Impacts of Oak Mulch Amendments on Rhizosphere Microbiome of Citrus Trees Grown in Florida Flatwood Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Plant Materials
2.2. Leaf Nutrient Analysis
2.3. Soil Nutrient, Temperature, and Moisure Analysis
2.4. Sampling of Rhizosophere Soil and DNA Isolation
2.5. DNA Quantification and PCR Amplification of Rhizophere Soil Samples
2.6. Sequencing Analysis and Taxonomic Assignment of Microorganisms Associated with the Rhizosphere Soil Samples
2.7. Plant and Soil Data Statistical Analysis
2.8. Rhizosphere Composition Statistical Analysis
3. Results
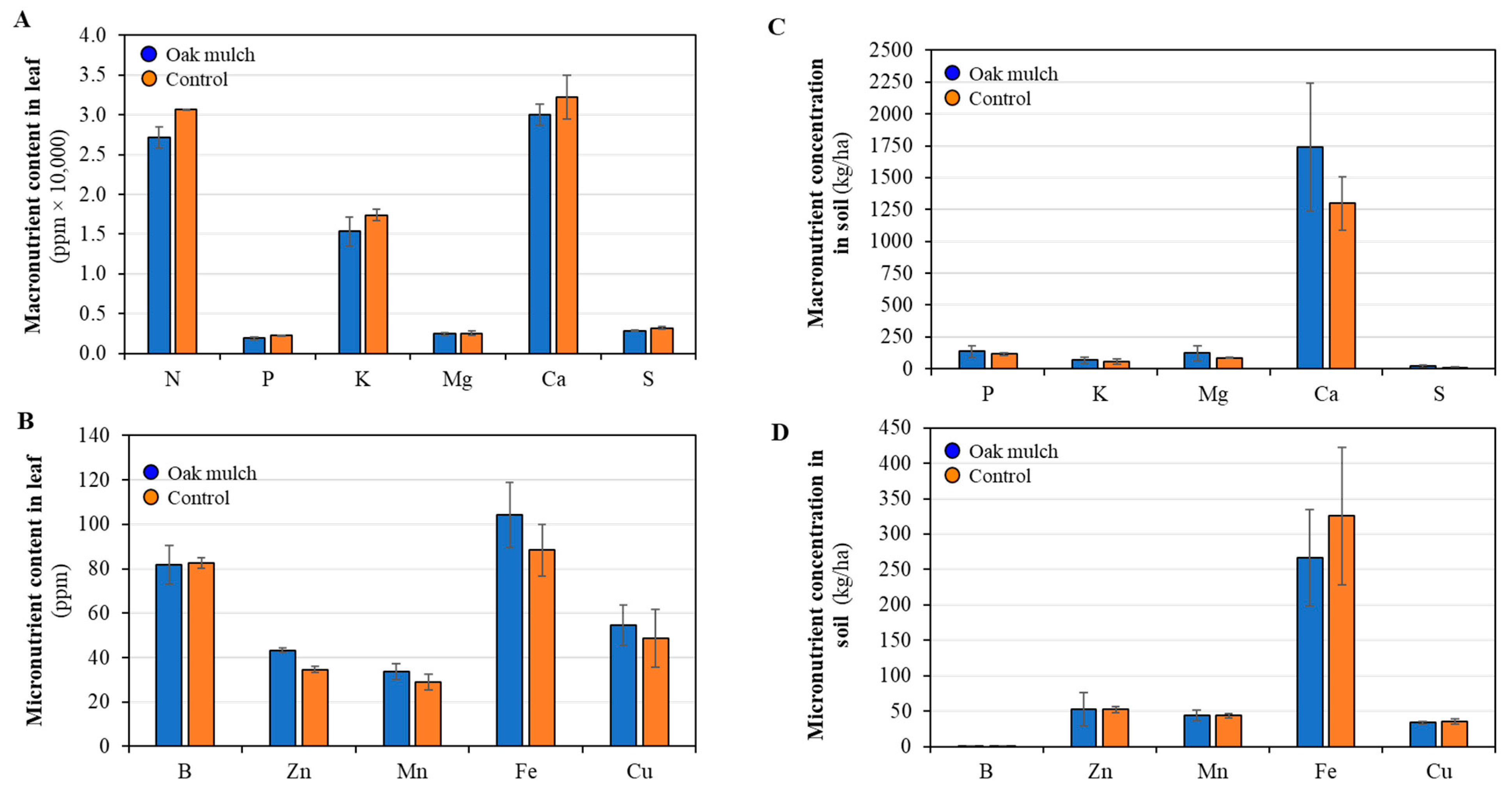
3.1. Impact of Oak Mulch on Soil and Leaf Nutrient Concentrations
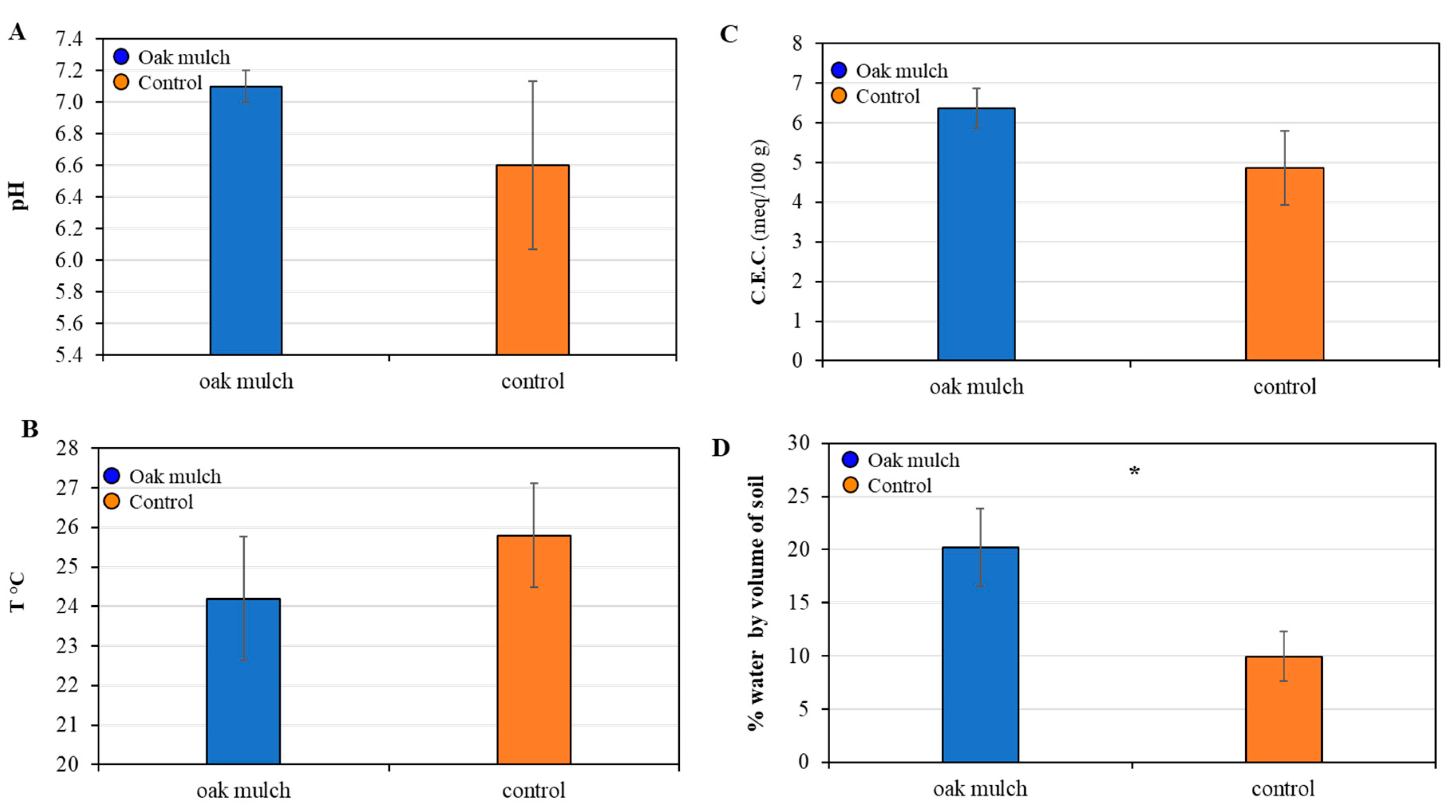
3.2. Impact of Oak Mulch on Soil Characteristics
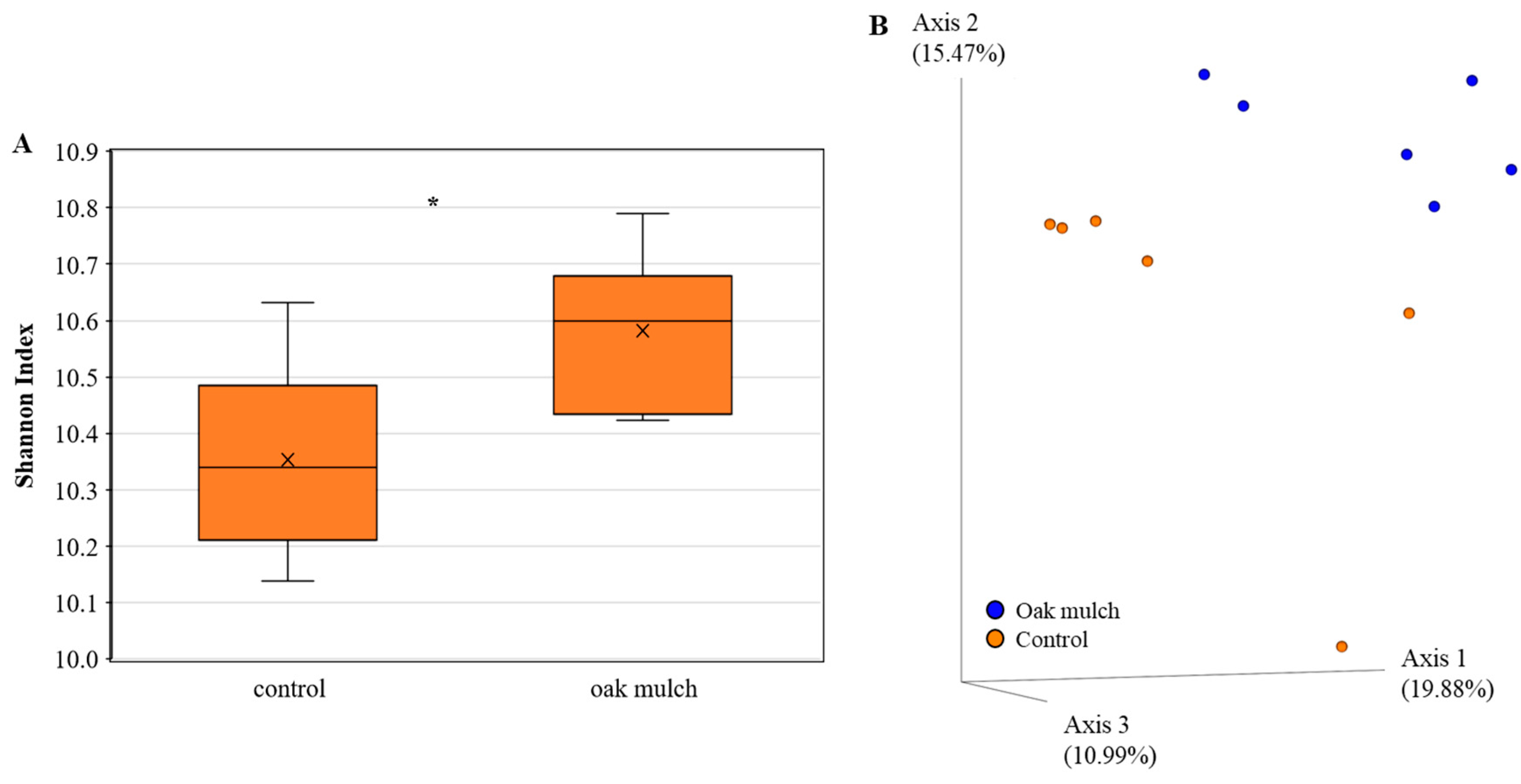
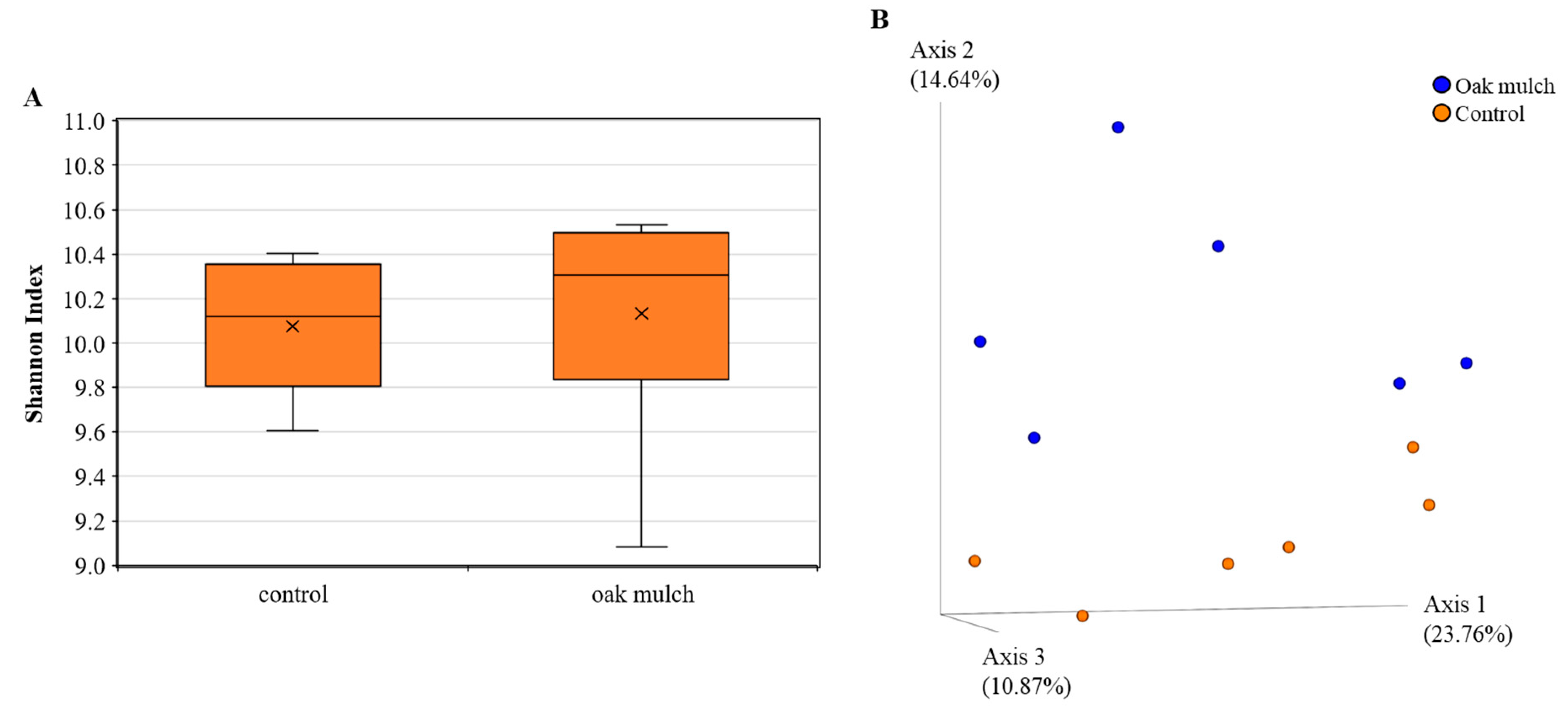
3.3. Impact of Oak Mulch on Rhizosphere Bacterial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozores-Hampton, M.; Stansly, P.A.; McSorley, R.; Obreza, T.A. Effects of Long-term Organic Amendments and Soil Solarization on Pepper and Watermelon Growth, Yield, and Soil Fertility. HortScience 2005, 40, 80–84. [Google Scholar] [CrossRef]
- Singerman, A.; Useche, P. Impact of Citrus Greening on Citrus Operations in Florida; FE983; University of Florida, Institute of Food and Agriculture Sciences, Electronic Data Information Source (UF/IFAS EDIS): Gainesville, FL, USA, 2016; Available online: https://edis.ifas.ufl.edu/publication/FE983 (accessed on 15 October 2023).
- Singerman, A.; Burani-Arouca, M.; Futch, S.H. The Profitability of New Citrus Plantings in Florida in the Era of Huanglongbing. HortScience 2018, 53, 1655–1663. [Google Scholar] [CrossRef]
- Zapien-Macias, J.M.; Ferrarezi, R.S.; Spyke, P.D.; Castle, W.S.; Gmitter, F.G.; Grosser, J.W.; Rossi, L. Early Performance of Recently Released Rootstocks with Grapefruit, Navel Orange, and Mandarin Scions under Endemic Huanglongbing Conditions in Florida. Horticulturae 2022, 8, 1027. [Google Scholar] [CrossRef]
- Achor, D.; Etxeberria, E.; Wang, N. Citrus affected with huanglongbing disease. Plant Pathol. J. 2010, 9, 56–64. [Google Scholar] [CrossRef]
- Kwakye, S.; Kadyampakeni, D.; Morgan, K.; Vashisth, T.; Wright, A. Effects of Iron Rates on Growth and Development of Young Huanglongbing-affected Citrus Trees in Florida. HortScience 2022, 57, 1092–1098. [Google Scholar] [CrossRef]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘CandidatusLiberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Louzada, E.S.; Vazquez, O.E.; Braswell, W.E.; Yanev, G.; Devanaboina, M.; Kunta, M. Distribution of ‘Candidatus Liberibacter asiaticus’ Above and Below Ground in Texas Citrus. Phytopathology 2016, 106, 702–709. [Google Scholar] [CrossRef]
- Bamdad, H.; Papari, S.; Lazarovits, G.; Berruti, F. Soil amendments for sustainable agriculture: Microbial organic fertilizers. Soil Use Manag. 2022, 38, 94–120. [Google Scholar] [CrossRef]
- Deng, S.; Wipf, H.M.L.; Pierroz, G.; Raab, T.K.; Khanna, R.; Coleman-Derr, D. A Plant Growth-Promoting Microbial Soil Amendment Dynamically Alters the Strawberry Root Bacterial Microbiome. Sci. Rep. 2019, 9, 17677. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Belal, E.E.; Rady, M.O.A.; Abd El-Mageed, S.A.; Mansour, E.; Awad, M.F.; Semida, W.M. Acidified Biochar as a Soil Amendment to Drought Stressed (Vicia faba L.) Plants: Influences on Growth and Productivity, Nutrient Status, and Water Use Efficiency. Agronomy 2021, 11, 1290. [Google Scholar] [CrossRef]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of Nutrient-Enriched Biochar as a Soil Amendment during Maize Growth: Exploring Practical Alternatives to Recycle Agricultural Residuals and to Reduce Chemical Fertilizer Demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef]
- Changxun, G.; Zhiyong, P.; Shu’Ang, P. Effect of biochar on the growth of Poncirus trifoliata (L.) Raf. seedlings in Gannan acidic red soil. Soil Sci. Plant Nutr. 2016, 62, 194–200. [Google Scholar] [CrossRef]
- Antoniou, A.; Tsolakidou, M.; Stringlis, I.; Pantelides, I. Rhizosphere Microbiome Recruited from a Suppressive Compost Improves Plant Fitness and Increases Protection against Vascular Wilt Pathogens of Tomato. Front. Plant Sci. 2017, 8, 2022. [Google Scholar] [CrossRef]
- Hallman, L.; Santiago, J.; Rossi, L. An Update on Oak Mulch to Increase Soil Health. 2022. Available online: https://citrusindustry.net/2022/06/06/an-update-on-oak-mulch-to-increase-soil-health/ (accessed on 15 October 2023).
- Pitino, M.; Sturgeon, K.; Dorado, C.; Cano, L.M.; Manthey, J.A.; Shatters, R.G.; Rossi, L. Quercus leaf extracts display curative effects against Candidatus Liberibacter asiaticus that restore leaf physiological parameters in HLB-affected citrus trees. Plant Physiol. Biochem. 2020, 148, 70–79. [Google Scholar] [CrossRef]
- Hallman, L.M.; Santiago, J.M.; Fox, J.-P.; Pitino, M.; Shatters, R.G.; Rossi, L. Use of hardwood mulch applications to improve soil characteristics of Alfisols used in Florida citrus production. Front. Soil Sci. 2023, 3, 1200847. [Google Scholar] [CrossRef]
- Mulumba, L.N.; Lal, R. Mulching effects on selected soil physical properties. Soil Tillage Res. 2008, 98, 106–111. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Ulbrich, T.; Rivas-Ubach, A.; Tiemann, L.; Friesen, M.; Evans, S. Plant root exudates and rhizosphere bacterial communities shift with neighbor context. Soil Biol. Biochem. 2022, 172, 108753. [Google Scholar] [CrossRef]
- Han, M.; Chen, Y.; Sun, L.; Yu, M.; Li, R.; Li, S.; Su, J.; Zhu, B. Linking rhizosphere soil microbial activity and plant resource acquisition strategy. J. Ecol. 2023, 111, 875–888. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Pantigoso, H.; Newberger, D.; Vivanco, J. The rhizosphere microbiome: Plant–microbial interactions for resource acquisition. Appl. Microbiol. 2022, 133, 2864–2876. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Bisceglie, F.; Pelosi, G.; Bonati, B.; Cardarelli, M.; Antenozio, M.L.; Degola, F.; Visioli, G. Phyto-Beneficial Traits of Rhizosphere Bacteria: In Vitro Exploration of Plant Growth Promoting and Phytopathogen Biocontrol Ability of Selected Strains Isolated from Harsh Environments. Plants 2022, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- David, B.; Chandrasehar, G.; Selvam, P. Pseudomonas fluorescens: A PlantGrowth-Promoting Rhizobacterium (PGPR) With Potential Role in Biocontrol of Pests of Crops. In Crop Improvement through Microbial Biotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–243. [Google Scholar]
- Kumar, A.; Prakash, A.; Johri, B.N. Bacillus as PGPR in Crop Ecosystem. In Bacteria in Agrobiology: Crop Ecosystems; Springer: Berlin/Heidelberg, Germany, 2011; pp. 37–59. [Google Scholar]
- Zhang, Y.; Xu, J.; Riera, N.; Jin, T.; Li, J.; Wang, N. Huanglongbing impairs the rhizosphere-to-rhizoplane enrichment process of the citrus root-associated microbiome. Microbiome 2017, 5, 97. [Google Scholar] [CrossRef]
- Isaac, R.A.; Johnson, W.C. Elemental Analysis of Plant Tissue by Plasma Emission Spectroscopy: Collaborative Study. J. Assoc. Off. Anal. Chem. 2020, 68, 499–505. [Google Scholar] [CrossRef]
- Bhatta, A.; Prasad, R.; Chakraborty, D.; Shaw, J.N.; Lamba, J.; Brantley, E.; Torbert, H.A. Mehlich 3 as a generic soil test extractant for environmental phosphorus risk assessment across Alabama soil regions. Agrosyst. Geosci. Environ. 2021, 4, e20187. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P.; Mahmoud, K. Ammonium and Nitrate Distribution in Soil Using Drip and Microsprinkler Irrigation for Citrus Production. Soil Sci. Soc. Am. J. 2014, 78, 645–654. [Google Scholar] [CrossRef]
- Santiago, J.M.; Fox, J.-P.; Guzmán, S.M.; Rossi, L. Effect of fabric mulch ground covers on lemon trees rhizosphere microbiome in Florida flatwood soils. Front. Soil Sci. 2023, 3, 1110370. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Zaman, N.R.; Chowdhury, F.T.; Khhan, H.; Islam, M.R. Plant Microbiome Diversity and Potential for Crops and Sustainable Agriculture. Plant Microbiome Plant Product. Sustain. Agric. 2023, 37, 331–364. [Google Scholar]
- Gumbrewicz, R.; Calderwood, L. Comparison of Wood Mulch Particle Sizes for Wild Blueberry Management in a Changing Climate. Int. J. Fruit Sci. 2022, 22, 551–567. [Google Scholar] [CrossRef]
- Chopra, M.; Koul, B. Comparative assessment of different types of mulching in various crops: A review. Plant Arch 2020, 20, 1620–1626. [Google Scholar]
- Hassan, M.; McInroy, J.; Kloepper, J. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Wu, D.; Ma, Y.; Yang, T.; Gao, G.; Wang, D.; Guo, X.; Chu, H. Phosphorus and Zinc Are Strongly Associated with Belowground Fungal Communities in Wheat Field under Long-Term Fertilization. Microbiol. Spectr. 2022, 10, e0011022. [Google Scholar] [CrossRef] [PubMed]
- Nisrina, L.; Effendi, Y.; Pancoro, A. Revealing the role of Plant Growth Promoting Rhizobacteria in suppressive soils against Fusarium oxysporum f.sp. cubense based on metagenomic analysis. Heliyon 2021, 7, e07636. [Google Scholar] [CrossRef]
- Wu, X.; Rensing, C.; Han, D.; Xiao, K.-Q.; Dai, Y.; Tang, Z.; Liesack, W.; Peng, J.; Cui, Z.; Zhang, F. Genome-Resolved Metagenomics Reveals Distinct Phosphorus Acquisition Strategies between Soil Microbiomes. mSystems 2022, 7, e0110721. [Google Scholar] [CrossRef]
- Boyle, J.J.; Shann, J.R. The Influence of Planting and Soil Characteristics on Mineralization of 2,4,5-T in Rhizosphere Soil. J. Environ. Qual. 1998, 27, 704–709. [Google Scholar] [CrossRef]
- Alawiye, T.; Babalola, O. Bacterial Diversity and Community Structure in Typical Plant Rhizosphere. Diversity 2019, 11, 179. [Google Scholar] [CrossRef]
- Liang, T.; Yang, G.; Ma, Y.; Yao, Q.; Ma, Y.; Ma, H.; Hu, Y.; Yang, Y.; Wang, S.; Pan, Y.; et al. Seasonal dynamics of microbial diversity in the rhizosphere of Ulmus pumila L. var. sabulosa in a steppe desert area of Northern China. PeerJ 2019, 7, e7526. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.R.; Mustafa, Z.; Viliunas, J. Soil microbes alter plant fitness under competition and drought. J. Evol. Biol. 2019, 32, 438–450. [Google Scholar] [CrossRef]
- Merino-Martín, L.; Stokes, A.; Gweon, H.S.; Moragues-Saitua, L.; Staunton, S.; Plassard, C.; Oliver, A.; Le Bissonnais, Y.; Griffiths, R.I. Interacting effects of land use type, microbes and plant traits on soil aggregate stability. Soil Biol. Biochem. 2021, 154, 108072. [Google Scholar] [CrossRef]
- Maurer, D.; Malique, F.; Alfarraj, S.; Albasher, G.; Horn, M.A.; Butterbach-Bahl, K.; Dannenmann, M.; Rennenberg, H. Interactive regulation of root exudation and rhizosphere denitrification by plant metabolite content and soil properties. Plant Soil 2021, 467, 107–127. [Google Scholar] [CrossRef]
- Zhang, H.; Phillip, F.; Wu, L.; Zhao, F.; Yu, S.; Yu, K. Effects of Temperature and Nitrogen Application on Carbon and Nitrogen Accumulation and Bacterial Community Composition in Apple Rhizosphere Soil. Front. Plant Sci. 2022, 13, 859395. [Google Scholar] [CrossRef]
- Huang, S.; Cui, X.; Xu, Z.; Zhang, Z.; Wang, X. Nitrogen addition exerts a stronger effect than elevated temperature on soil available nitrogen and relation to soil microbial properties in the rhizosphere of Camellia sinensis L. seedlings. Environ. Sci. Pollut. Res. 2022, 29, 35179–35192. [Google Scholar] [CrossRef]
- Wan, W.; Tan, J.; Wang, Y.; Qin, Y.; He, H.; Wu, H.; Zuo, W.; He, D. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci. Total Environ. 2020, 700, 134418. [Google Scholar] [CrossRef]
- Babalola, O.O.; Emmanuel, O.C.; Adeleke, B.S.; Odelade, K.A.; Nwachukwu, B.C.; Ayiti, O.E.; Adegboyega, T.T.; Igiehon, N.O. Rhizosphere Microbiome Cooperations: Strategies for Sustainable Crop Production. Curr. Microbiol. 2021, 78, 1069–1085. [Google Scholar] [CrossRef]



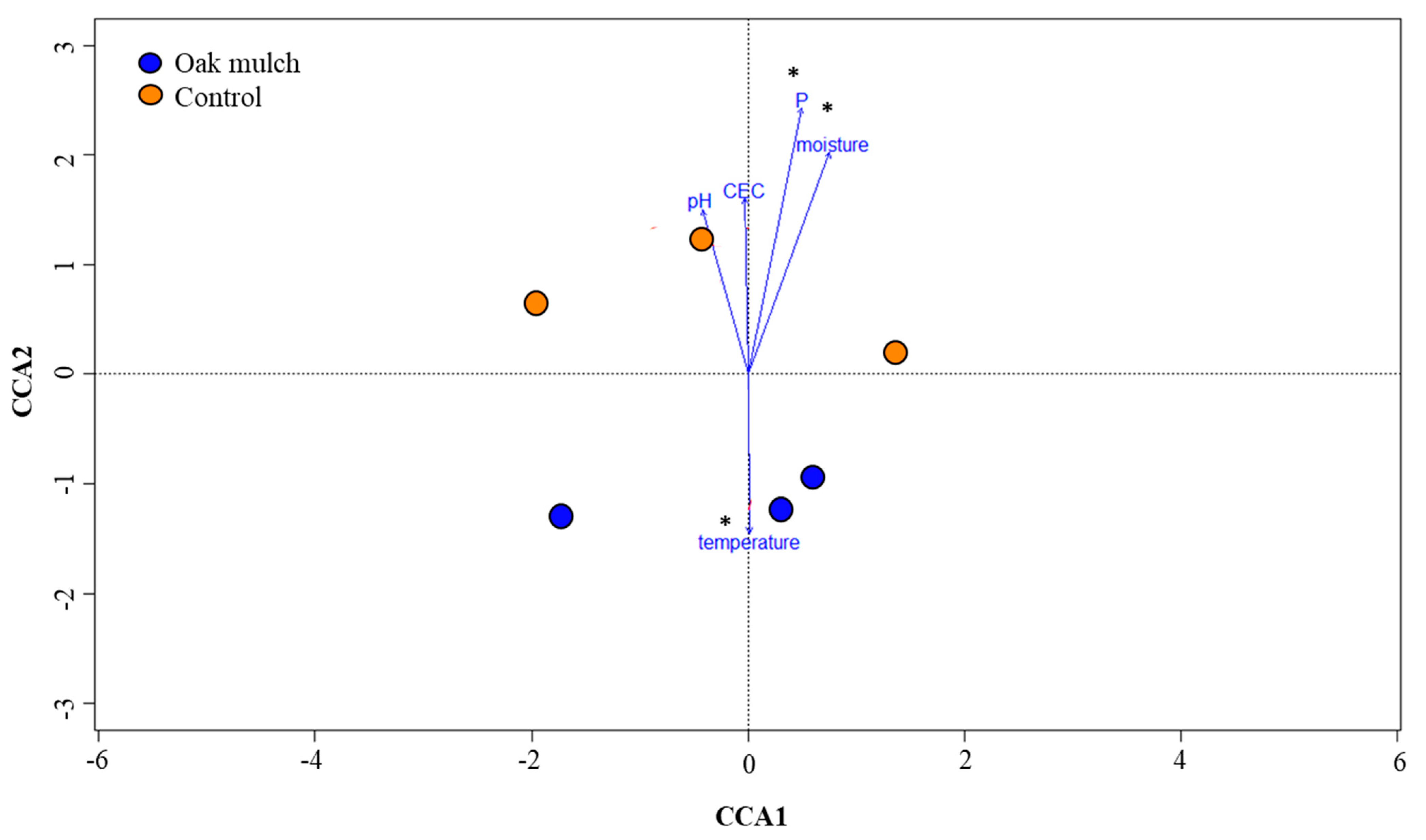
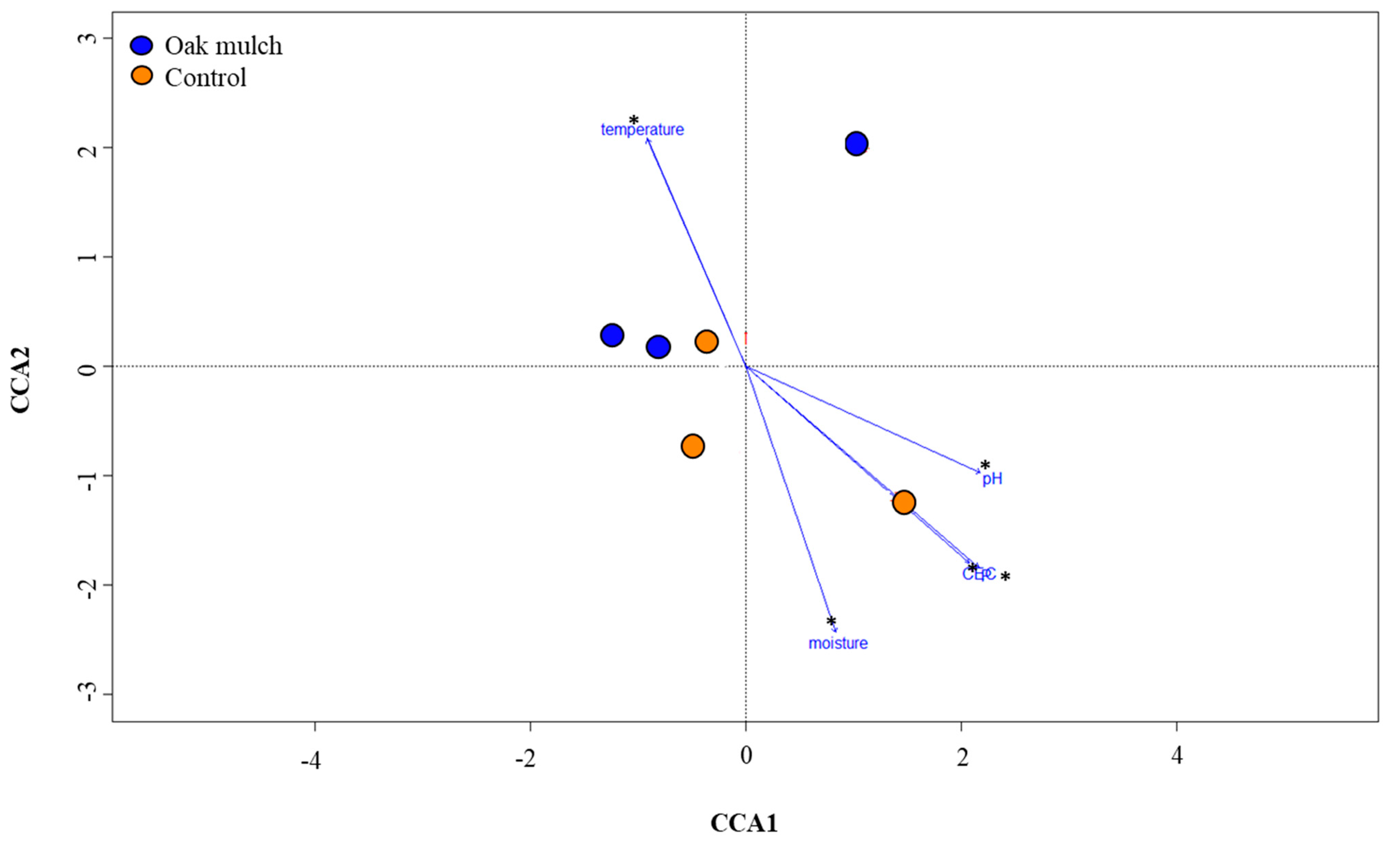
| Bacteria | Oak Mulch | Control | p-Value |
|---|---|---|---|
| Rhizobiales | 15.78% | 11.20% | 0.006 |
| Gemmatimonadales | 2.26% | 4.18% | 0.05 |
| Vicinamibacterales | 5.43% | 4.01% | 0.05 |
| Pirellulales | 1.13% | 2.82% | 0.03 |
| Nitrospirales | 1.13% | 2.14% | 0.05 |
| Frankiales | 0.34% | 1.12% | 0.003 |
| Haliangiales | 0.67% | 1.00% | 0.03 |
| Planctomycetales | 1.60% | 0.97% | 0.01 |
| Latescibacterota | 0.29% | 0.75% | 0.003 |
| Reyranellales | 0.73% | 0.32% | 0.03 |
| Nitrosotaleales | 0.03% | 0.32% | 0.05 |
| Caldilineales | 0.35% | 0.19% | 0.02 |
| Entotheonellales | 0.34% | 0.19% | 0.01 |
| Thermoanaerobaculales | 0.31% | 0.17% | 0.05 |
| Flavobacteriales | 0.27% | 0.14% | 0.03 |
| Bdellovibrionales | 0.24% | 0.12% | 0.003 |
| Kapabacteriales | 0.05% | 0.10% | 0.04 |
| Kallotenuales | 0.03% | 0.09% | 0.04 |
| Pseudomonadales | 0.26% | 0.07% | 0.006 |
| Azospirillales | 0.01% | 0.04% | 0.03 |
| Babeliales | 0.32% | 0.02% | 0.02 |
| Bacteria | Oak Mulch | Control | p-Value |
|---|---|---|---|
| Rhizobiales | 14.28% | 10.65% | 0.05 |
| Burkholderiales | 5.74% | 7.36% | 0.05 |
| Gemmatimonadales | 2.28% | 4.70% | 0.006 |
| Rokubacteriales | 1.28% | 3.04% | 0.02 |
| Nitrospirales | 1.15% | 2.38% | 0.02 |
| Solirubrobacterales | 3.48% | 2.25% | 0.02 |
| Latescibacterota | 0.23% | 0.94% | 0.02 |
| Tepidisphaerales | 0.06% | 0.34% | 0.02 |
| Reyranellales | 0.67% | 0.34% | 0.05 |
| Diplorickettsiales | 0.48% | 0.23% | 0.02 |
| Dadabacteriales | 0.07% | 0.22% | 0.05 |
| Fimbriimonadales | 0.07% | 0.19% | 0.05 |
| Bdellovibrionales | 0.28% | 0.16% | 0.05 |
| Myxococcales | 0.28% | 0.17% | 0.04 |
| Kapabacteriales | 0.06% | 0.13% | 0.05 |
| Longimicrobiales | 0.02% | 0.07% | 0.05 |
| Streptosporangiales | 0.15% | 0.06% | 0.04 |
| Deinococcales | 0.04% | 0.005% | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, J.M.; Hallman, L.M.; Fox, J.-P.; Pitino, M.; Shatters, R.G., Jr.; Cano, L.M.; Rossi, L. Impacts of Oak Mulch Amendments on Rhizosphere Microbiome of Citrus Trees Grown in Florida Flatwood Soils. Microorganisms 2023, 11, 2764. https://doi.org/10.3390/microorganisms11112764
Santiago JM, Hallman LM, Fox J-P, Pitino M, Shatters RG Jr., Cano LM, Rossi L. Impacts of Oak Mulch Amendments on Rhizosphere Microbiome of Citrus Trees Grown in Florida Flatwood Soils. Microorganisms. 2023; 11(11):2764. https://doi.org/10.3390/microorganisms11112764
Chicago/Turabian StyleSantiago, John M., Lukas M. Hallman, John-Paul Fox, Marco Pitino, Robert G. Shatters, Jr., Liliana M. Cano, and Lorenzo Rossi. 2023. "Impacts of Oak Mulch Amendments on Rhizosphere Microbiome of Citrus Trees Grown in Florida Flatwood Soils" Microorganisms 11, no. 11: 2764. https://doi.org/10.3390/microorganisms11112764
APA StyleSantiago, J. M., Hallman, L. M., Fox, J.-P., Pitino, M., Shatters, R. G., Jr., Cano, L. M., & Rossi, L. (2023). Impacts of Oak Mulch Amendments on Rhizosphere Microbiome of Citrus Trees Grown in Florida Flatwood Soils. Microorganisms, 11(11), 2764. https://doi.org/10.3390/microorganisms11112764







