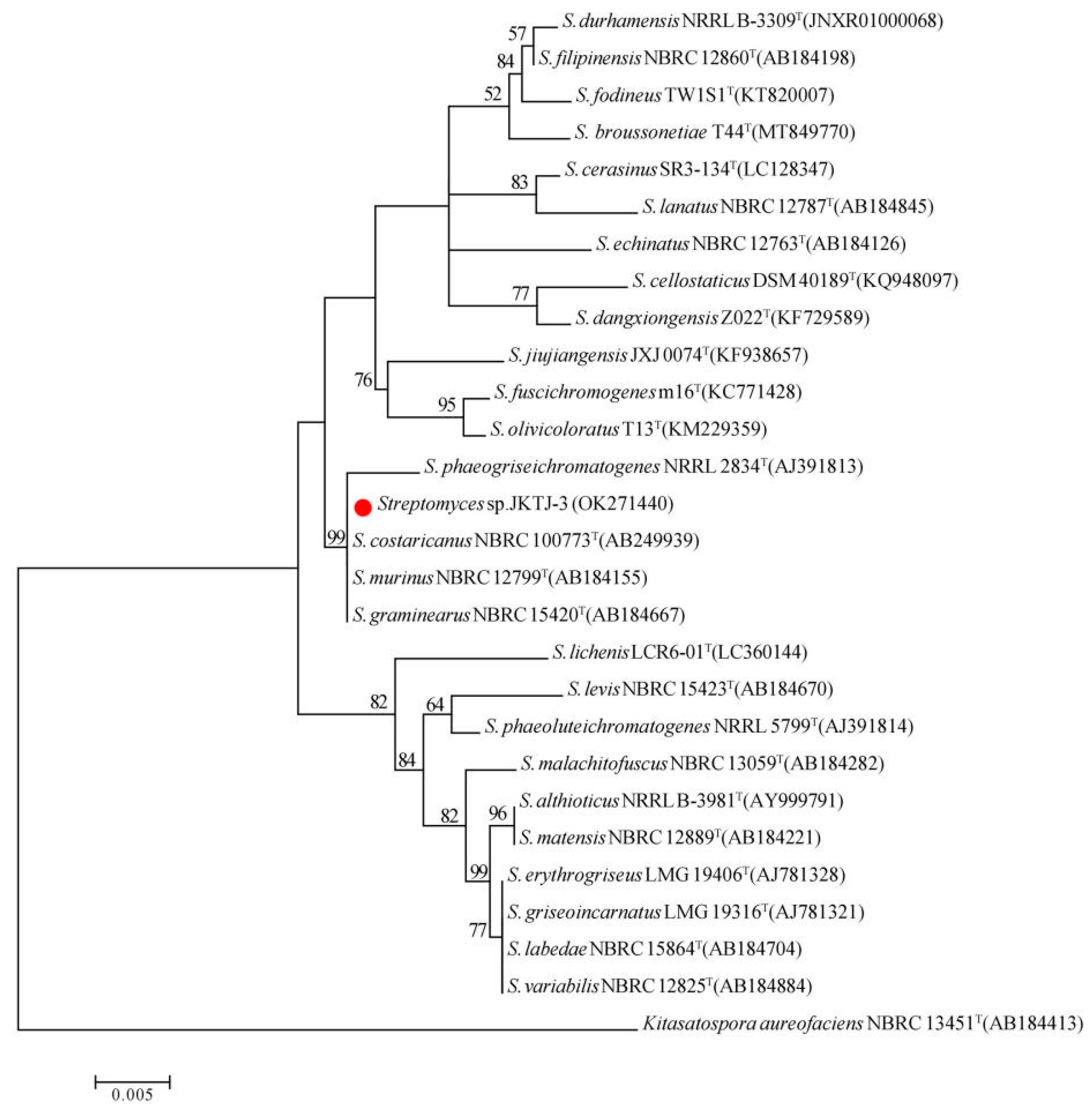
3.3. Taxonomic Identification of Isolate JKTJ-3
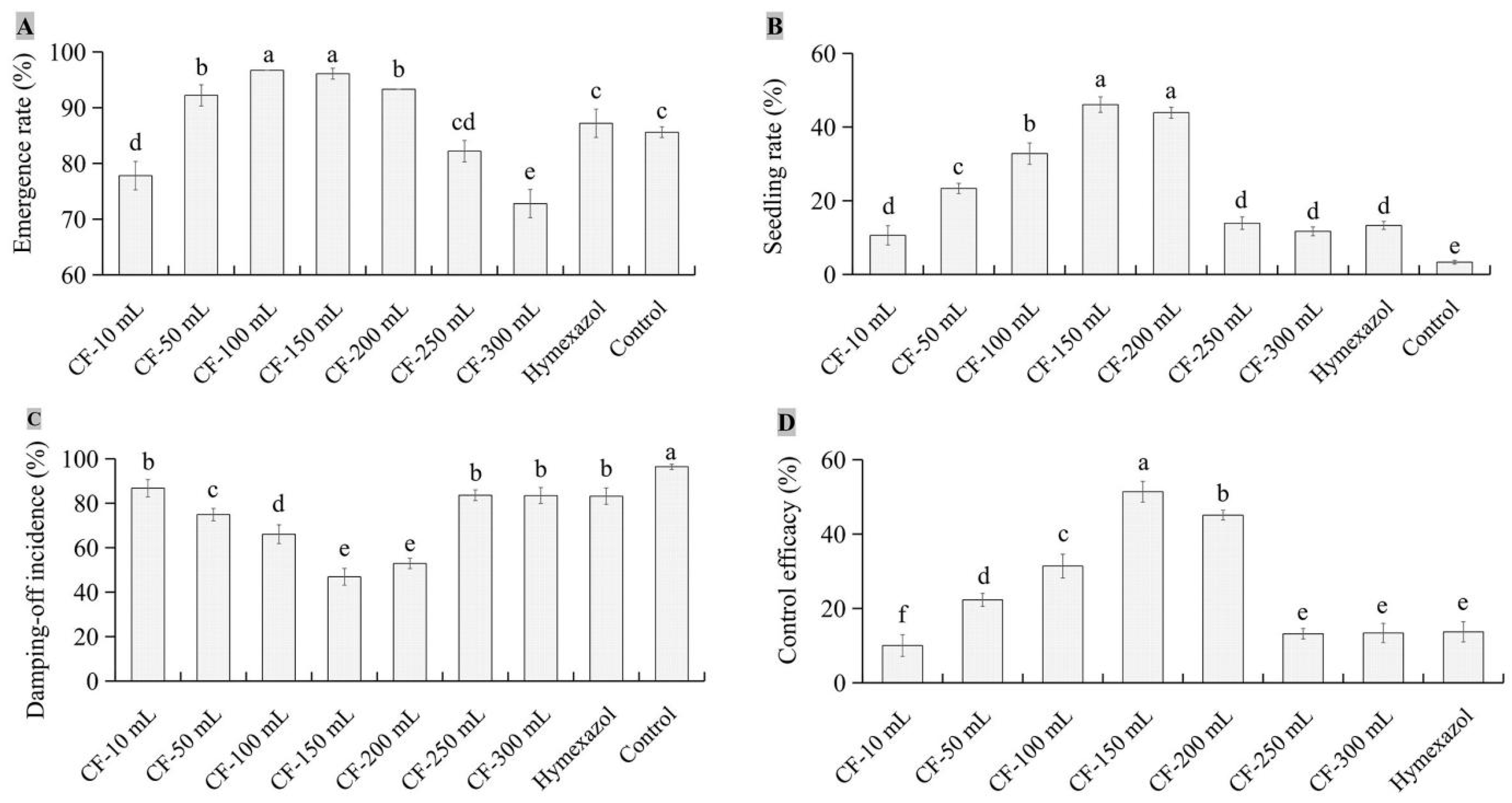
3.4. Biocontrol Efficacy of JKTJ-3 by Treatment of Seeding Substrate
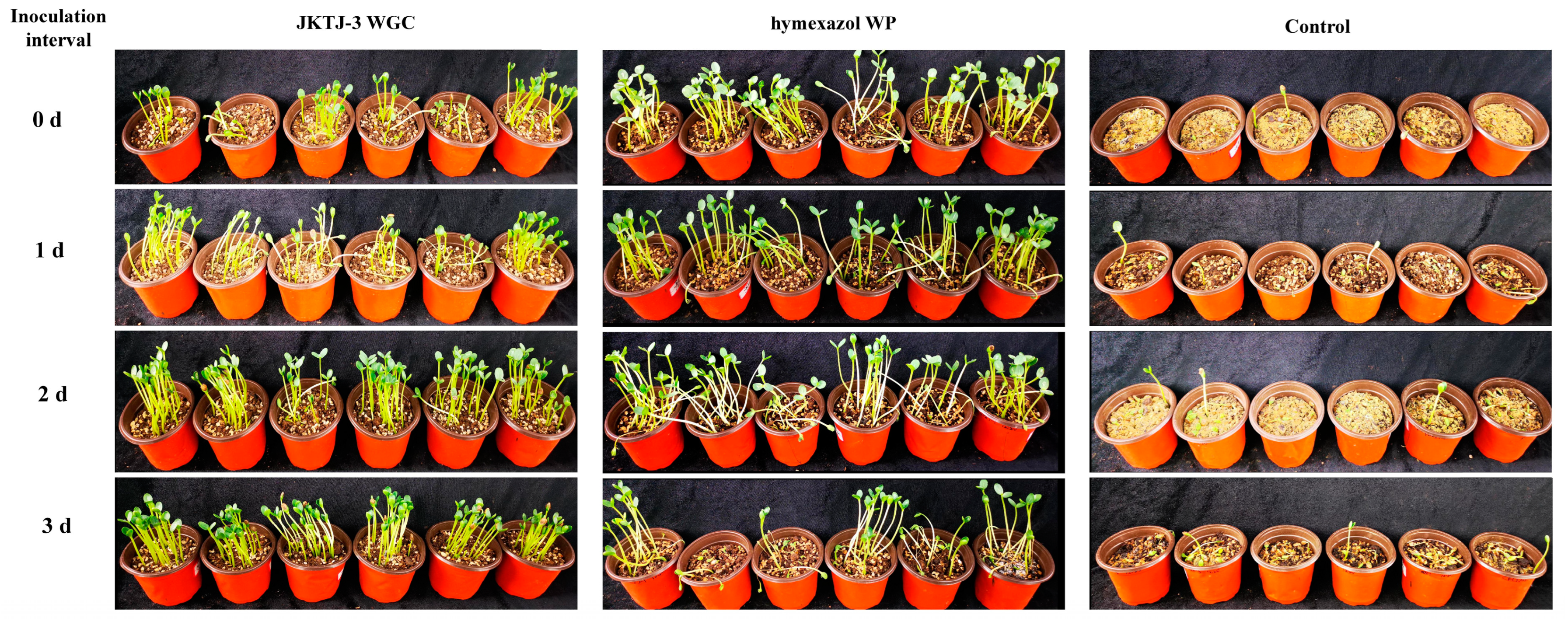
3.5. Protective Efficacy of JKTJ-3 by Treatment of Seeding Substrate
3.6. Biocontrol Efficacy of Isolate JKTJ-3 by Seed Treatment
3.7. Biocontrol Mechanisms of Isolate JKTJ-3
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Tojo, M.; Fujita, Y.; Awad, H.M.; Ichitani, T. Preparation of Pythium inocula using bentgrass seeds for glasshouse Studies. Annu. Rep. Kansai Plant Prot. Soc. 1993, 35, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, D.G.; Fiddaman, P.; Leifert, C.; Malathrakis, N.E. Biological control of cucumber and sugar beet damping-off caused by Pythium ultimum with bacterial and fungal antagonists. J. Appl. Microbiol. 2010, 92, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Haenseler, C.M. Control of seed decay and damping-off of cucumbers. Rep. N. J. Exp. Stat. 1930, 51, 254–264. [Google Scholar]
- Burgieł, Z.J. Research on possibilities of utilization of chosen brassicaceae plants in protection of cucumber against damping-off caused by Rhizoctonia solani Kuhn and Fusarium culmorum (W.G. Smith) Sacc. J. Pol. Bot. Soc. 2005, 58, 171–178. [Google Scholar] [CrossRef]
- Al-Fadhal, F.A.; AL-Abedy, A.N.; Alkhafije, D.A. Isolation and molecular identification of Rhizoctonia solani and Fusarium solani isolated from cucumber (Cucumis sativus L.) and their control feasibility by Pseudomonas fluorescens and Bacillus subtilis. Egypt. J. Biol. Pest Control 2019, 29, 47. [Google Scholar] [CrossRef]
- Wan, Z.Y.; Fang, W.; Wu, Z.Y.; Zhang, Y.N.; Zhang, Z.G.; Wang, K.M.; Yang, Z.W. Isolation andidentification of active substances from broad-spectrum antifungal Streptomyces HBERC-57169. Biot. Resour. 2019, 41, 516–523. [Google Scholar] [CrossRef]
- Mckeen, C.D. Arasan as a seed and soil treatment for the control of damping-off in certain vegetables. Sci. Agric. 1950, 30, 261–270. [Google Scholar] [CrossRef]
- Arora, H.; Sharma, A.; Sharma, S.; Haron, F.F.; Datta, R. Pythium damping-off and root rot of Capsicum annuum L.: Impacts, diagnosis, and management. Microorganisms 2021, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Dürr, C.; Schwanck, A.A.; Robin, M.-H.; Sarthou, J.-P.; Cellier, V.; Messéan, A.; Aubertot, J.-N. Integrated management of damping-off diseases, a review. Agron. Sustain. Dev. 2017, 37, 10. [Google Scholar] [CrossRef]
- Abdelrahim, A.M.; Abusurrieh, A.A. Chemical control of Pythium debaryanum, the causal organism of damping-off in cucumber. Arab J. Plant Prot. 1991, 1, 14–18. [Google Scholar]
- Palakshappa, M.G.; Lokesh, M.S.; Parameshwarappa, K.G. Efficacy of ridomil gold (metalaxyl M + mancozeb (4+64 WP)) against chilli damping off caused by Pythium aphanidermatum. Karnataka J. Agric. Sci. 2010, 23, 445–446. [Google Scholar]
- Begum, S.; Devi, R.; Singh, N.I. Management of damping-off of vegetable seedlings caused by Rhizoctonia solani. Plant Prot. Assoc. India 2014, 42, 248–254. [Google Scholar]
- Cohen, Y. Occurrence of metalaxyl-resistant isolates of Phytophthora infestans in potato fields in Israel. Phytopathology 1983, 73, 925–927. [Google Scholar] [CrossRef]
- White, J.G.; Stanghellini, M.E.; Ayoubi, L.M. Variation in the sensitivity to metalaxyl of Pythium spp. isolated from carrot and other sources. Ann. Appl. Biol. 2010, 113, 269–277. [Google Scholar] [CrossRef]
- Ma, Z.; Michailides, T.J. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot. 2005, 24, 853–863. [Google Scholar] [CrossRef]
- Hwangbo, H.; Kim, K.-Y.; Choi, H.-S. Effects of biocontrol agents on suppression of damping-off in Cucumis sativus L. caused by Rhizoctonia solani. Hortic. Environ. Biotechnol. 2016, 57, 191–196. [Google Scholar] [CrossRef]
- Roberts, D.P.; Selmer, K.; Lupitskyy, R.; Rice, C.; Buyer, J.S.; Maul, J.E.; Lakshman, D.K.; DeSouza, J. Seed treatment with prodigiosin controls damping-off of cucumber caused by Pythium ultimum. AMB Express 2021, 11, 147. [Google Scholar] [CrossRef]
- Sadeghi, A.; Koobaz, P.; Azimi, H.; Karimi, E.; Akbari, A.R. Plant growth promotion and suppression of Phytophthora drechsleri damping-off in cucumber by cellulase-producing Streptomyces. BioControl 2017, 62, 805–819. [Google Scholar] [CrossRef]
- Awad, L.K.; Fayyadh, M.A. The Activity of some actinomycetes isolates in control of cucumber damping off disease caused by Rhizoctiona solani and Pythium sp. Basrah J. Agric. Sci. 2018, 31, 11–23. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Rhizosphere-Competent Isolates of Streptomycete and non-streptomycete actinomycetes capable of producing cell-wall-degrading enzymes to control Pythium aphanidermatum damping-off disease of cucumber. Botany 2006, 84, 211–222. [Google Scholar] [CrossRef]
- Roberts, D.; McKenna, L.; Lakshman, D.; Meyer, S.; Kong, H.; De Souza, J.; Lydon, J.; Baker, C.; Buyer, J.; Chung, S. Suppression of damping-off of cucumber caused by Pythium ultimum with live cells and extracts of Serratia marcescens N4-5. Soil Biol. Biochem. 2007, 39, 2275–2288. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Ran, W.; Shen, Q.; Yang, X. Trichoderma harzianum strain SQR-T37 and its bio-organic fertilizer could control Rhizoctonia solani damping-off disease in cucumber seedlings mainly by the mycoparasitism. Appl. Microbiol. Biotechnol. 2011, 91, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Valois, D.; Fayad, K.; Barasubiye, T.; Garon, M.; Dery, C.; Brzezinski, R.; Beaulieu, C. Glucanolytic actinomycetes antagonistic to Phytophthora fragariae var. rubi, the causal agent of raspberry root rot. Appl. Environ. Microbiol. 1996, 62, 1630–1635. [Google Scholar] [CrossRef]
- Samac, D.A.; Willert, A.M.; McBride, M.J.; Kinkel, L.L. Effects of antibiotic-producing Streptomyces on nodulation and leaf spot in alfalfa. Appl. Soil Ecol. 2003, 22, 55–66. [Google Scholar] [CrossRef]
- Hata, E.M.; Yusof, M.T.; Zulperi, D. Induction of systemic resistance against bacterial leaf streak disease and growth promotion in rice plant by Streptomyces shenzhenesis TKSC3 and Streptomyces sp. SS8. Plant Pathol. J. 2021, 37, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Baharlouei, A.; Sharifi-Sirchi, G.; Bonjar, G.S. Identification of an Antifungal Chitinase from a Potential Biocontrol Agent, Streptomyces Plicatus Strain 101, and Its New Antagonistic Spectrum of Activity. Philipp. Agric. Sci 2010, 93, 439–445. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Riley, M.B.; Keinath, A.P. Effect of incorporation of Brassica spp. residues on population densities of soilborne microorganisms and on damping-off and Fusarium wilt of watermelon. Plant Dis. 2008, 92, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Al-Sa’di, A.M.; Drenth, A.; Deadman, M.; De Cock, A.W.A.M.; Aitken, E. Molecular characterization and pathogenicity of Pythium species associated with damping-off in greenhouse cucumber (Cucumis sativus) in Oman. Plant Pathol. 2007, 56, 140–149. [Google Scholar] [CrossRef]
- Benhamou, N.; Belanger, R.R. Induction of systemic resistance to pythium damping-off in cucumber plants by benzothiadiazole: Ultrastructure and cytochemistry of the host response. Plant J. 1998, 14, 13–21. [Google Scholar] [CrossRef]
- Shakeel, Q.; Lyu, A.; Zhang, J.; Wu, M.; Li, G.; Hsiang, T.; Yang, L. Biocontrol of Aspergillus flavus on peanut kernels using Streptomyces yanglinensis 3–10. Front. Microbiol. 2018, 9, 1049. [Google Scholar] [CrossRef]
- Paul, B.; Charles, R.; Bhatnagar, T. Biological control of Pythium mamillatum causing damping-off of cucumber seedlings by a soil bacterium, Bacillus mycoides. Microbiol. Res. 1995, 150, 71–75. [Google Scholar] [CrossRef]
- Li, B.; Ravnskov, S.; Xie, G.; Larsen, J. Biocontrol of Pythium damping-off in cucumber by arbuscular mycorrhiza-associated bacteria from the genus Paenibacillus. Biocontrol 2007, 52, 863–875. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control Region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Esnard, J.; Potter, T.L.; Zuckerman, B.M. Streptomyces costaricanus sp. nov., isolated from nematode-suppressive soil. Int. J. Syst. Evol. Microbiol. 1995, 45, 775–779. [Google Scholar] [CrossRef]
- Shirling, E.T.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Jones, K.L. Fresh Isolates of actinomycetes in which the presence of sporogenous aerial mycelia is a fluctuating characteristic. J. Bacteriol. 1949, 57, 141–145. [Google Scholar] [CrossRef]
- Actiomycete Taxonmic Group, Institute of Microbiology of Chinese Academy of Sciences. Manual of Streptomyces Identification; Science Press: Beijing, China, 1975; p. 336. [Google Scholar]
- Shakeel, Q.; Lyu, A.; Jing, Z.; Wu, M.; Chen, S.; Chen, W.; Li, G.; Long, Y. Optimization of the cultural medium and conditions for production of antifungal substances by Streptomyces platensis 3–10 and evaluation of its efficacy in suppression of clubroot disease (Plasmodiophora brassicae) of oilseed rape. Biol. Control 2016, 101, 59–68. [Google Scholar] [CrossRef]
- Wan, Z.Y.; Fang, W.; Zhang, Y.N.; Zhang, Z.G.; Wu, Z.Y.; Wang, K.M.; Yang, Z.W. Isolation and identification of antifungal active compounds of actinomycete HBERC-39158. Hubei Agr. Sci. 2020, 59, 140–144, (In Chinese with English abstract). [Google Scholar]
- Aljarah, N. The Activity of Metarhizium sp. to Control Pythium Aphanidermatum causal agent cucumber damping off under greenhouse conditions. Int. J. Sci. Res. IJSR 2017, 6, 1098–1101. [Google Scholar] [CrossRef]
- Halo, B.A.; Al-Yahyai, R.A.; Maharachchikumbura, S.S.; Al-Sadi, A.M. Talaromyces variabilis interferes with Pythium aphanidermatum growth and suppresses Pythium-induced damping-off of cucumbers and tomatoes. Sci. Rep. 2019, 9, 11255. [Google Scholar] [CrossRef] [PubMed]
- Labeda, D.; Goodfellow, M.; Brown, R.; Ward, A.; Lanoot, B.; Vanncanneyt, M.; Swings, J.; Kim, S.-B.; Liu, Z.; Chun, J.; et al. Phylogenetic study of the species within the family Streptomycetaceae. Antonie Van Leeuwenhoek 2012, 101, 73–104. [Google Scholar] [CrossRef] [PubMed]
- Komaki, H. Reclassification of Streptomyces costaricanus and Streptomyces phaeogriseichromatogenes as later heterotypic synonyms of Streptomyces murinus. Int. J. Syst. Evol. Microbiol. 2021, 71, 004638. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, M.; Kumar, Y.; Labeda, D.P.; Sembiring, L. The Streptomyces violaceusniger clade: A home for Streptomycetes with rugose ornamented spores. Antonie Van Leeuwenhoek 2007, 92, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, O.; Karlsen, L.; Nielsen, N.; Pedersen, S.; Rugh, S. A New immobilized glucose isomerase with high productivity produced by a strain of Streptomyces murinus. Starch 1988, 40, 307–313. [Google Scholar] [CrossRef]
- Bandlish, R.K.; Michael Hess, J.; Epting, K.L.; Vieille, C.; Kelly, R.M. Glucose-to-fructose conversion at high temperatures with xylose (glucose) isomerases from Streptomyces murinus and two hyperthermophilic thermotoga species. Biotechnol. Bioeng. 2002, 80, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhang, L.; Gu, Z.; Ding, C.; Shi, G. Constitutive expression of AMP deaminase from Streptomyces murinus in Pichia pastoris GS115 using the GAP promoter. Microbiol. China 2014, 10, 2022–2028. [Google Scholar] [CrossRef]
- Bowers, J.; Parke, J. Epidemiology of Pythium damping-off and Aphanomyces root rot of peas after seed treatment with bacterial agents for biological control. Phytopathology 1993, 83, 1466–1473. [Google Scholar] [CrossRef]
- Lewis, J.; Larkin, R.; Rogers, D. A Formulation of Trichoderma and Gliocladium to reduce damping-off caused by Rhizoctonia solani and saprophytic growth of the pathogen in soilless mix. Plant Dis. 1998, 82, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.P.; Lakshman, D.K.; McKenna, L.F.; Emche, S.E.; Maul, J.E.; Bauchan, G. Seed treatment with ethanol extract of Serratia marcescens is compatible with Trichoderma isolates for control of damping-off of cucumber caused by Pythium ultimum. Plant Dis. 2016, 100, 1278–1287. [Google Scholar] [CrossRef]
- Callan, N.W.; Mathre, D.; Miller, J.B. Bio-priming seed treatment for biological control of Pythium ultimum preemergence damping-off in Sh-2 sweet corn. Plant Dis. 1990, 74, 368–372. [Google Scholar] [CrossRef]
- Becker, J.O.; Schwinn, F.J. Control of soil-borne pathogens with living bacteria and fungi: Status and outlook. Pestic. Sci. 1993, 37, 355–363. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Liotti, R.G.; da Silva Figueiredo, M.I.; Soares, M.A. Streptomyces griseocarneus R132 controls phytopathogens and promotes growth of pepper (Capsicum annuum). Biol. Control 2019, 138, 104065. [Google Scholar] [CrossRef]
- Hong, T.-Y.; Cheng, C.-W.; Huang, J.-W.; Meng, M. Isolation and biochemical characterization of an endo-1, 3-β-glucanase from Streptomyces sioyaensis containing a C-terminal family 6 carbohydrate-binding module that binds to 1, 3-β-glucan. Microbiology 2002, 148, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Zajkowicz, A.; Gdowicz-Kłosok, A.; Krześniak, M.; Ścieglińska, D.; Rusin, M. Actinomycin D and nutlin-3a synergistically promote phosphorylation of P53 on serine 46 in cancer cell lines of different origin. Cell. Signal. 2015, 27, 1677–1687. [Google Scholar] [CrossRef]
- Lei, J.; Duan, J.; Ma, H.; Li, J.; Li, H.; Yang, Z. Screening, identification and optimized fermentation condition of an actinomycete strain against Pseudomonas solanacearum. Chin. J. Appl. Environ. Biol. 2010, 16, 79–83. [Google Scholar] [CrossRef]



| Pathogen | Inhibition Rate (%) | |||
|---|---|---|---|---|
| JKTJ-3 | H55-1a | V10a | V61b | |
| Rhizoctonia solani | 91.1 ± 0.6 a 1 | 0.0 ± 0 c 1 | 0.0 ± 0 c 1 | 80.0 ± 0.4 b 1 |
| Stagonosporopsis cucurbitacearum | 69.6 ± 0.5 a | 54.9 ±1.0 c | 57.1 ± 1.1 c | 65.8 ± 0.9 b |
| Fusarium oxysporum f. sp. hiveum | 71.3 ± 0.4 a | 0.0 ± 0 c | 54.0 ± 0.8 b | 54.7 ± 0.3 b |
| F. solani | 76.7 ± 0.6 a | 51.6 ± 1.2 c | 53.8 ± 0.7 c | 61.8 ± 1.5 b |
| Botrytis cinerea | 69.3 ± 0.6 a | 47.3 ± 0.8 c | 59.3 ± 0.6 b | 63.8 ± 1.1 b |
| Colletotrichum gloeosporioides | 85.1 ± 0.9 a | 56.7 ± 0.5 c | 61.8 ± 0.9 b | 63.3 ± 0.4 b |
| C. capsic | 72.7 ± 0.3 a | 55.1 ± 0.5 c | 62.9 ± 0.6 b | 64.0± 0.7 b |
| Sclerotinia sclerotiorum | 84.0 ± 0.6 a | 59.3 ± 0.6 c | 0.0 ± 0 d | 70.7 ± 0.8 b |
| Verticillium dahliae | 95.1 ± 0.4 a | 79.3 ± 0.7 b | 64.0 ± 0.5 c | 79.6 ± 1.2 b |
| Leptosphaeria biglobosa | 91.8 ± 0.8 a | 81.1 ± 0.4 b | 66.2 ± 0.2 c | 84.0 ± 0.6 b |
| Phomopsis vexans | 78.2 ± 0.5 a | 58.2 ± 1.1 c | 50.7 ± 0.4 d | 66.0 ± 0.8 b |
| Phomopsis asparagi | 81.8 ± 1.1 a | 0.0 ± 0 d | 62.7 ± 0.6 b | 57.8 ± 1.0 c |
| Characteristic | Growth 1 | Substrate Mycelia Color | Aerial Mycelia Color 2 | Sporulation 3 | Pigment |
|---|---|---|---|---|---|
| Growth characteristics | |||||
| ISP-1 | ++ | Orange | − | NS | Orange |
| ISP-2 | +++ | Orange | GP/SP/DB | AS | Orange |
| ISP-3 | + | Orange | W | SS | Yellow |
| ISP-4 | ++ | Orange | W | SS | Yellow |
| ISP-5 | +++ | Orange | W/GP | AS | Orange |
| ISP-6 | ++ | Orange | − | NS | Orange |
| GS-1 | + | Yellow | − | NS | Yellow |
| BM | +++ | Orange | W/GP/DB | AS | Orange |
| Utilization of carbon sources | |||||
| D-glucose | + | Not determined | Not determined | SS | Pale |
| Sucrose | ++ | Not determined | Not determined | SS | Pale |
| L-arabinose | + | Not determined | Not determined | SS | Pale |
| D-fructose | ++ | Not determined | Not determined | DS | Yellow |
| D-xylose | +++ | Not determined | Not determined | SS | Yellow |
| D-mannitol | +++ | Not determined | Not determined | SS | Yellow |
| Raffinose | + | Not determined | Not determined | SS | Pale |
| Rhamnose | ++ | Not determined | Not determined | SS | Pale |
| Growth response to NaCl | |||||
| NaCl (≤2%) | +++ | Not determined | Not determined | AS | Orange to yellow |
| NaCl (5–8%) | + | Not determined | Not determined | NS | Yellow |
| NaCl (>8%) | − | Not determined | Not determined | NS | Pale |
| Growth response to pH | |||||
| pH 3.5 | + | Not determined | Not determined | NS | Pale |
| pH 4–7 | ++/+++ | Not determined | Not determined | SS | Yellow |
| pH 7.5–8.0 | + | Not determined | Not determined | NS | Yellow |
| Inoculation Interval | Treatment | Seed Emergence Rate (%) | Damping-Off Incidence (%) | Seedling Rate (%) | Control Efficacy (%) |
|---|---|---|---|---|---|
| 0 d | JKTJ-3 CF | 82.0 b 1 | 58.5 b 1 | 34.0 b 1 | 39.0 b 1 |
| Hymexazol WP | 90.0 a | 47.8 c | 47.0 a | 50.2 a | |
| Control | 75.0 c | 96.0 a | 3.0 c | - | |
| 1 d | JKTJ-3 CF | 90.0 a | 40.0 c | 54.0 a | 57.5 a |
| Hymexazol WP | 90.0 a | 51.1 b | 44.0 b | 45.7 b | |
| Control | 86.0 b | 94.2 a | 5.0 c | - | |
| 2 d | JKTJ-3 CF | 95.0 a | 35.8 c | 61.0 a | 63.8 a |
| Hymexazol WP | 95.0 a | 70.5 b | 28.0 b | 28.7 b | |
| Control | 90.0 b | 98.9 a | 1.0 c | - | |
| 3 d | JKTJ-3 CF | 95.0 a | 34.7 c | 62.0 a | 63.3 a |
| Hymexazol WP | 95.0 a | 86.3 b | 13.0 b | 8.9 b | |
| Control | 95.0 a | 94.7 a | 5.0 c | - |
| Inoculation Interval | Treatments | Seed Emergence Rate (%) | Damping-Off Incidence (%) | Seedling Rate (%) | Control Efficacy (%) |
|---|---|---|---|---|---|
| 0 d | JKTJ-3 WGC | 88.3 b 1 | 21.7 c 1 | 69.2 a 1 | 75.6 a 1 |
| Hymexazol WP | 91.7 a | 40.0 b | 52.0 b | 55.3 b | |
| Control | 86.7 b | 89.3 a | 9.8 c | - | |
| 1 d | JKTJ-3 WGC | 87.5 a | 10.0 c | 78.8 a | 88.6 a |
| Hymexazol WP | 89.2 a | 47.5 b | 43.3 b | 46.2 b | |
| Control | 82.5 b | 88.5 a | 10.3 c | - | |
| 2 d | JKTJ-3 WGC | 90.8 b | 0.8 c | 90.1 a | 99.2 a |
| Hymexazol WP | 93.3 a | 54.2 b | 41.6 b | 38.3 b | |
| Control | 90.8 b | 87.8 a | 11.4 c | - | |
| 3 d | JKTJ-3 WGC | 96.7 a | 0.0 c | 94.3 a | 100.0 a |
| Hymexazol WP | 97.5 a | 59.8 b | 38.8 b | 30.5 b | |
| Control | 94.3 b | 86.3 a | 13.3 c |
| Seed-Soaking Time | Treatment | Seed Emergence Rate (%) | Damping-Off Incidence (%) | Seedling Rate (%) | Control Efficacy (%) |
|---|---|---|---|---|---|
| 4 h | JKTJ-3 FC | 92.0 bc 1 | 55.7 de 1 | 44.3 c 1 | 39.2 bc 1 |
| JKTJ-3 CF | 91.0 c | 45.1 f | 54.9 a | 50.7 a | |
| Control | 95.0 b | 91.6 a | 8.4 g | ||
| 8 h | JKTJ-3 FC | 94.0 bc | 76.6 b | 23.4 ef | 14.7 e |
| JKTJ-3 CF | 95.0 b | 50.5 e | 49.5 b | 43.7 b | |
| Control | 93.0 bc | 89.8 a | 10.2 g | ||
| 12 h | JKTJ-3 FC | 95.0 b | 72.6 b | 27.4 e | 22.3 d |
| JKTJ-3 CF | 99.0 a | 63.5 c | 36.5 d | 32.1 c | |
| Control | 92.0 bc | 93.5 a | 6.5 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, M.; Cai, X.; Wang, D.; Liang, H.; Zhu, J.; Li, G.; Shi, X. Efficacy of Streptomyces murinus JKTJ-3 in Suppression of Pythium Damping-Off of Watermelon. Microorganisms 2023, 11, 1360. https://doi.org/10.3390/microorganisms11061360
Ge M, Cai X, Wang D, Liang H, Zhu J, Li G, Shi X. Efficacy of Streptomyces murinus JKTJ-3 in Suppression of Pythium Damping-Off of Watermelon. Microorganisms. 2023; 11(6):1360. https://doi.org/10.3390/microorganisms11061360
Chicago/Turabian StyleGe, Mihong, Xiang Cai, Dehuan Wang, Huan Liang, Juhong Zhu, Guoqing Li, and Xianfeng Shi. 2023. "Efficacy of Streptomyces murinus JKTJ-3 in Suppression of Pythium Damping-Off of Watermelon" Microorganisms 11, no. 6: 1360. https://doi.org/10.3390/microorganisms11061360
APA StyleGe, M., Cai, X., Wang, D., Liang, H., Zhu, J., Li, G., & Shi, X. (2023). Efficacy of Streptomyces murinus JKTJ-3 in Suppression of Pythium Damping-Off of Watermelon. Microorganisms, 11(6), 1360. https://doi.org/10.3390/microorganisms11061360







