A First in Human Clinical Trial Assessing the Safety and Immunogenicity of Two Intradermally Delivered Enterotoxigenic Escherichia coli CFA/I Fimbrial Tip Adhesin Antigens with and without Heat-Labile Enterotoxin with Mutation LT(R192G)
Abstract
:1. Introduction
2. Materials and Methods
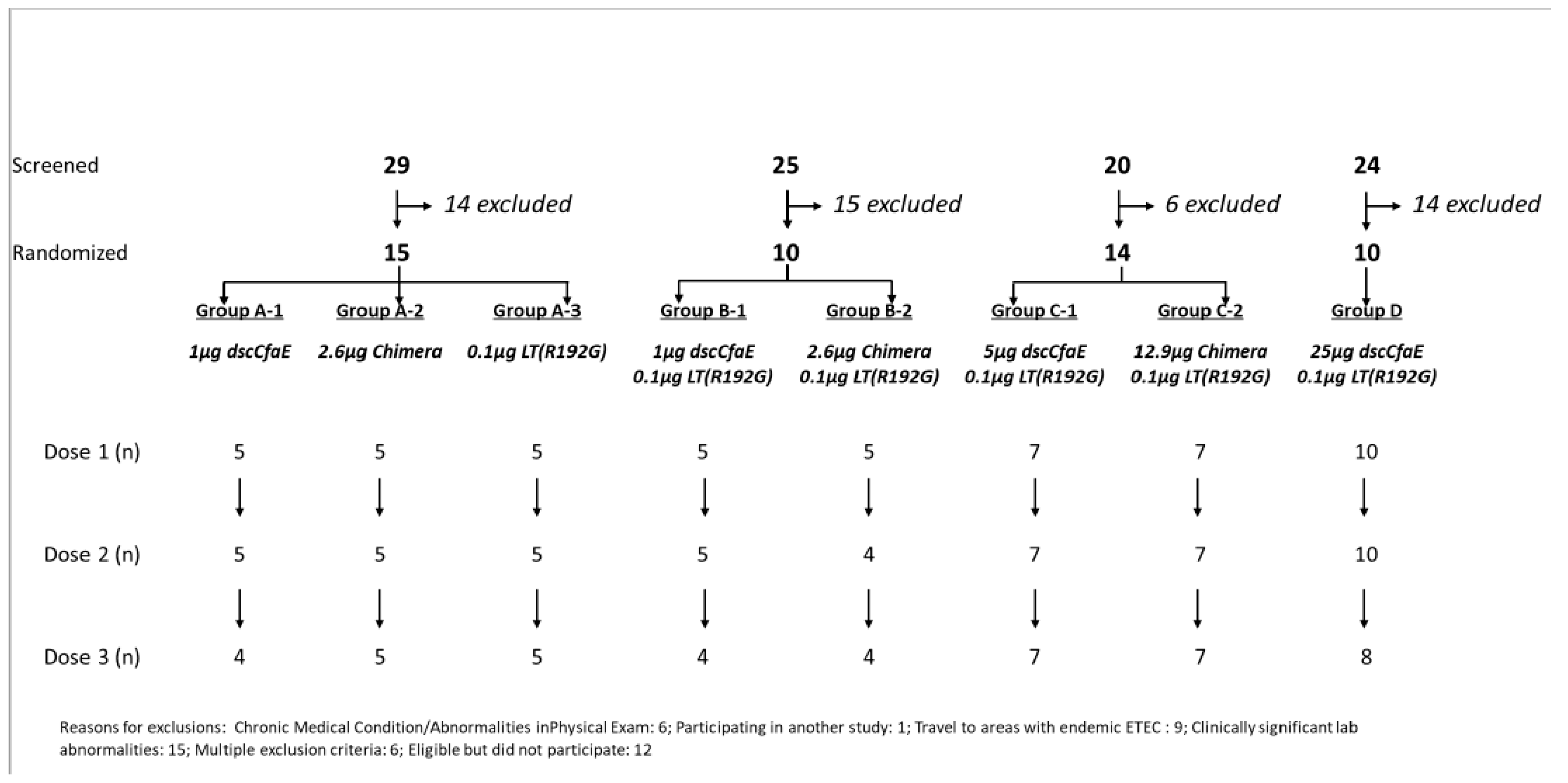
2.1. Clinical Trial Design
2.2. Study Population and Enrollment Criteria
2.3. Manufacture of dscCfaE, Chimera and mLT Vaccine Components
2.4. Vaccine Formulation
2.5. Immunization Procedures
2.6. Safety Assessment
2.7. Immunological Assessments
2.7.1. Antigen-Specific ELISA
2.7.2. Hemagglutination Inhibition Assay (HAI)
2.7.3. ELISPOT
2.8. Study Endpoints and Definitions
2.9. Data Analysis and Statistical Considerations
3. Results
3.1. Demographics
3.2. Safety and Clinical Adverse Events
3.3. Immunological Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Dopfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Lamberti, L.M.; Fischer Walker, C.L.; Black, R.E. Systematic review of diarrhea duration and severity in children and adults in low- and middle-income countries. BMC Public Health 2012, 12, 276. [Google Scholar] [CrossRef]
- mcaKuhlmann, F.M.; Laine, R.O.; Afrin, S.; Nakajima, R.; Akhtar, M.; Vickers, T.; Parker, K.; Nizam, N.N.; Grigura, V.; Goss, C.W.; et al. Contribution of Noncanonical Antigens to Virulence and Adaptive Immunity in Human Infection with Enterotoxigenic E. coli. Infect. Immun. 2021, 89, e00041-21. [Google Scholar] [CrossRef]
- Qadri, F.; Saha, A.; Ahmed, T.; Al Tarique, A.; Begum, Y.A.; Svennerholm, A.M. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect. Immun. 2007, 75, 3961–3968. [Google Scholar] [CrossRef]
- Qadri, F.; Svennerholm, A.M.; Faruque, A.S.; Sack, R.B. Enterotoxigenic Escherichia coli in developing countries: Epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 2005, 18, 465–483. [Google Scholar] [CrossRef]
- Qadri, F.; Khan, A.I.; Faruque, A.S.; Begum, Y.A.; Chowdhury, F.; Nair, G.B.; Salam, M.A.; Sack, D.A.; Svennerholm, A.M. Enterotoxigenic Escherichia coli and Vibrio cholerae diarrhea, Bangladesh, 2004. Emerg. Infect. Dis. 2005, 11, 1104–1107. [Google Scholar] [CrossRef]
- Levine, M.M.; Nasrin, D.; Acacio, S.; Bassat, Q.; Powell, H.; Tennant, S.M.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: Analysis of the GEMS case-control study and 12-month GEMS-1A follow-on study. Lancet Glob. Health 2020, 8, e204–e214. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.; Panchalingham, S.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: A 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). Lancet Glob. Health 2019, 7, e568–e584. [Google Scholar] [CrossRef]
- Hosangadi, D.; Smith, P.G.; Giersing, B.K. Considerations for using ETEC and Shigella disease burden estimates to guide vaccine development strategy. Vaccine 2019, 37, 7372–7380. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef]
- Anderson, J.D.t.; Bagamian, K.H.; Muhib, F.; Amaya, M.P.; Laytner, L.A.; Wierzba, T.; Rheingans, R. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: A modelling analysis. Lancet Glob. Health 2019, 7, e321–e330. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.; Hall, A.; Riddle, M.S.; Porter, C.K. Travelers’ diarrhea: Update on the incidence, etiology and risk in military and similar populations—1990–2005 versus 2005–2015, does a decade make a difference? Trop. Dis. Travel Med. Vaccines 2019, 5, 1. [Google Scholar] [CrossRef]
- Shah, N.; DuPont, H.L.; Ramsey, D.J. Global etiology of travelers’ diarrhea: Systematic review from 1973 to the present. Am. J. Trop. Med. Hyg. 2009, 80, 609–614. [Google Scholar]
- Riddle, M.S.; Sanders, J.W.; Putnam, S.D.; Tribble, D.R. Incidence, etiology, and impact of diarrhea among long-term travelers (U.S. military and similar populations): A systematic review. Am. J. Trop. Med. Hyg. 2006, 74, 891–900. [Google Scholar] [CrossRef]
- Steffen, R.; Hill, D.R.; DuPont, H.L. Traveler’s diarrhea: A clinical review. JAMA 2015, 313, 71–80. [Google Scholar] [CrossRef]
- Riddle, M.S.; Rockabrand, D.M.; Schlett, C.; Monteville, M.R.; Frenck, R.W.; Romine, M.; Ahmed, S.F.; Sanders, J.W. A prospective study of acute diarrhea in a cohort of United States military personnel on deployment to the Multinational Force and Observers, Sinai, Egypt. Am. J. Trop. Med. Hyg. 2011, 84, 59–64. [Google Scholar] [CrossRef]
- Piyaphanee, W.; Kusolsuk, T.; Kittitrakul, C.; Suttithum, W.; Ponam, T.; Wilairatana, P. Incidence and impact of travelers’ diarrhea among foreign backpackers in Southeast Asia: A result from Khao San road, Bangkok. J. Travel Med. 2011, 18, 109–114. [Google Scholar] [CrossRef]
- Svennerholm, A.M.; Wenneras, C.; Holmgren, J.; McConnell, M.M.; Rowe, B. Roles of different coli surface antigens of colonization factor antigen II in colonization by and protective immunogenicity of enterotoxigenic Escherichia coli in rabbits. Infect. Immun. 1990, 58, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sack, D.A. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert. Rev. Vaccines 2012, 11, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.T.; McVeigh, A.L.; Anantha, R.P.; Lee, L.H.; Akay, Y.M.; Pontzer, E.A.; Scott, D.A.; Bullitt, E.; Savarino, S.J. Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol. Microbiol. 2007, 63, 1372–1384. [Google Scholar] [CrossRef]
- Nayak, G.; Ferguson, M.; Tribble, D.R.; Porter, C.K.; Rapena, R.; Marchicelli, M.; Decker, C.F. Cardiac diastolic dysfunction is prevalent in HIV-infected patients. AIDS Patient Care STDS 2009, 23, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Savarino, S.J.; McKenzie, R.; Tribble, D.R.; Porter, C.K.; O’Dowd, A.; Cantrell, J.A.; Sincock, S.A.; Poole, S.T.; DeNearing, B.; Woods, C.M.; et al. Prophylactic Efficacy of Hyperimmune Bovine Colostral Antiadhesin Antibodies Against Enterotoxigenic Escherichia coli Diarrhea: A Randomized, Double-Blind, Placebo-Controlled, Phase 1 Trial. J. Infect. Dis. 2017, 216, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Stoppato, M.; Gaspar, C.; Regeimbal, J.; Nunez, R.G.; Giuntini, S.; Schiller, Z.A.; Gawron, M.A.; Pondish, J.R.; Martin, J.C., 3rd; Schneider, M.I.; et al. Oral administration of an anti-CfaE secretory IgA antibody protects against Enterotoxigenic Escherichia coli diarrheal disease in a nonhuman primate model. Vaccine 2020, 38, 2333–2339. [Google Scholar] [CrossRef]
- Rollenhagen, J.E.; Jones, F.; Hall, E.; Maves, R.; Nunez, G.; Espinoza, N.; O’Dowd, A.; Prouty, M.G.; Savarino, S.J. Establishment, Validation, and Application of a New World Primate Model of Enterotoxigenic Escherichia coli Disease for Vaccine Development. Infect. Immun. 2019, 87, 10–1128. [Google Scholar] [CrossRef]
- Clemens, J.D.; Sack, D.A.; Harris, J.R.; Chakraborty, J.; Neogy, P.K.; Stanton, B.; Huda, N.; Khan, M.U.; Kay, B.A.; Khan, M.R.; et al. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: Results of a large-scale field trial. J. Infect. Dis. 1988, 158, 372–377. [Google Scholar] [CrossRef]
- Riddle, M.S.; Maciel, M., Jr.; Porter, C.K.; Poole, S.T.; Gutierrez, R.L.; Gormley, R.; Laird, R.M.; Sebeny, P.J.; Dori, K.E.; Greenleaf, M.E.; et al. A first in human clinical trial assessing the safety and immunogenicity of transcutaneously delivered enterotoxigenic Escherichia coli fimbrial tip adhesin with heat-labile enterotoxin with mutation R192G. Vaccine 2020, 38, 7040–7048. [Google Scholar] [CrossRef]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, 10–1128. [Google Scholar] [CrossRef]
- Norton, E.B.; Lawson, L.B.; Freytag, L.C.; Clements, J.D. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 2011, 18, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cassels, F.; Scharton-Kersten, T.; Hammond, S.A.; Hartman, A.; Angov, E.; Corthesy, B.; Alving, C.; Glenn, G. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect. Immun. 2002, 70, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Ahren, C.M.; Svennerholm, A.M. Synergistic protective effect of antibodies against Escherichia coli enterotoxin and colonization factor antigens. Infect. Immun. 1982, 38, 74–79. [Google Scholar] [CrossRef]
- McKenzie, R.; Bourgeois, A.L.; Frech, S.A.; Flyer, D.C.; Bloom, A.; Kazempour, K.; Glenn, G.M. Transcutaneous immunization with the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): Protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine 2007, 25, 3684–3691. [Google Scholar] [CrossRef]
- McKenzie, R.; Darsley, M.; Thomas, N.; Randall, R.; Carpenter, C.; Forbes, E.; Finucane, M.; Sack, R.B.; Hall, E.; Bourgeois, A.L. A double-blind, placebo-controlled trial to evaluate the efficacy of PTL-003, an attenuated enterotoxigenic E. coli (ETEC) vaccine strain, in protecting against challenge with virulent ETEC. Vaccine 2008, 26, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, R.; Porter, C.K.; Cantrell, J.A.; Denearing, B.; O’Dowd, A.; Grahek, S.L.; Sincock, S.A.; Woods, C.; Sebeny, P.; Sack, D.A.; et al. Volunteer Challenge With Enterotoxigenic Escherichia coli That Express Intestinal Colonization Factor Fimbriae CS17 and CS19. J. Infect. Dis. 2011, 204, 60–64. [Google Scholar] [CrossRef]
- Levine, M.M. Immunogenicity and efficacy of oral vaccines in developing countries: Lessons from a live cholera vaccine. BMC Biol. 2010, 8, 129. [Google Scholar] [CrossRef]
- Rollenhagen, J.E.; Woods, C.M.; O’Dowd, A.; Poole, S.T.; Tian, J.H.; Guebre-Xabier, M.; Ellingsworth, L.; Prouty, M.G.; Glenn, G.; Savarino, S.J. Evaluation of transcutaneous immunization as a delivery route for an enterotoxigenic E. coli adhesin-based vaccine with CfaE, the colonization factor antigen 1 (CFA/I) tip adhesin. Vaccine 2019, 37, 6134–6138. [Google Scholar] [CrossRef]
- Combadiere, B.; Liard, C. Transcutaneous and intradermal vaccination. Hum. Vaccines 2011, 7, 811–827. [Google Scholar] [CrossRef]
- Dickinson, B.L.; Clements, J.D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 1995, 63, 1617–1623. [Google Scholar] [CrossRef]
- Jobling, M.G.; Poole, S.T.; Rasulova-Lewis, F.; O’Dowd, A.; McVeigh, A.L.; Balakrishnan, A.; Sincock, S.A.; Prouty, M.G.; Holmes, R.K.; Savarino, S.J. Biochemical and immunological characterization of an ETEC CFA/I adhesin cholera toxin B subunit chimera. PLoS ONE 2020, 15, e0230138. [Google Scholar] [CrossRef]
- Gutierrez, R.L.; Porter, C.K.; Jarell, A.; Alcala, A.; Riddle, M.S.; Turiansky, G.W. A grading system for local skin reactions developed for clinical trials of an intradermal and transcutaneous ETEC vaccine. Vaccine 2020, 38, 3773–3779. [Google Scholar] [CrossRef] [PubMed]
- Anantha, R.P.; McVeigh, A.L.; Lee, L.H.; Agnew, M.K.; Cassels, F.J.; Scott, D.A.; Whittam, T.S.; Savarino, S.J. Evolutionary and functional relationships of colonization factor antigen i and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect. Immun. 2004, 72, 7190–7201. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Young, F.; Richardson, K.; Marra, C.A. A meta-analysis of intradermal versus intramuscular influenza vaccines: Immunogenicity and adverse events. Influenza Other Respir. Viruses 2013, 7, 584–603. [Google Scholar] [CrossRef]
- Zoeteweij, J.P.; Epperson, D.E.; Porter, J.D.; Zhang, C.X.; Frolova, O.Y.; Constantinides, A.P.; Fuhrmann, S.R.; El-Amine, M.; Tian, J.H.; Ellingsworth, L.R.; et al. GM1 binding-deficient exotoxin is a potent noninflammatory broad spectrum intradermal immunoadjuvant. J. Immunol. 2006, 177, 1197–1207. [Google Scholar] [CrossRef]
- Maciel, M., Jr.; Smith, M.; Poole, S.T.; Laird, R.M.; Rollenhagen, J.E.; Kaminski, R.W.; Wenzel, H.; Bourgeois, A.L.; Savarino, S.J. Evaluation of the reactogenicity, adjuvanticity and antigenicity of LT(R192G) and LT(R192G/L211A) by intradermal immunization in mice. PLoS ONE 2019, 14, e0224073. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Pasetti, M.F.; Brady, R.; Buskirk, A.D.; Wahid, R.; Dickey, M.; Cohen, M.; Baughman, H.; El-Khorazaty, J.; Maier, N.; et al. A Phase 1 dose escalating study of double mutant heat-labile toxin LTR192G/L211A (dmLT) from Enterotoxigenic Escherichia coli (ETEC) by sublingual or oral immunization. Vaccine 2019, 37, 602–611. [Google Scholar] [CrossRef]
- Maciel, M., Jr.; Trop, S.; Kim, A.; Ward, E.; Villar, Z.; Lee, T.; Jaep, K.; Porter, C.; Poole, S.; Prouty, M. Serological and α4β7+ antibody-secreting cell responses after intramuscular immunizatioin with CssBA, a CS6-subunit based enterotoxigenic E. coli vaccine candidate and LT(R192G/L211A) as adjuvant. In Proceedings of the 10th International Confernce on Vaccines for Enteric Diseases, Lausanne, Switzerland, 16–18 October 2019. [Google Scholar]



| Group | n | dscCfaE | Chimera | mLT |
|---|---|---|---|---|
| A-1 | 5 | 1 µg | -- | -- |
| A-2 | 5 | -- | 2.6 µg | -- |
| A-3 | 5 | -- | -- | 0.1 µg |
| B-1 | 5 | 1 µg | -- | 0.1 µg |
| B-2 | 5 | -- | 2.6 µg | 0.1 µg |
| C-1 | 7 | 5 µg | -- | 0.1 µg |
| C-2 | 7 | -- | 12.9 µg | 0.1 µg |
| D | 10 | 25 µg | -- | 0.1 µg |
| Cohort | A-1 (N = 5) | A-2 (N = 5) | A-3 (N = 5) | B-1 (N = 5) | B-2 (N = 5) | C-1 (N = 7) | C-2 (N = 7) | D (N = 10) |
|---|---|---|---|---|---|---|---|---|
| Median Age (IQR) | 28.4 (24.8, 30.3) | 31.0 (26.6, 33.4) | 31.2 (26.3, 32.6) | 31.9 (31.7, 42.5) | 38.9 (33.8, 42.2) | 32.5 (25.9, 34.2) | 30.2 (25.5, 32.3) | 30.5 (27.6, 42.7) |
| Gender | ||||||||
| Female, n (%) | 0 (0.0%) | 1 (20.0%) | 2 (40.0%) | 0 (0.0%) | 2 (40.0%) | 5 (71.4%) | 5 (71.4%) | 6 (60.0%) |
| Male, n (%) | 5 (100%) | 4 (80.0%) | 3 (60.0%) | 5 (100%) | 3 (60.0%) | 2 (28.6%) | 2 (28.6%) | 4 (40.0%) |
| Race | ||||||||
| Black, n (%) | 2 (40.0%) | 3 (60.0%) | 1 (20.0%) | 2 (40.0%) | 3 (60.0%) | 1 (14.3%) | 3 (42.9%) | 5 (50.0%) |
| Caucasian, n (%) | 3 (60.0%) | 1 (20.0%) | 4 (80.0%) | 2 (40.0%) | 2 (40.0%) | 5 (71.4%) | 3 (42.9%) | 4 (40.0%) |
| American Indian, n (%) | 0 (0.0%) | 1 (20.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Asian, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (10.0%) |
| Other, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (14.3%) | 1 (14.3%) | 0 (0.0%) |
| Ethnicity, n (%) | ||||||||
| Hispanic/Latino, n (%) | 1 (20.0%) | 1 (20.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (14.3%) | 0 (0.0%) | 0 (0.0%) |
| AE | Mild N (%) | Moderate N (%) | Severe N (%) | Total N (%) |
|---|---|---|---|---|
| Arthralgia | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Axillary Pain | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Fasciculation | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Fever | 2 (4.1) | 0 (0.0) | 0 (0.0) | 2 (4.1) |
| GI Symptoms | 0 (0.0) | 1 (2.0) | 0 (0.0) | 1 (2.0) |
| Headache | 6 (12.2) | 1 (2.0) | 0 (0.0) | 7 (14.3) |
| Hemoglobin Decrease | 4 (8.2) | 1 (2.0) | 0 (0.0) | 5 (10.2) |
| Knee Pain | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Leukopenia | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Loose Stool | 5 (10.2) | 0 (0.0) | 0 (0.0) | 5 (10.2) |
| Lymphadenopathy | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Malaise | 1 (2.0) | 2 (4.1) | 0 (0.0) | 3 (6.1) |
| Myalgia | 2 (4.1) | 0 (0.0) | 0 (0.0) | 2 (4.1) |
| Sleepiness | 0 (0.0) | 1 (2.0) | 0 (0.0) | 1 (2.0) |
| Thrombocytopenia | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Vaccination Site Pain | 19 (38.8) | 2 (4.1) | 0 (0.0) | 21(42.9) |
| Vaccination Site Pruritus | 32 (65.3) | 3 (6.1) | 1 (2.0) | 36 (73.5) |
| Vaccine Site Reaction | 44 (89.8) | 1 (2.0) | 0 (0.0) | 45 (91.8) |
| Vaccine Site Swelling | 4 (8.2) | 0 (0.0) | 0 (0.0) | 4 (8.2) |
| Vaccine Site Tenderness | 32 (65.3) | 1 (2.0) | 0 (0.0) | 33 (67.3) |
| Group | Cohort A-1 CfaE 1 µg | Cohort A-2 Chimera 2.6 µg | Cohort A-3 mLT 0.1 µg | Cohort B-1 CfaE 1 µg (+)mLT | Cohort B-2 Chimera 2.6 µg (+)mLT | Cohort C-1 CfaE 5 µg (+)mLT | Cohort C-2 Chimera 12.9 µg (+)mLT | Cohort D CfaE 25 µg (+)mLT |
|---|---|---|---|---|---|---|---|---|
| N | 5 | 5 | 5 | 5 | 5 | 7 | 7 | 10 |
| Pain | 0 (0.0) | 2 (40.0) | 1 (20.0) | 1 (20.0) | 3 (60.0) | 4 (57.1) | 5 (71.4) | 5 (50.0) |
| Pruritus | 0 (0.0) | 4 (80.0) | 4 (80.0) | 4 (80.0) | 5 (100) | 5 (71.4) | 6 (85.7) | 8 (80.0) |
| Tenderness | 1 (20.0) | 3 (60.0) | 3 (60.0) | 3 (60.0) | 3 (60.0) | 6 (85.7) | 6 (85.7) | 8 (80.0) |
| Vaccine Site Reaction | 1 (20.0) | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 7 (100.0) | 7 (100) | 10 (100) |
| Erythema | 1 (20.0) | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 7 (100.0) | 7 (100) | 10 (100) |
| Induration | 0 (0.0) | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 7 (100.0) | 7 (100) | 10 (100) |
| Papules | 0 (0.0) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (20.0) |
| Edema | 0 (0.0) | 2 (40.0) | 1 (20.0) | 3 (60.0) | 4 (80.0) | 5 (71.4) | 7 (100) | 7 (70.0) |
| Hypopigmentation | 0 (0.0) | 2 (40.0) | 1 (20.0) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hyperpigmentation | 0 (0.0) | 4 (80.0) | 5 (100) | 5 (100) | 5 (100) | 6 (85.7) | 7 (100) | 8 (80.0) |
| Vesicles | 0 (0.0) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Group | N | Serology | ASC | |||||
|---|---|---|---|---|---|---|---|---|
| IgG | IgA | IgA | ||||||
| CfaE | LTB | CfaE | LTB | CfaE | LTB | |||
| A | 1 µg dscCfaE | 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (40.0) |
| 2.6 µg Chimera | 5 | 5 (100) | 5 (100) | 1 (20.0) | 4 (80.0) | 1 (20.0) | 5 (100) | |
| 0.1 µg mLT | 5 | 0 (0.0) | 5 (100) | 0 (0.0) | 3 (60.0) | 0 (0.0) | 5 (100) | |
| B | 1 µg dscCfaE + 0.1 µg mLT | 5 | 4 (80.0) | 5 (100) | 3 (60.0) | 4 (80.0) | 3 (60.0) | 5 (100) |
| 2.6 µg Chimera + 0.1 µg mLT | 4 | 4 (100) | 4 (100) | 2 (50.0) | 4 (100) | 2 (50.0) | 4 (100) | |
| C | 5 µg dscCfaE + 0.1 µg mLT | 7 | 7 (100) | 7 (100) | 3 (42.9) | 5 (71.4) | 2 (28.6) | 6 (85.7) |
| 12.9 µg Chimera + 0.1 µg mLT | 7 | 7 (100) | 7 (100) | 3 (42.9) | 7 (100) | 4 (57.1) | 7 (100) | |
| D | 25 µg dscCfaE + 0.1 µg mLT | 10 | 9 (90.0) | 9 (90.0) | 5 (50.0) | 7 (70.0) | 6 (60.0) | 10 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, R.L.; Riddle, M.S.; Porter, C.K.; Maciel, M., Jr.; Poole, S.T.; Laird, R.M.; Lane, M.; Turiansky, G.W.; Jarell, A.; Savarino, S.J. A First in Human Clinical Trial Assessing the Safety and Immunogenicity of Two Intradermally Delivered Enterotoxigenic Escherichia coli CFA/I Fimbrial Tip Adhesin Antigens with and without Heat-Labile Enterotoxin with Mutation LT(R192G). Microorganisms 2023, 11, 2689. https://doi.org/10.3390/microorganisms11112689
Gutiérrez RL, Riddle MS, Porter CK, Maciel M Jr., Poole ST, Laird RM, Lane M, Turiansky GW, Jarell A, Savarino SJ. A First in Human Clinical Trial Assessing the Safety and Immunogenicity of Two Intradermally Delivered Enterotoxigenic Escherichia coli CFA/I Fimbrial Tip Adhesin Antigens with and without Heat-Labile Enterotoxin with Mutation LT(R192G). Microorganisms. 2023; 11(11):2689. https://doi.org/10.3390/microorganisms11112689
Chicago/Turabian StyleGutiérrez, Ramiro L., Mark S. Riddle, Chad K. Porter, Milton Maciel, Jr., Steven T. Poole, Renee M. Laird, Michelle Lane, George W. Turiansky, Abel Jarell, and Stephen J. Savarino. 2023. "A First in Human Clinical Trial Assessing the Safety and Immunogenicity of Two Intradermally Delivered Enterotoxigenic Escherichia coli CFA/I Fimbrial Tip Adhesin Antigens with and without Heat-Labile Enterotoxin with Mutation LT(R192G)" Microorganisms 11, no. 11: 2689. https://doi.org/10.3390/microorganisms11112689
APA StyleGutiérrez, R. L., Riddle, M. S., Porter, C. K., Maciel, M., Jr., Poole, S. T., Laird, R. M., Lane, M., Turiansky, G. W., Jarell, A., & Savarino, S. J. (2023). A First in Human Clinical Trial Assessing the Safety and Immunogenicity of Two Intradermally Delivered Enterotoxigenic Escherichia coli CFA/I Fimbrial Tip Adhesin Antigens with and without Heat-Labile Enterotoxin with Mutation LT(R192G). Microorganisms, 11(11), 2689. https://doi.org/10.3390/microorganisms11112689






