A Review of the Past, Present, and Future of the Monkeypox Virus: Challenges, Opportunities, and Lessons from COVID-19 for Global Health Security
Abstract
:1. Monkeypox Virus: An Introduction
2. Mechanism of Cell Entry and Viral Replication
3. Clinical Presentation
4. Transmission and Cross-Reactivity
5. A Potential Public Health Crisis: What Is Yet to Be Discovered about MPV?
6. Vaccine Development and Distribution
7. Monkeypox Outbreak and Impact on Health Systems
8. Lessons Learned from the COVID-19 Pandemic
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lum, F.M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Adnan, N.; Haq Z ul Malik, A.; Mehmood, A.; Ishaq, U.; Faraz, M.; Malik, J.; Mehmoodi, A. Human monkeypox virus: An updated review. Medicine 2022, 101, e30406. [Google Scholar] [CrossRef] [PubMed]
- Multi-Country Monkeypox Outbreak in Non-Endemic Countries. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 (accessed on 2 October 2023).
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.W.; Kabamba, J.; McCollum, A.M.; Lushima, R.S.; Wemakoy, E.O.; Muyembe Tamfum, J.J.; Nguete, B.; Hughes, C.M.; Monroe, B.P.; Reynolds, M.G. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral Res. 2019, 162, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.L.; Szczeniowski, M.; Esteves, K. Re-emergence of monkeypox in Africa: A review of the past six years. Br. Med. Bull. 1998, 54, 693–702. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Fine, P.E.; Jezek, Z.; Grab, B.; Dixon, H. The Transmission Potential of Monkeypox Virus in Human Populations. Int. J. Epidemiol. 1988, 17, 643–650. [Google Scholar] [CrossRef]
- Kmiec, D.; Kirchhoff, F. Monkeypox: A New Threat? Int. J. Mol. Sci. 2022, 23, 7866. [Google Scholar] [CrossRef]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Nigeria Centre for Disease Control and Prevention. Available online: https://ncdc.gov.ng/diseases/sitreps/?cat=8&name=An%20Update%20of%20Monkeypox%20Outbreak%20in%20Nigeria (accessed on 27 September 2023).
- Kraemer, M.U.G.; Tegally, H.; Pigott, D.M.; Dasgupta, A.; Sheldon, J.; Wilkinson, E.; Schultheiss, M.; Han, A.; Oglia, M.; Marks, S.; et al. Tracking the 2022 monkeypox outbreak with epidemiological data in real-time. Lancet Infect. Dis. 2022, 22, 941–942. [Google Scholar] [CrossRef] [PubMed]
- Hirani, R.; Rashid, D.; Lewis, J.; Hosein-Woodley, R.; Issani, A. Monkeypox outbreak in the age of COVID-19: A new global health emergency. Mil. Med. Res. 2022, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S. CNBC. WHO Declares Rapidly Spreading Monkeypox Outbreak a Global Health Emergency. 2022. Available online: https://www.cnbc.com/2022/07/23/who-declares-spreading-monkeypox-outbreak-a-global-health-emergency.html (accessed on 27 September 2023).
- Bhalla, N.; Payam, A.F. Addressing the Silent Spread of Monkeypox Disease with Advanced Analytical Tools. Small 2023, 19, 2206633. [Google Scholar] [CrossRef]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D.; Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. Available online: https://www.cureus.com/articles/100707-monkeypox-a-comprehensive-review-of-transmission-pathogenesis-and-manifestation (accessed on 2 October 2023). [CrossRef]
- Moss, B. Poxvirus Cell Entry: How Many Proteins Does it Take? Viruses 2012, 4, 688–707. [Google Scholar] [CrossRef]
- Rampogu, S.; Kim, Y.; Kim, S.W.; Lee, K.W. An overview on monkeypox virus: Pathogenesis, transmission, host interaction and therapeutics. Front. Cell Infect. Microbiol. 2023, 13, 1076251. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1076251 (accessed on 2 October 2023). [CrossRef]
- Moss, B. Poxvirus DNA Replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a010199. [Google Scholar] [CrossRef]
- Sklenovská NMonkeypox Virus. In Animal-Origin Viral Zoonoses; Malik, Y.S.; Singh, R.K.; Dhama, K. (Eds.) Livestock Diseases and Management; Springer: Singapore, 2020; pp. 39–68. [Google Scholar] [CrossRef]
- Bray, M.; Buller, M. Looking Back at Smallpox. Clin. Infect. Dis. 2004, 38, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.L.; Smith, G.L. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 2008, 16, 472–479. [Google Scholar] [CrossRef]
- Xiang, Y.; White, A. Monkeypox virus emerges from the shadow of its more infamous cousin: Family biology matters. Emerg. Microbes Infect. 2022, 11, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Ježek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human Monkeypox: Clinical Features of 282 Patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef]
- Weinstein, R.A.; Nalca, A.; Rimoin, A.W.; Bavari, S.; Whitehouse, C.A. Reemergence of Monkeypox: Prevalence, Diagnostics, and Countermeasures. Clin. Infect. Dis. 2005, 41, 1765–1771. [Google Scholar] [CrossRef]
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Patrocinio-Jesus, R.; Peruzzu, F. Monkeypox Genital Lesions. N. Engl. J. Med. 2022, 387, 66. [Google Scholar] [CrossRef]
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Eurosurveillance 2022, 27, 2200421. [Google Scholar] [CrossRef]
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka, S.M.; Mulembakani, P.; Rimoin, A.W.; Martin, J.W.; Muyembe, J.-J.T. Maternal and Fetal Outcomes Among Pregnant Women With Human Monkeypox Infection in the Democratic Republic of Congo. J. Infect. Dis. 2017, 216, 824–828. [Google Scholar] [CrossRef]
- Dashraath, P.; Nielsen-Saines, K.; Rimoin, A.; Mattar, C.N.Z.; Panchaud, A.; Baud, D. Monkeypox in pregnancy: Virology, clinical presentation, and obstetric management. Am. J. Obstet. Gynecol. 2022, 227, 849–861.e7. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, P.; Mohanty, P.; Manna, S.; Shamim, M.A.; Rao, P.P.; Aggarwal, A.K.; Khubchandani, J.; Mohanty, A.; Nowrouzi-Kia, B.; Chattu, V.K.; et al. Potentially Asymptomatic Infection of Monkeypox Virus: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 2083. [Google Scholar] [CrossRef] [PubMed]
- Accordini, S.; Cordioli, M.; Pomari, E.; Tacconelli, E.; Castilletti, C. People with asymptomatic or unrecognised infection potentially contribute to monkeypox virus transmission. Lancet Microbe 2023, 4, e209. [Google Scholar] [CrossRef] [PubMed]
- A Brief Review of the Monkeypox Outbreak: Transmission, Presentation, and Developments in Treatment and Vaccines. J. Immunol. Sci. 2023, 7, 1–14. Available online: https://www.immunologyresearchjournal.com/articles/a-brief-review-of-the-monkeypox-outbreak-transmission-presentation-and-developments-in-treatment-and-vaccines.html (accessed on 27 September 2023).
- Outbreak of Human Monkeypox, Democratic Republic of Congo, 1996 to 1997—Volume 7, Number 3—June 2001—Emerging Infectious Diseases Journal—CDC. Available online: https://wwwnc.cdc.gov/eid/article/7/3/01-7311_article (accessed on 27 September 2023).
- Walter, K.; Malani, P.N. What Is Monkeypox? JAMA 2022, 328, 222. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, Y.; Wang, L. Can monkeypox virus be transmitted through the air?—Correspondence. Int. J. Surg. Lond. Engl. 2022, 108, 106995. [Google Scholar] [CrossRef]
- Milton, D.K. What was the primary mode of smallpox transmission? Implications for biodefense. Front. Cell Infect. Microbiol. 2012, 2, 150. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Kelley, C.F.; Kraft, C.S.; de Man, T.J.; Duphare, C.; Lee, H.W.; Yang, J.; Easley, K.A.; Tharp, G.K.; Mulligan, M.J.; Sullivan, P.S.; et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: Implications for HIV transmission and prevention. Mucosal Immunol. 2017, 10, 996–1007. [Google Scholar] [CrossRef]
- Mikulak, J.; Di Vito, C.; Zaghi, E.; Mavilio, D. Host Immune Responses in HIV-1 Infection: The Emerging Pathogenic Role of Siglecs and Their Clinical Correlates. Front. Immunol. 2017, 8, 314. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2017.00314 (accessed on 27 September 2023). [CrossRef] [PubMed]
- Dubois, M.E.; Slifka, M.K. Retrospective Analysis of Monkeypox Infection. Emerg. Infect. Dis. 2008, 14, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; He, C.; Liu, M.; Yuan, P.; Tian, S.; Zheng, M.; Zhang, L.; Zhou, X.; Xu, F.; Luo, J.; et al. Cross-reactive immune responses to monkeypox virus induced by MVA vaccination in mice. Virol. J. 2023, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.; Christie, R.; Paton, R.S.; Cumming, F.; Overton, C.E. Transmission dynamics of monkeypox in the United Kingdom: Contact tracing study. BMJ 2022, 379, e073153. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.; Payne, J.M.; Olguin, V.L.; Sanders, M.P.; Clements, T.; Stefan, C.P.; Williams, J.A.; Hooper, J.W.; Huggins, J.W.; Mucker, E.M.; et al. Development of a specific MPXV antigen detection immunodiagnostic assay. Front. Microbiol. 2023, 14, 1243523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, H.; Jiang, S.; Dong, T.; Wang, X.; Wang, Y.; Li, Y. Rapid Detection of the Monkeypox Virus Genome and Antigen Proteins Based on Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2023, 15, 34419–34426. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Veronese, N.; Marotta, C.; Shin, J.I.; Koyanagi, A.; Silenzi, A.; Antunes, M.; Saracino, A.; Bavaro, D.F.; Soysal, P.; et al. Human Monkeypox: A Comprehensive Narrative Review and Analysis of the Public Health Implications. Microorganisms 2022, 10, 1633. [Google Scholar] [CrossRef]
- Hirani, R.; Hosein-Woodley, R.; Rashid, D.; Drugge, E.D.; Etienne, M. Monkeypox Outbreak 2022: Disparities and Prevention. J. Hosp. Infect. 2022, 133, 105–106. Available online: https://www.journalofhospitalinfection.com/article/S0195-6701(22)00382-6/fulltext (accessed on 16 December 2022). [CrossRef]
- Gessain, A.; Nakoune, E.; Yazdanpanah, Y. Monkeypox. N. Engl. J. Med. 2022, 387, 1783–1793. [Google Scholar] [CrossRef]
- Ranganath, N.; Tosh, P.K.; O’Horo, J.; Sampathkumar, P.; Binnicker, M.J.; Shah, A.S. Monkeypox 2022: Gearing Up for Another Potential Public Health Crisis. Mayo Clin. Proc. 2022, 97, 1694–1699. [Google Scholar] [CrossRef]
- Schneider, K.A.; Eichner, M. Does it matter who is spreading monkeypox? Lancet Infect. Dis. 2022, 22, 1266–1267. [Google Scholar] [CrossRef]
- Rojek, A.; Dunning, J.; Olliaro, P. Monkeypox: How will we know if the treatments work? Lancet Infect. Dis. 2022, 22, 1269–1270. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Herrera, C.; Sabino-Santos, G. Monkeypox Virus Evolution before 2022 Outbreak—Volume 29, Number 2—February 2023—Emerging Infectious Diseases Journal—CDC. Available online: https://wwwnc.cdc.gov/eid/article/29/2/22-0962_article (accessed on 9 October 2023).
- Stanford, M.M.; McFadden, G.; Karupiah, G.; Chaudhri, G. Immunopathogenesis of poxvirus infections: Forecasting the impending storm. Immunol. Cell Biol. 2007, 85, 93–102. [Google Scholar] [CrossRef]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694.e9. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.B.; Ovsyannikova, I.; Poland, G.A. Smallpox Vaccines for Biodefense. Vaccine 2009, 27, D73–D79. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Naleway, A.L. Smallpox Vaccine: The Good, the Bad, and the Ugly. Clin. Med. Res. 2003, 1, 87–92. [Google Scholar] [CrossRef]
- Sudarmaji, N.; Kifli, N.; Hermansyah, A.; Yeoh, S.F.; Goh, B.H.; Ming, L.C. Prevention and Treatment of Monkeypox: A Systematic Review of Preclinical Studies. Viruses 2022, 14, 2496. [Google Scholar] [CrossRef]
- History of Smallpox Vaccination. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/history-of-smallpox-vaccination (accessed on 29 October 2023).
- Pugh, C.; Keasey, S.; Korman, L.; Pittman, P.R.; Ulrich, R.G. Human Antibody Responses to the Polyclonal Dryvax Vaccine for Smallpox Prevention Can Be Distinguished from Responses to the Monoclonal Replacement Vaccine ACAM2000. Clin. Vaccine Immunol. CVI 2014, 21, 877–885. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.E.; Fernández-Quezada, D.; Casillas-Muñoz, F.A.G.; Carrillo-Ballesteros, F.J.; Ortega-Prieto, A.M.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A. Human Monkeypox: A Comprehensive Overview of Epidemiology, Pathogenesis, Diagnosis, Treatment, and Prevention Strategies. Pathogens 2023, 12, 947. [Google Scholar] [CrossRef]
- Aragón, T.J.; Ulrich, S.; Fernyak, S.; Rutherford, G.W. Risks of serious complications and death from smallpox vaccination: A systematic review of the United States experience, 1963–1968. BMC Public Health 2003, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Guidelines for Smallpox Vaccine (Vaccinia) Adverse Reactions. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5501a1.htm (accessed on 29 October 2023).
- Michael Lane, J.; Ruben, F.L.; Neff, J.M.; Millar, J.D. Complications of Smallpox Vaccination, 1968: Results of Ten Statewide Surveys. J. Infect. Dis. 1970, 122, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Women with Smallpox Vaccine Exposure during Pregnancy Reported to the National Smallpox Vaccine in Pregnancy Registry—United States. 2003. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5217a3.htm (accessed on 29 October 2023).
- Petersen, B.W.; Harms, T.J.; Reynolds, M.G.; Harrison, L.H. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses—Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States. 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 734. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm7122e1.htm (accessed on 28 September 2023). [CrossRef]
- Greenberg, R.N.; Hurley, Y.; Dinh, D.V.; Mraz, S.; Vera, J.G.; von Bredow, D.; von Krempelhuber, A.; Roesch, S.; Virgin, G.; Arndtz-Wiedemann, N.; et al. A Multicenter, Open-Label, Controlled Phase II Study to Evaluate Safety and Immunogenicity of MVA Smallpox Vaccine (IMVAMUNE) in 18–40 Year Old Subjects with Diagnosed Atopic Dermatitis. PLoS ONE 2015, 10, e0138348. [Google Scholar]
- Overton, E.T.; Lawrence, S.J.; Wagner, E.; Nopora, K.; Rösch, S.; Young, P.; Schmidt, D.; Kreusel, C.; Carli, S.D.; Meyer, T.P.; et al. Immunogenicity and safety of three consecutive production lots of the non replicating smallpox vaccine MVA: A randomised, double blind, placebo controlled phase III trial. PLoS ONE 2018, 13, e0195897. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann-Roth, E.M.; von Sonnenburg, F.; de la Motte, S.; Arndtz-Wiedemann, N.; von Krempelhuber, A.; Uebler, N.; Vollmar, J.; Virgin, G.; Chaplin, P. Cardiac Safety of Modified Vaccinia Ankara for Vaccination against Smallpox in a Young, Healthy Study Population. PLoS ONE 2015, 10, e0122653. [Google Scholar] [CrossRef]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.H.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef]
- Ilchmann, H.; Samy, N.; Reichhardt, D.; Schmidt, D.; Powell, J.D.; Meyer, T.P.H.; Silbernagl, G.; Nichols, R.; Weidenthaler, H.; De Moerlooze, L.; et al. One- and Two-Dose Vaccinations With Modified Vaccinia Ankara-Bavarian Nordic Induce Durable B-Cell Memory Responses Comparable to Replicating Smallpox Vaccines. J. Infect. Dis. 2022, 227, 1203–1213. [Google Scholar] [CrossRef]
- CDC. Centers for Disease Control and Prevention. CDC’s Mpox Pediatric Considerations. 2023. Available online: https://www.cdc.gov/poxvirus/mpox/clinicians/pediatric.html (accessed on 29 October 2023).
- CDC. Centers for Disease Control and Prevention. Mpox Vaccine Recommendations. 2023. Available online: https://www.cdc.gov/poxvirus/mpox/vaccines/vaccine-recommendations.html (accessed on 28 October 2023).
- Overton, E.T.; Lawrence, S.J.; Stapleton, J.T.; Weidenthaler, H.; Schmidt, D.; Koenen, B.; Silbernagl, G.; Nopora, K.; Chaplin, P. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine 2020, 38, 2600–2607. [Google Scholar] [CrossRef]
- Payne, A.B. Reduced Risk for Mpox After Receipt of 1 or 2 Doses of JYNNEOS Vaccine Compared with Risk Among Unvaccinated Persons—43 U.S. Jurisdictions, 31 July–1 October 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1560. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm7149a5.htm (accessed on 23 September 2023). [CrossRef]
- Owens, L.E. JYNNEOS Vaccination Coverage Among Persons at Risk for Mpox—United States, 22 May 2022–31 January 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 342. Available online: https://www.cdc.gov/mmwr/volumes/72/wr/mm7213a4.htm (accessed on 28 September 2023). [CrossRef] [PubMed]
- JYNNEOS Vaccine Distribution by Jurisdiction | SNS | HHS/ASPR. Available online: https://aspr.hhs.gov:443/SNS/Pages/JYNNEOS-Distribution.aspx (accessed on 28 September 2023).
- Kriss, J.L.; Boersma, P.M.; Martin, E.; Reed, K.; Adjemian, J.; Smith, N.; Carter, R.J.; Tan, K.R.; Srinivasan, A.; McGarvey, S.; et al. Receipt of First and Second Doses of JYNNEOS Vaccine for Prevention of Monkeypox—United States, 22 May–10 October 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Philpott, D. Epidemiologic and Clinical Characteristics of Monkeypox Cases—United States, 17 May–22 July 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1018. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm7132e3.htm (accessed on 23 February 2023). [CrossRef] [PubMed]
- Takahashi-Nishimaki, F.; Suzuki, K.; Morita, M.; Maruyama, T.; Miki, K.; Hashizume, S.; Sugimoto, M. Genetic Analysis of Vaccinia Virus Lister Strain and Its Attenuated Mutant LC16m8: Production of Intermediate Variants by Homologous Recombination. J. Gen. Virol. 1987, 68, 2705–2710. [Google Scholar] [CrossRef]
- Morikawa, S.; Sakiyama, T.; Hasegawa, H.; Saijo, M.; Maeda, A.; Kurane, I.; Maeno, G.; Kimura, J.; Hirama, C.; Yoshida, T.; et al. An Attenuated LC16m8 Smallpox Vaccine: Analysis of Full-Genome Sequence and Induction of Immune Protection. J. Virol. 2005, 79, 11873–11891. [Google Scholar] [CrossRef]
- Saijo, M.; Ami, Y.; Suzaki, Y.; Nagata, N.; Iwata, N.; Hasegawa, H.; Ogata, M.; Fukushi, S.; Mizutani, T.; Sata, T.; et al. LC16m8, a Highly Attenuated Vaccinia Virus Vaccine Lacking Expression of the Membrane Protein B5R, Protects Monkeys from Monkeypox. J. Virol. 2006, 80, 5179–5188. [Google Scholar] [CrossRef]
- A Single Vaccination of Nonhuman Primates with Highly Attenuated Smallpox Vaccine, LC16m8, Provides Long-Term Protection against Monkeypox. Available online: https://www.jstage.jst.go.jp/article/yoken/70/4/70_JJID.2016.417/_article (accessed on 28 September 2023).
- Saito, T.; Fujii, T.; Kanatani, Y.; Saijo, M.; Morikawa, S.; Yokote, H.; Takeuchi, T.; Kuwabara, N. Clinical and Immunological Response to Attenuated Tissue-Cultured Smallpox Vaccine LC16m8. JAMA 2009, 301, 1025–1033. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Gurwith, M.; Dekker, C.L.; Frey, S.E.; Edwards, K.M.; Kenner, J.; Lock, M.; Empig, C.; Morikawa, S.; Saijo, M.; et al. Safety and Immunogenicity of LC16m8, an Attenuated Smallpox Vaccine in Vaccinia-Naive Adults. J. Infect. Dis. 2011, 204, 1395–1402. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Sheridan, C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat. Biotechnol. 2021, 39, 1479–1482. [Google Scholar] [CrossRef]
- Williams, J.A. Vector Design for Improved DNA Vaccine Efficacy, Safety and Production. Vaccines 2013, 1, 225–249. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Sang, Y.; Zhang, Z.; Liu, F.; Lu, H.; Yu, C.; Sun, H.; Long, J.; Cao, Y.; Mai, J.; Miao, Y.; et al. Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. Signal Transduct. Target. Ther. 2023, 8, 1–11. [Google Scholar] [CrossRef]
- Fang, Z.; Monteiro, V.S.; Renauer, P.A.; Shang, X.; Suzuki, K.; Ling, X.; Bai, M.; Xiang, Y.; Levchenko, A.; Booth, C.J.; et al. Polyvalent mRNA vaccination elicited potent immune response to monkeypox virus surface antigens. Cell Res. 2023, 33, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cheng, X.; Zhu, Y.; Mo, O.; Yu, H.; Zhu, L.; Zhang, J.; Kuang, L.; Gao, Y.; Cao, R.; et al. Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity. Sci. China Life Sci. 2023, 66, 2329–2341. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Zhang, Y.; Liu, X.; Murad, Y.M.; Xu, J.; Yu, Z.; Hua, X.; Song, Y.; Ding, J.; Huang, H.; et al. mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge. Nat. Commun. 2023, 14, 5925. [Google Scholar] [CrossRef] [PubMed]
- Hirao, L.A.; Draghia-Akli, R.; Prigge, J.T.; Yang, M.; Satishchandran, A.; Wu, L.; Hammarlund, E.; Khan, A.S.; Babas, T.; Rhodes, L.; et al. Multivalent Smallpox DNA Vaccine Delivered by Intradermal Electroporation Drives Protective Immunity in Nonhuman Primates Against Lethal Monkeypox Challenge. J. Infect. Dis. 2011, 203, 95–102. [Google Scholar] [CrossRef]
- Hooper, J.W.; Thompson, E.; Wilhelmsen, C.; Zimmerman, M.; Ichou, M.A.; Steffen, S.E.; Schmaljohn, C.S.; Schmaljohn, A.L.; Jahrling, P.B. Smallpox DNA Vaccine Protects Nonhuman Primates against Lethal Monkeypox. J. Virol. 2004, 78, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Abbas, S.; Rehan, S.T.; Hasan, M.M. Monkeypox virus: A future scourge to the Pakistani Healthcare system. Ann. Med. Surg. 2022, 79, 103978. [Google Scholar] [CrossRef]
- Sahito, A.M.; Saleem, A.; Javed, S.O.; Farooq, M.; Ullah, I.; Hasan, M.M. Polio amidst COVID-19 in Pakistan: Ongoing efforts, challenges, and recommendations. Int. J. Health Plann. Manag. 2022, 37, 1907–1911. [Google Scholar] [CrossRef]
- Yousaf, A.; Khan, F.M.A.; Hasan, M.M.; Ullah, I.; Bardhan, M. Dengue, measles, and COVID-19: A threefold challenge to public health security in Pakistan. Ethics Med. Public. Health 2021, 19, 100704. [Google Scholar] [CrossRef]
- Butt, M.H.; Safdar, A.; Amir, A.; Zaman, M.; Ahmad, A.; Saleem, R.T.; Misbah, S.; Khan, Y.H.; Mallhi, T.H. Arboviral diseases and COVID-19 coincidence: Challenges for Pakistan’s derelict healthcare system. J. Med. Virol. 2021, 93, 6465–6467. [Google Scholar] [CrossRef]
- Alakunle, E.F.; Okeke, M.I. Monkeypox virus: A neglected zoonotic pathogen spreads globally. Nat. Rev. Microbiol. 2022, 20, 507–508. [Google Scholar] [CrossRef]
- Saied, A.A.; Dhawan, M.; Metwally, A.A.; Fahrni, M.L.; Choudhary, P.; Choudhary, O.P. Disease History, Pathogenesis, Diagnostics, and Therapeutics for Human Monkeypox Disease: A Comprehensive Review. Vaccines 2022, 10, 2091. [Google Scholar] [CrossRef]
- Nguyen, P.Y.; Ajisegiri, W.S.; Costantino, V.; Chughtai, A.A.; MacIntyre, C.R. Reemergence of Human Monkeypox and Declining Population Immunity in the Context of Urbanization, Nigeria, 2017–2020. Emerg. Infect. Dis. 2021, 27, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Lahariya, C.; Thakur, A.; Dudeja, N. Monkeypox Disease Outbreak (2022): Epidemiology, Challenges, and the Way Forward. Indian. Pediatr. 2022, 59, 636–642. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 28 September 2023).
- Commissioner of the FDA. FDA Approves First COVID-19 Vaccine. 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 28 September 2023).
- Fernandez Prendes, C.; Del Castro Madrazo, J.A.; Padron Encalada, C.E.; Dominguez, M.R.; Camblor Santervas, L.A.; Perez, M.A. Hybrid Repair of an Innominate Artery Mycotic Aneurysm with an “On-The-Table” Customized Endograft. Ann. Vasc. Surg. 2019, 59, 311.e5–311.e9. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef]
- Khuram, H.; Maddox, P.A.; Hirani, R.; Issani, A. The COVID-19 pandemic and depression among medical students: Barriers and solutions. Prev. Med. 2023, 171, 107365. [Google Scholar] [CrossRef]
- Hirani, R.; Khuram, H.; Sandoval, O.G.; Issani, A. Epidemiological effects of the COVID-19 pandemic on modifiable risk factors for dementia. Int. J. Geriatr. Psychiatry 2023, 38, e5881. [Google Scholar] [CrossRef]
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; McCarthy, S.E.; Gestole, M.C.; Wolfe, N.D.; Fair, J.N.; et al. Genomic Variability of Monkeypox Virus among Humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Swelum, A.A.; Shafi, M.E.; Albaqami, N.M.; El-Saadony, M.T.; Elsify, A.; Abdo, M.; Taha, A.E.; Abdel-Moneim, A.-M.E.; Al-Gabri, N.A.; Almaiman, A.A.; et al. COVID-19 in Human, Animal, and Environment: A Review. Front. Vet. Sci. 2020, 7, 578. Available online: https://www.frontiersin.org/articles/10.3389/fvets.2020.00578 (accessed on 29 October 2023). [CrossRef]
- Mallapaty, S. Did the coronavirus jump from animals to people twice? Nature 2021, 597, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Mpox in Animals and Pets | Mpox | Poxvirus | CDC. 2023. Available online: https://www.cdc.gov/poxvirus/mpox/veterinarian/mpox-in-animals.html (accessed on 29 October 2023).
- Guharoy, R.; Krenzelok, E.P. Lessons from the mismanagement of the COVID-19 pandemic: A blueprint to reform CDC. Am. J. Health Syst. Pharm. 2021, 78, 1739–1741. [Google Scholar] [CrossRef]
- Moré, J.M.; Miller, J.A.; Etienne, M. Disaster Neurology Update. Neurol. Clin. Pract. 2021, 11, 175–178. [Google Scholar] [CrossRef] [PubMed]
- CDC. Centers for Disease Control and Prevention. What to Do if You Have Mpox. 2023. Available online: https://www.cdc.gov/poxvirus/mpox/if-sick/what-to-do.html (accessed on 29 October 2023).
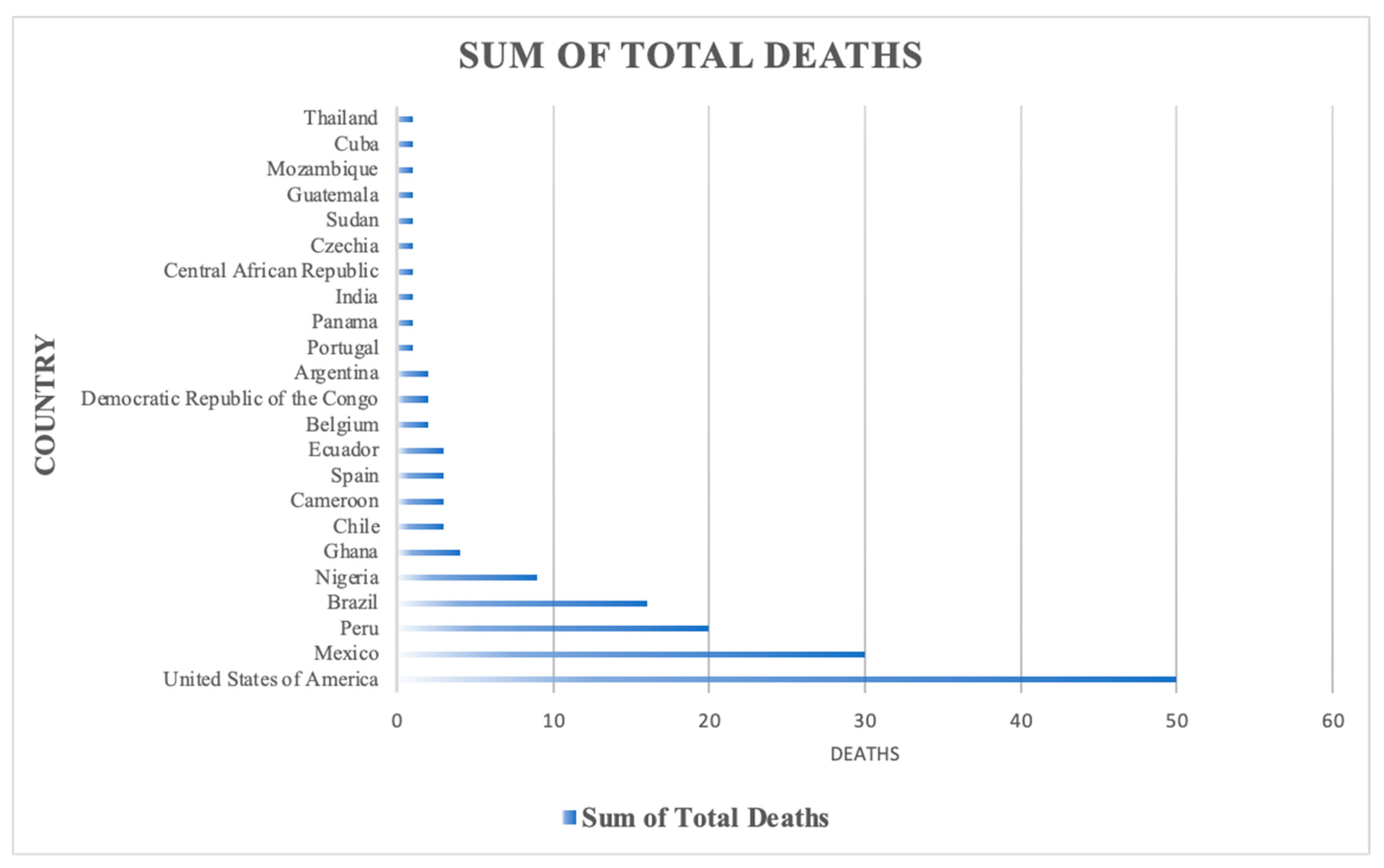

| Country | Sum of Total Confirmed Cases |
|---|---|
| United States of America | 30,636 |
| Brazil | 10,967 |
| Spain | 7580 |
| France | 4154 |
| Colombia | 4090 |
| Mexico | 4062 |
| Peru | 3812 |
| United Kingdom | 3782 |
| Germany | 3703 |
| Canada | 1496 |
| China | 1484 |
| Chile | 1442 |
| Netherlands | 1274 |
| Argentina | 1130 |
| Portugal | 1050 |
| Grand Total | 80,662 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirani, R.; Noruzi, K.; Iqbal, A.; Hussaini, A.S.; Khan, R.A.; Harutyunyan, A.; Etienne, M.; Tiwari, R.K. A Review of the Past, Present, and Future of the Monkeypox Virus: Challenges, Opportunities, and Lessons from COVID-19 for Global Health Security. Microorganisms 2023, 11, 2713. https://doi.org/10.3390/microorganisms11112713
Hirani R, Noruzi K, Iqbal A, Hussaini AS, Khan RA, Harutyunyan A, Etienne M, Tiwari RK. A Review of the Past, Present, and Future of the Monkeypox Virus: Challenges, Opportunities, and Lessons from COVID-19 for Global Health Security. Microorganisms. 2023; 11(11):2713. https://doi.org/10.3390/microorganisms11112713
Chicago/Turabian StyleHirani, Rahim, Kaleb Noruzi, Aroubah Iqbal, Anum S. Hussaini, Rafay A. Khan, Aleksandr Harutyunyan, Mill Etienne, and Raj K. Tiwari. 2023. "A Review of the Past, Present, and Future of the Monkeypox Virus: Challenges, Opportunities, and Lessons from COVID-19 for Global Health Security" Microorganisms 11, no. 11: 2713. https://doi.org/10.3390/microorganisms11112713
APA StyleHirani, R., Noruzi, K., Iqbal, A., Hussaini, A. S., Khan, R. A., Harutyunyan, A., Etienne, M., & Tiwari, R. K. (2023). A Review of the Past, Present, and Future of the Monkeypox Virus: Challenges, Opportunities, and Lessons from COVID-19 for Global Health Security. Microorganisms, 11(11), 2713. https://doi.org/10.3390/microorganisms11112713






