Promotional Properties of ACC Deaminase-Producing Bacterial Strain DY1-3 and Its Enhancement of Maize Resistance to Salt and Drought Stresses
Abstract
:1. Introduction
2. Materials and Methods
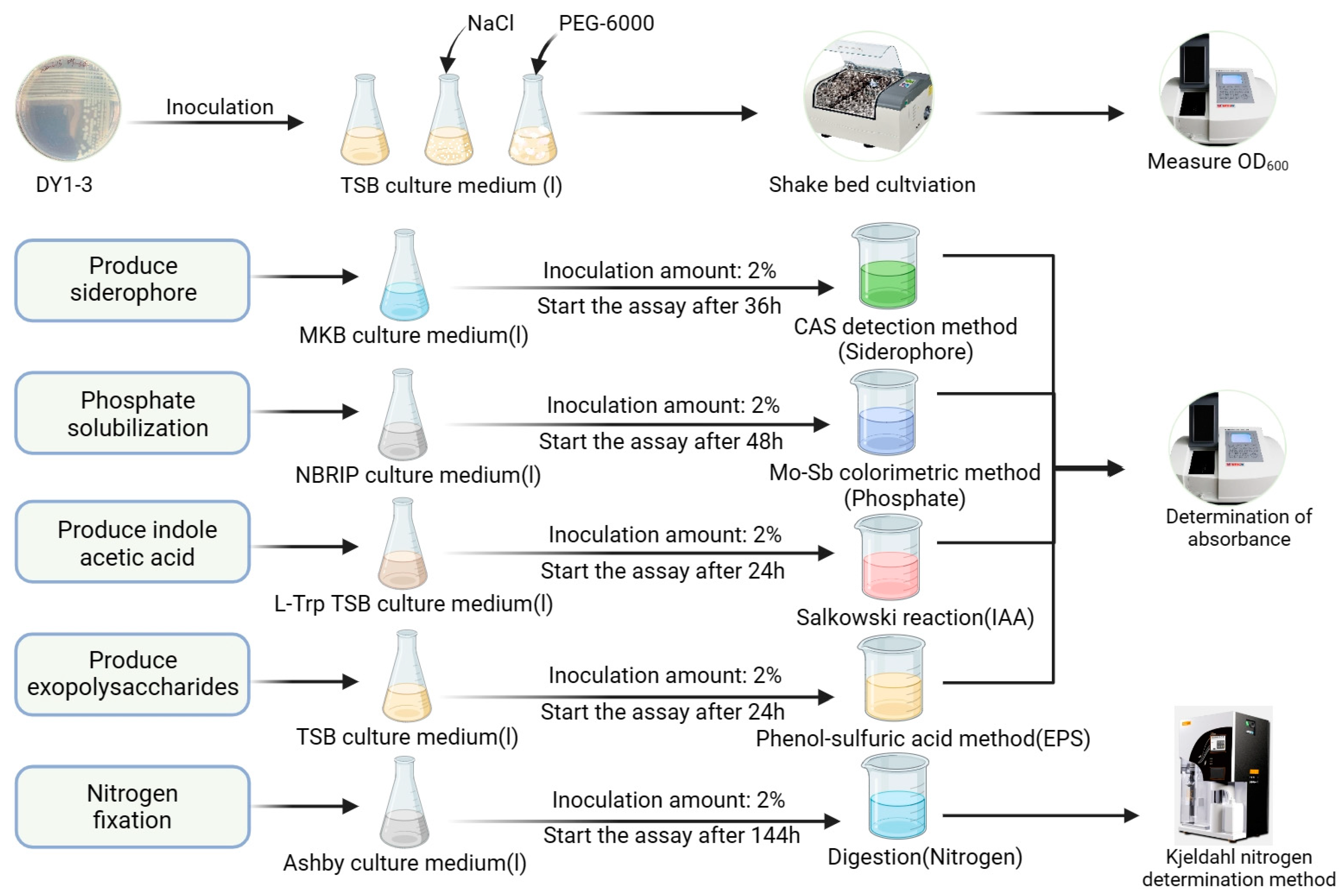
2.1. Test Strains and Soil
2.2. Experiments on the Characterization of PGPR
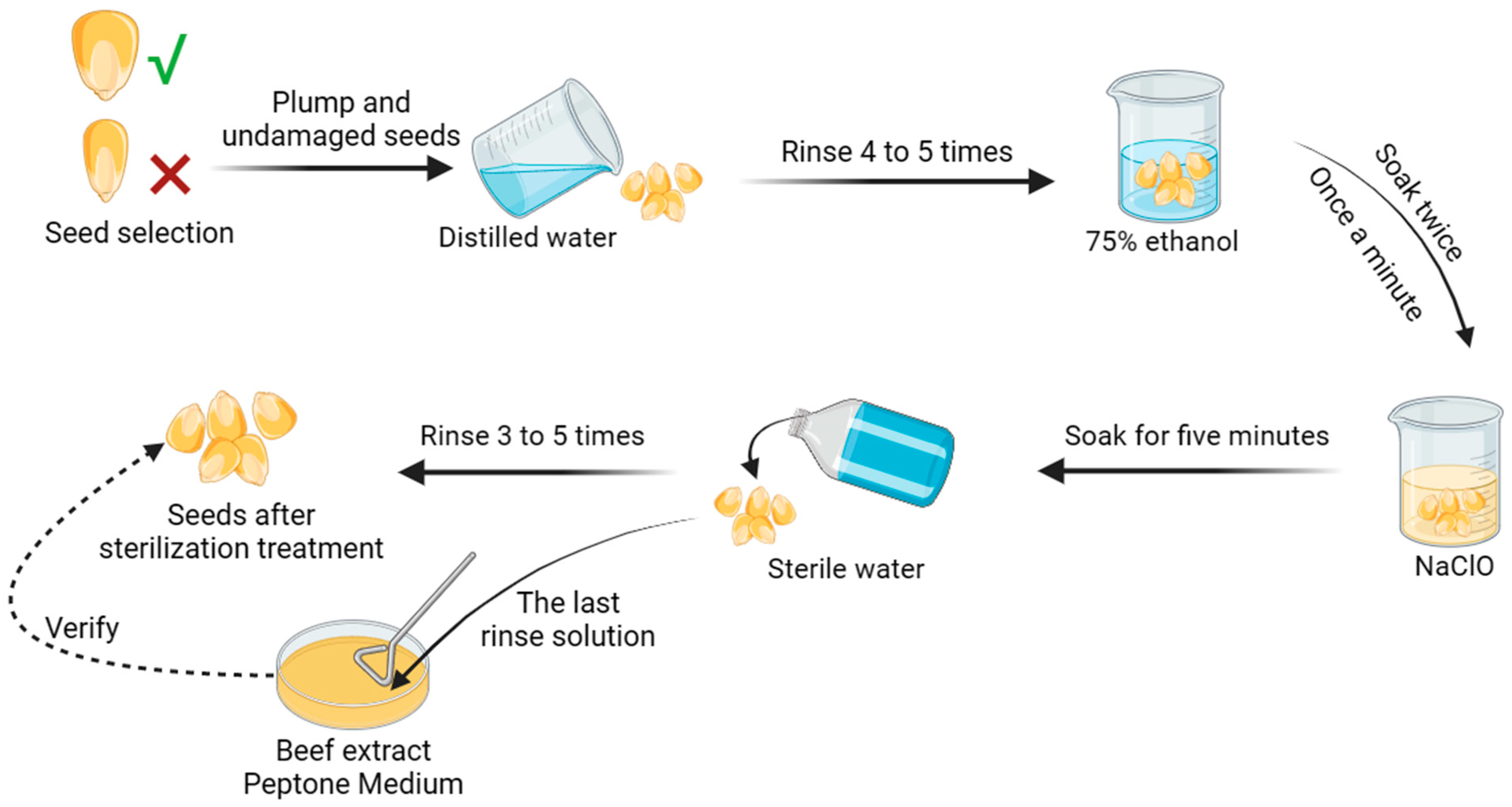
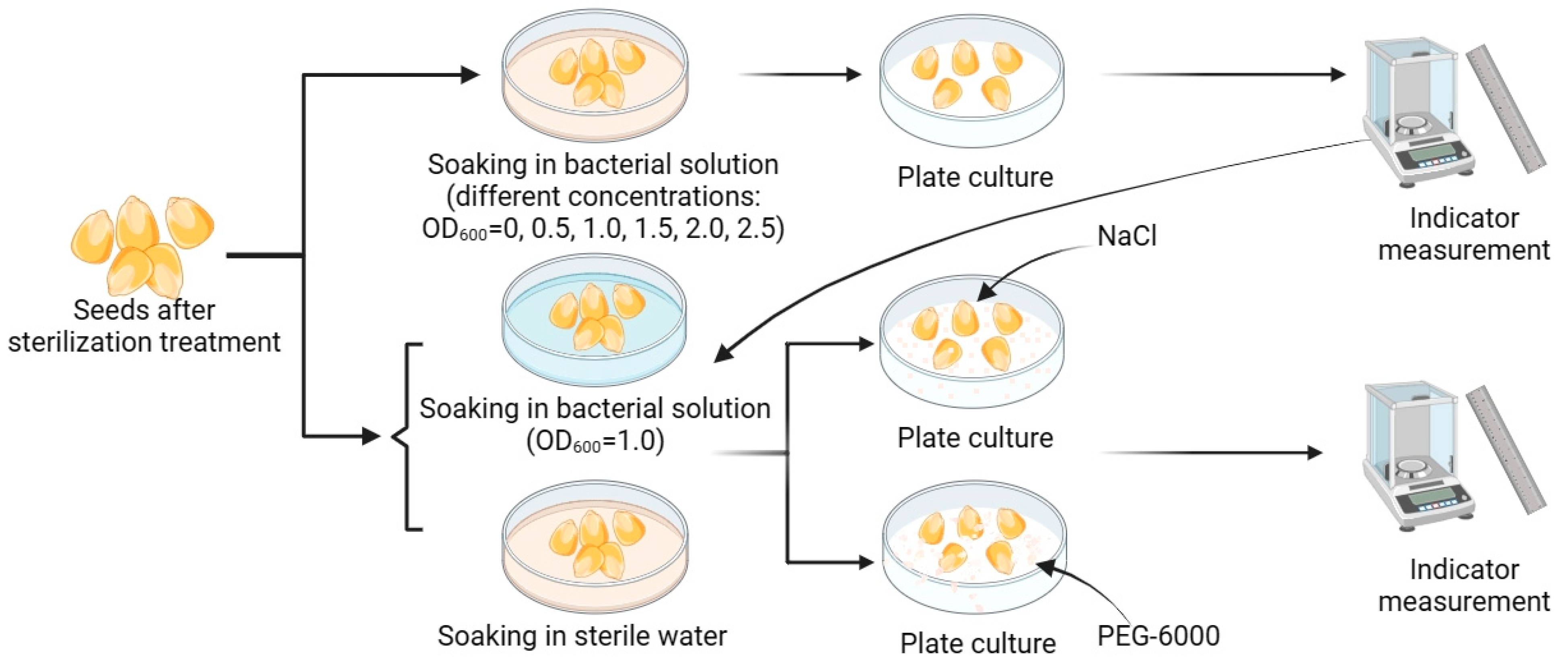
2.3. Experiments with Maize Seeds Inoculated with Bacterial Strains
2.3.1. Strain and Seed Treatment
2.3.2. Effect of Application on Seed Growth during Germination and Resistance to Salt and Drought Stresses
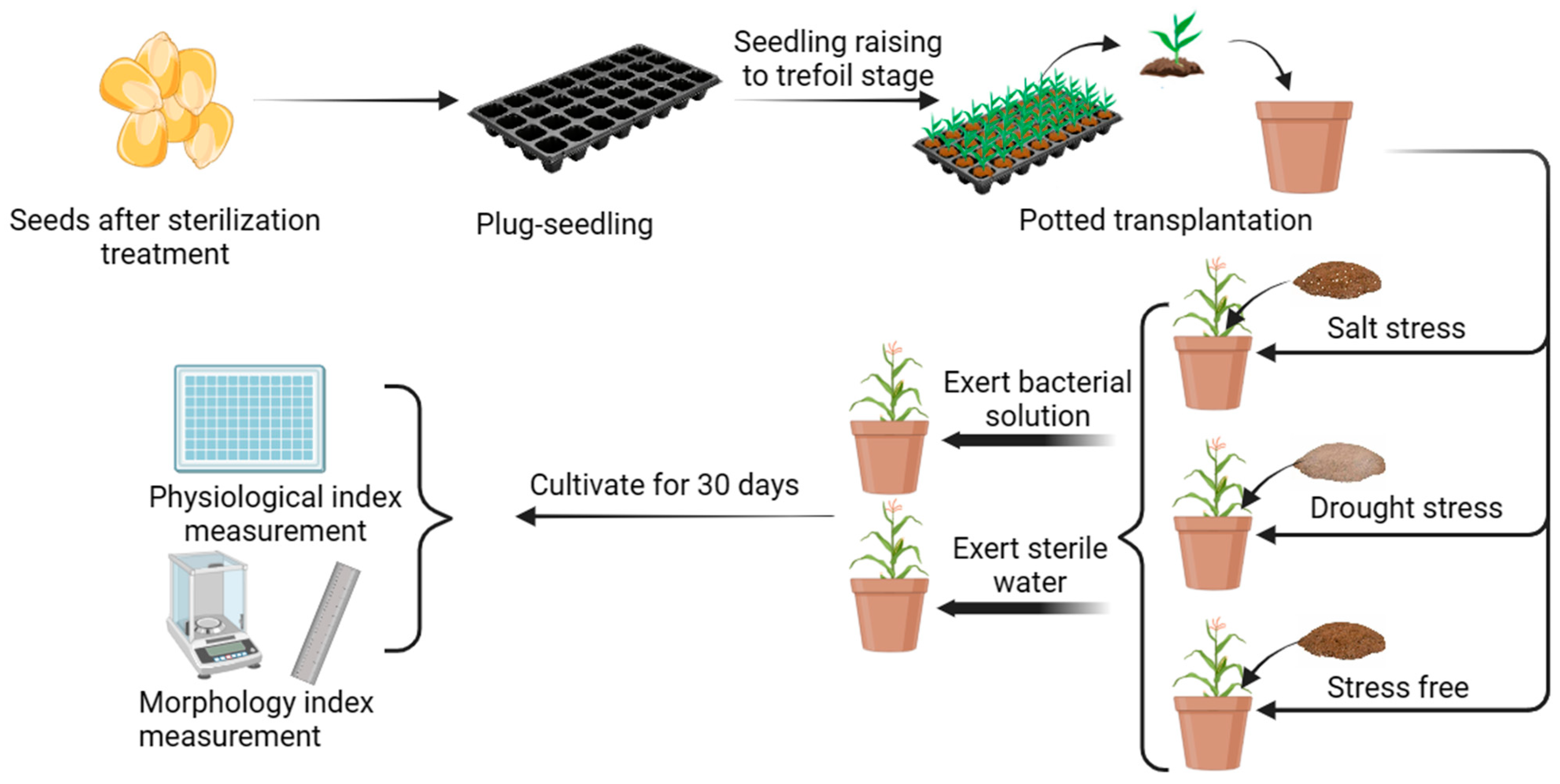
2.4. Potting Experiments with Bacterial Solutions Applied to Corn
2.5. Statistical Data Analysis
3. Result
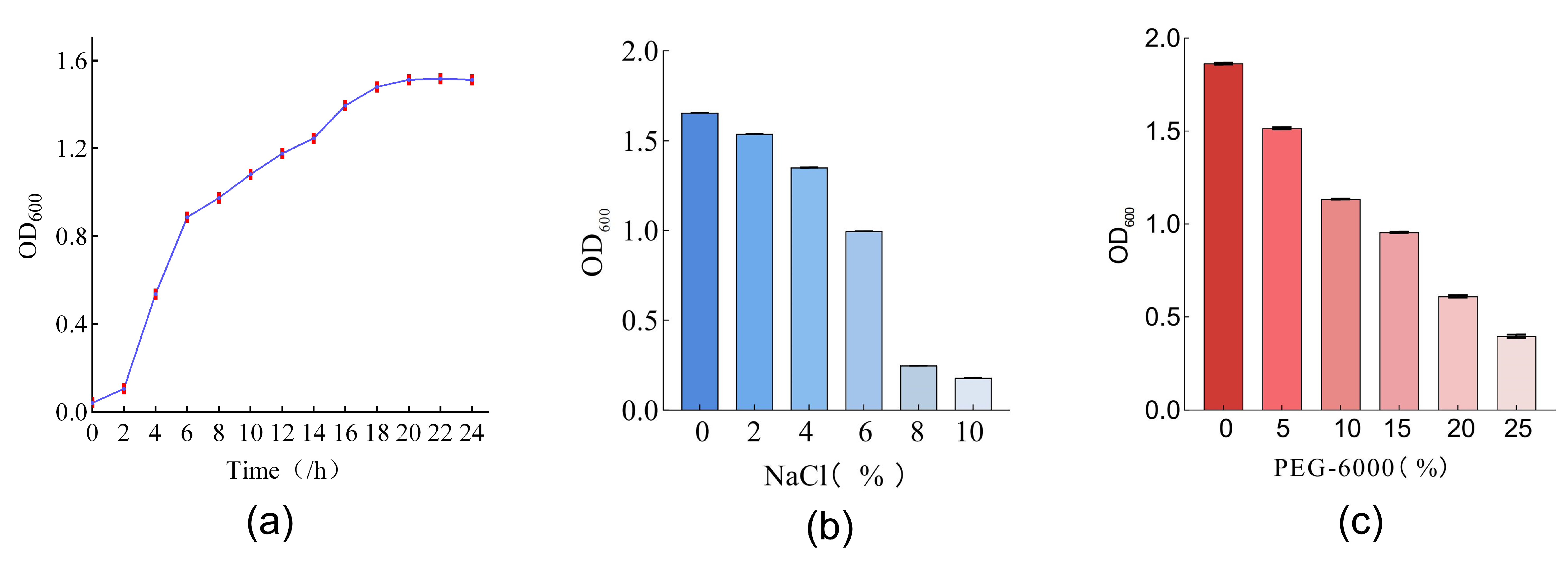
3.1. Potential of Bacterial Strains as Plant Growth Promoters
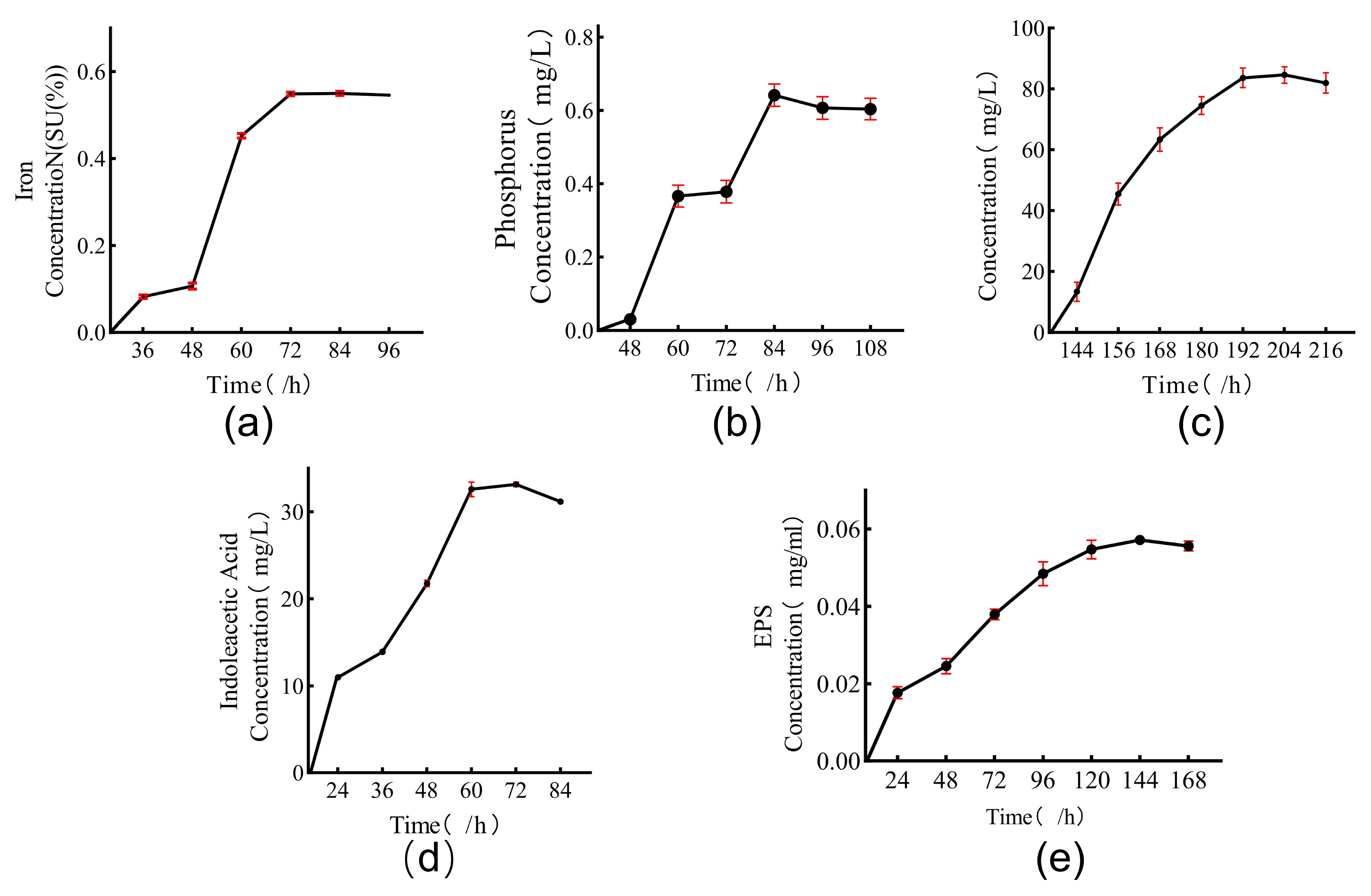
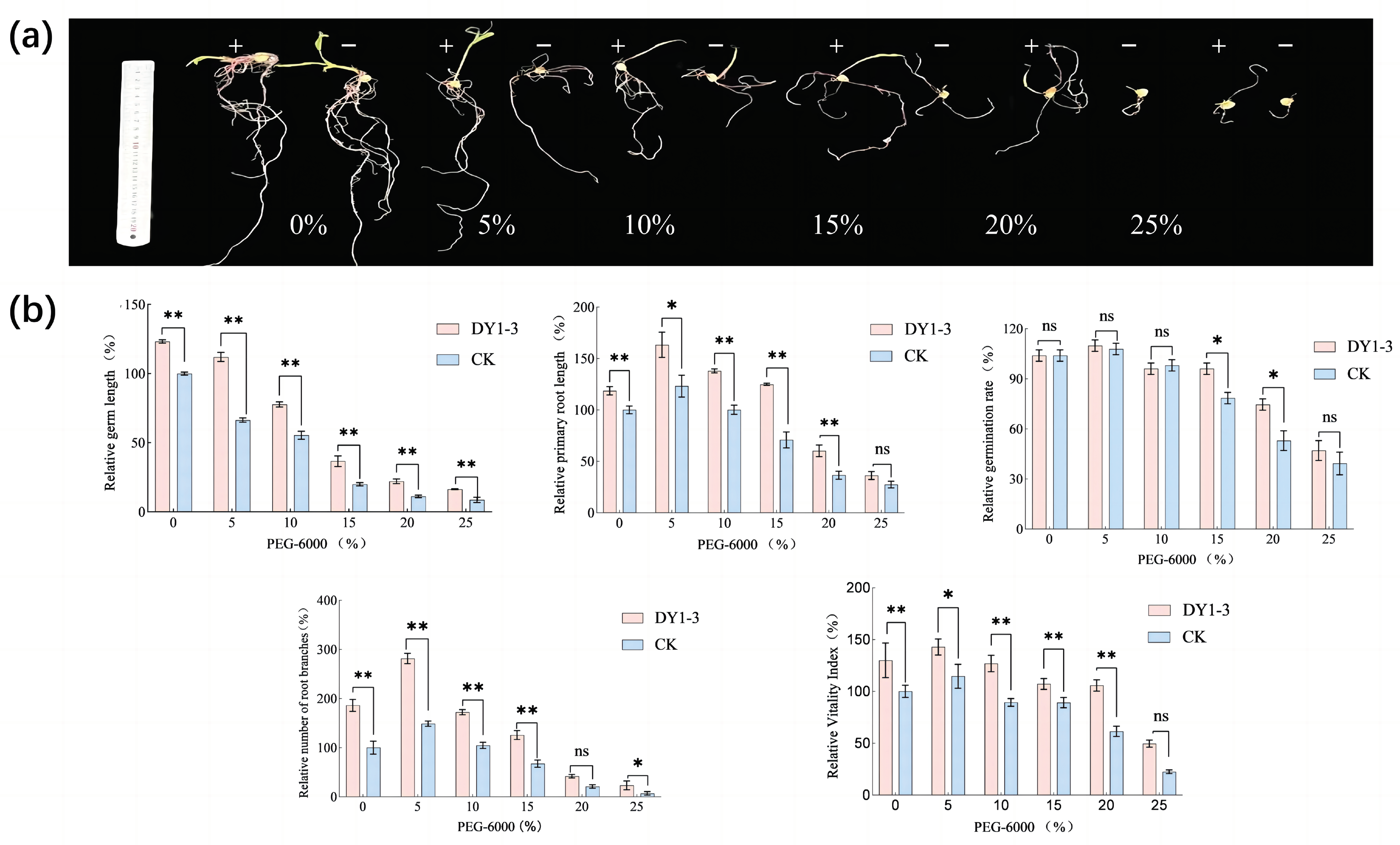
3.2. Effect of Adding Strain DY1-3 to Corn Seeds
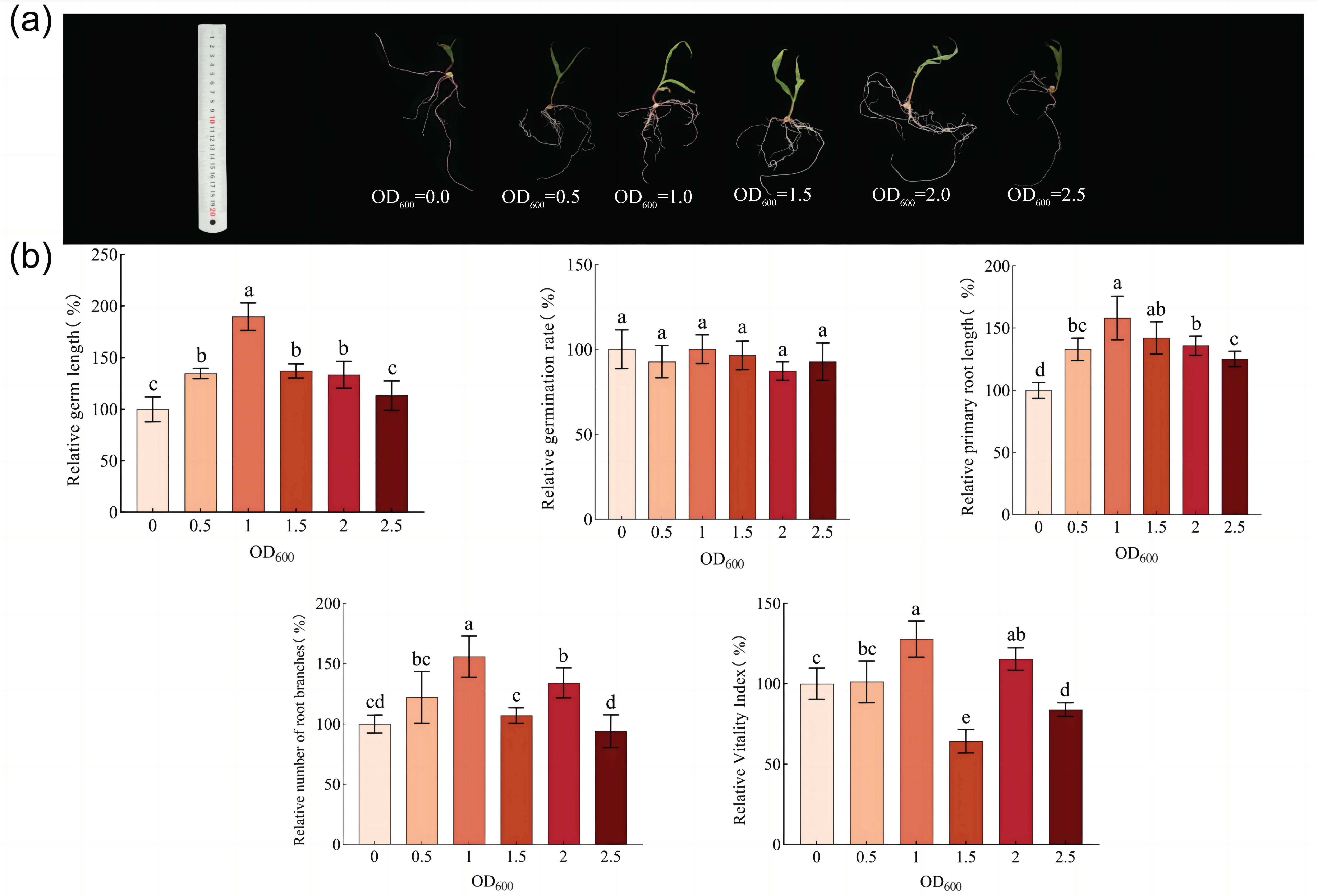
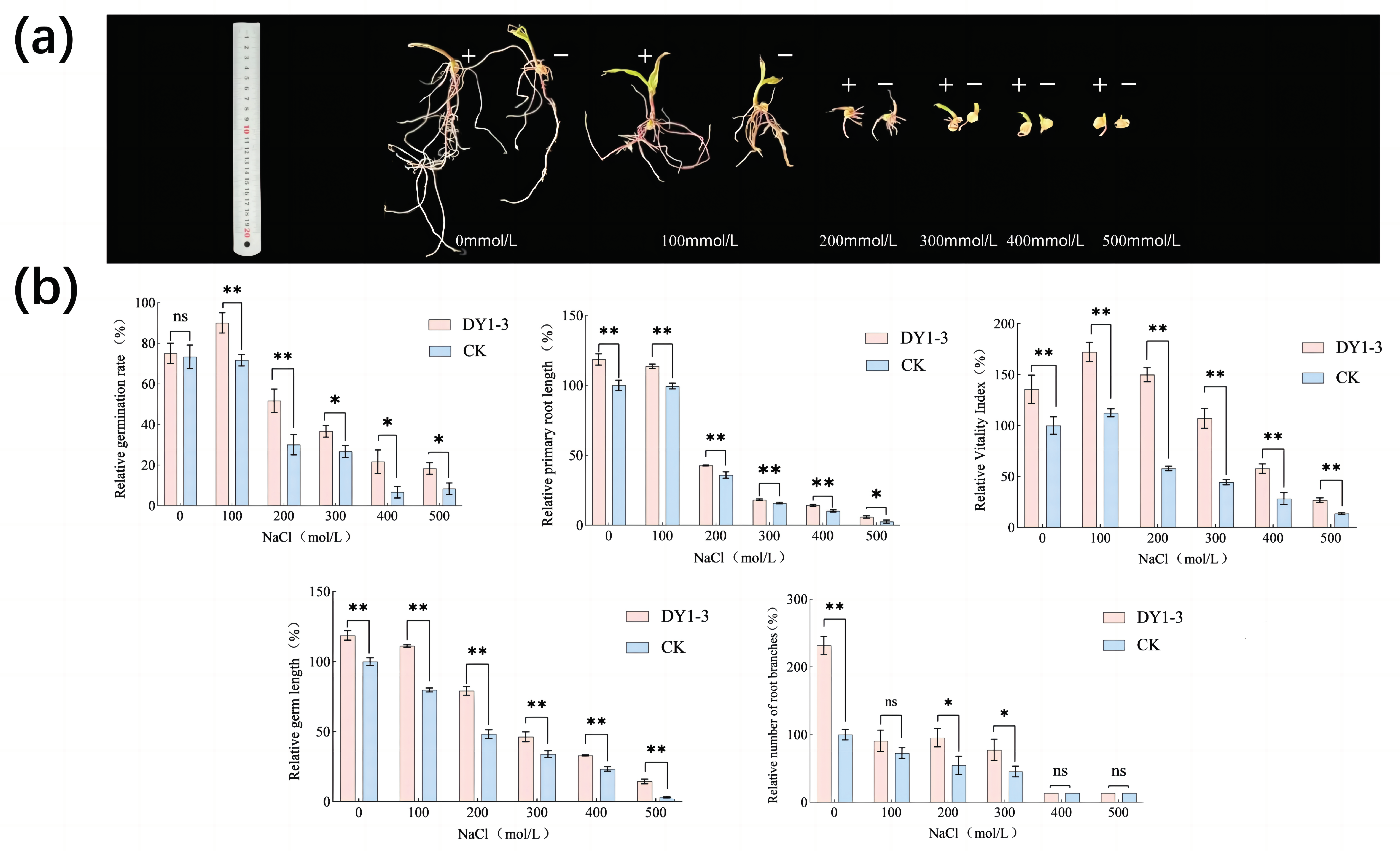
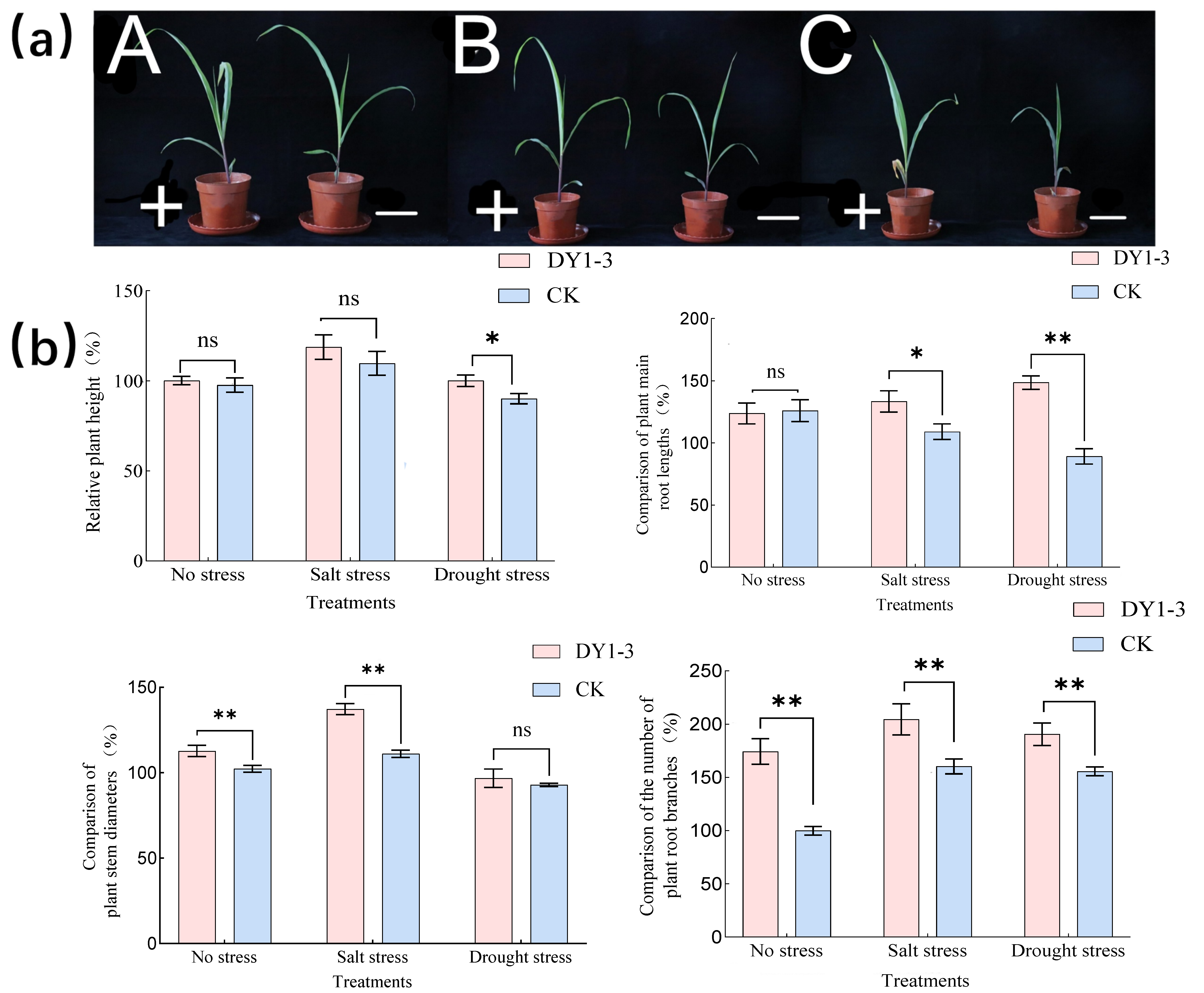
3.3. Effect of Strain DY1-3 Access on Morphological Indicators of Maize Seedlings
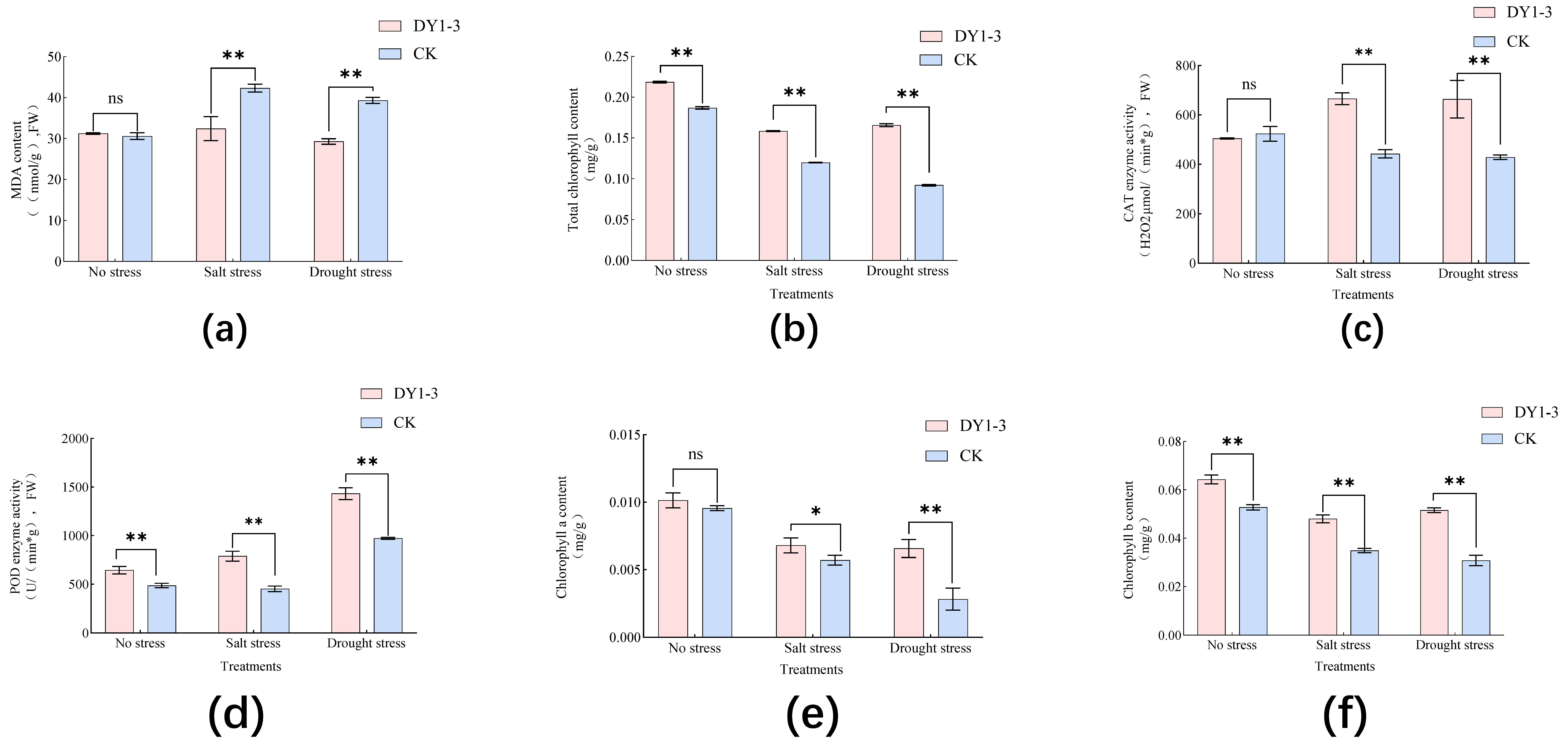
3.4. Effect of Strain DY1-3 Access on Physiological Indexes of Maize Seedlings
4. Discussion
5. Conclusions
- The results of this study show that DY1-3 has a strong ability to survive in high-salinity and high-drought environments, and is capable of producing iron carriers, dissolving phosphorus, synthesizing IAA, producing EPS, and nitrogen fixation.
- In the absence of abiotic stress, the addition of bacterial solution did not significantly increase the germination rate of maize seeds, but under drought and salt stress conditions, the use of bacterial solution of DY1-3 strain to soak maize seeds significantly increased the germination rate of maize seeds as well as increased the germination length of the seeds, the number of root meristems, and the vitality index, etc., and mitigated the inhibitory effects of salt and drought stress on the growth of maize seeds.
- The application of strain DY1-3 had no significant effect on the growth of the crop under stress-free conditions, but in the study of the physiological characteristics of the crop related to stress resistance, the contents of SOD, POD, CAT, and chlorophyll were significantly higher than those of the control seedlings without the application of the bacterial broth, and the incorporation of the bacterial broth significantly lowered the content of MDA in the experimental group of seedlings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.Z.; Chen, J.S.; Peng, E.-R.; Li, R.L.; Wu, D.Z. Research Progress on Soil Salinization and Management. China Rural Water Hydropower 2023, 6, 202–208. [Google Scholar]
- Li, H.; Zhao, Q.; Huang, H. Current states and challenges of salt-affected soil remediation by cyanobacteria. Sci. Total Environ. 2019, 669, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms. greenhouse gas emissions. crop yield. biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Yang, K. Klebsiella variicola improves the antioxidant ability of maize seedlings under saline-alkali stress. PeerJ 2021, 9, e11963. [Google Scholar] [CrossRef] [PubMed]
- Shelden, M.C.; Munns, R. Crop root system plasticity for improved yields in saline soils. Front. Plant Sci. 2023, 14, 1120583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Smith, J.A.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef]
- Chandwani, S.; Amaresan, N. Role of ACC deaminase producing bacteria for abiotic stress management and sustainable agriculture production. Environ. Sci. Pollut. Res. Int. 2022, 29, 22843–22859. [Google Scholar] [CrossRef]
- Murali, M.; Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Niranjana, S.R.; Amruthesh, K.N. Bio-prospecting of ACC deaminase producing Rhizobacteria towards sustainable agriculture: A special emphasis on abiotic stress in plants. Appl. Soil. Ecol. 2021, 168, 104142. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.; Shinde, A.L.; Kumar, S.; Wakchaure, G.C.; Kumar, S.; Kanwat, M.; Singh, D.P. Stress-responsive gene regulation conferring salinity tolerance in wheat inoculated with ACC deaminase producing facultative methylotrophic actinobacterium. Front. Plant Sci. 2023, 14, 1249600. [Google Scholar] [CrossRef]
- El-Ballat, E.M.; Elsilk, S.E.; Ali, H.M.; Ali, H.E.; Hano, C.; El-Esawi, M.A. Metal-Resistant PGPR Strain Azospirillum brasilense EMCC1454 Enhances Growth and Chromium Stress Tolerance of Chickpea (Cicer arietinum L.) by Modulating Redox Potential. Osmolytes. Antioxidants. and Stress-Related Gene Expression. Plants 2023, 12, 2110. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Sousa, C.A.; Sanchis-Perez, I.; Lopez-Rayo, S.; Barros, M.T.; Soares, H.; Lucena, J.J. Calcareous soil interactions of the iron (III) chelates of DPH and Azotochelin and its application on amending iron chlorosis in soybean (Glycine max). Sci. Total Environ. 2019, 647, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chang, M.; Han, X.; Wang, Q.; Wang, J.; Yang, H.; Guan, Q.; Dai, S. Beneficial effects of endophytic Pantoea ananatis with ability to promote rice growth under saline stress. J. Appl. Microbiol. 2021, 131, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, Y.; Li, X.; Li, M.; Wang, C.; Li, Z.; Wang, Y.; Wang, W. A Salt-Tolerant Streptomyces paradoxus D2-8 from Rhizosphere Soil of Phragmites communis Augments Soybean Tolerance to Soda Saline-Alkali Stress. Pol. J. Microbiol. 2022, 71, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Yu, M.; Quandahor, P.; Tian, T.; Shen, T. Bacillus velezensis FX-6 suppresses the infection of Botrytis cinerea and increases the biomass of tomato plants. PLoS ONE 2023, 18, e286971. [Google Scholar] [CrossRef] [PubMed]
- Herpell, J.B.; Alickovic, A.; Diallo, B.; Schindler, F.; Weckwerth, W. Phyllosphere symbiont promotes plant growth through ACC deaminase production. ISME J. 2023, 17, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandey, S.; Kotra, V.; Kumar, A. Assessing the role of ACC deaminase-producing bacteria in alleviating salinity stress and enhancing zinc uptake in plants by altering the root architecture of French bean (Phaseolus vulgaris) plants. Planta 2023, 258, 3. [Google Scholar] [CrossRef]
- Li, X.; Lang, D.; Wang, J.; Zhang, W.; Zhang, X. Plant-beneficial Streptomyces dioscori SF1 potential biocontrol and plant growth promotion in saline soil within the arid and semi-arid areas. Environ. Sci. Pollut. Res. Int. 2023, 30, 70194–70212. [Google Scholar] [CrossRef]
- Ratnaningsih, H.R.; Noviana, Z.; Dewi, T.K.; Loekito, S.; Wiyono, S.; Gafur, A.; Antonius, S. IAA and ACC deaminase producing-bacteria isolated from the rhizosphere of pineapple plants grown under different abiotic and biotic stresses. Heliyon 2023, 9, e16306. [Google Scholar] [CrossRef]
- Moon, Y.S.; Ali, S. Possible mechanisms for the equilibrium of ACC and role of ACC deaminase-producing bacteria. Appl. Microbiol. Biotechnol. 2022, 106, 877–887. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2022, 13, 1101862. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, Y.; Feng, Y.; Zhang, Y.; Fan, Y. Bacterial Diversity Analysis and Screening for ACC Deaminase-Producing Strains in Moss-Covered Soil at Different Altitudes in Tianshan Mountains—A Case Study of Glacier No. 1. Microorganisms 2023, 11, 1521. [Google Scholar] [CrossRef] [PubMed]
- Pantoja-Guerra, M.; Burkett-Cadena, M.; Cadena, J.; Dunlap, C.A.; Ramírez, C.A. Lysinibacillus spp.: An IAA-producing endospore forming-bacteria that promotes plant growth. Antonie Van Leeuwenhoek 2023, 116, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, S.; Wang, Y.; Zhou, Y.; Liu, Q.; Yin, K. Screening and identification of five saline-alkali tolerant bacteria for growth promotion of red adzuki bean. Microbiology 2021, 48, 1580–1592. [Google Scholar] [CrossRef]
- Ghorbel, S.; Aldilami, M.; Zouari-Mechichi, H.; Mechichi, T.; AlSherif, E.A. Isolation and characterization of a plant growth-promoting rhizobacterium strain MD36 that promotes barley seedlings and growth under heavy metals stress. 3 Biotech 2023, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.-R.; Fan, Y.-R.; Li, Z.-L.; Xiao, Z.-C.; Gu, D.-H.; Wang, X.-F.; Zeng, T.-T.; Fan, J.-P. Protective Effect of Tremella aurantialba Polysaccharide on Cyclophosphamide-induced Immunocompromised Mice. Food Res. Dev. 2023, 44, 85–90. [Google Scholar]
- Barbaccia, P.; Gaglio, R.; Dazzi, C.; Miceli, C.; Bella, P.; Lo Papa, G.; Settanni, L. Plant Growth-Promoting Activities of Bacteria Isolated from an Anthropogenic Soil Located in Agrigento Province. Microorganisms 2022, 10, 2167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Zhang, J.; Xu, W.; Mou, Z. Characteristics of Inorganic Phosphate-Solubilizing Bacteria from the Sediments of a Eutrophic Lake. Int. J. Environ. Res. Public Health 2019, 16, 2141. [Google Scholar] [CrossRef]
- Lin, H.-P.; Xie, C.-Y.; Wang, Y.; Cheng, X.-L. Screening of Endophytic Nitrogen Fixation Bacteria from Roots of Aegiceras corniculatum and Optimization of Their Culture Conditions. For. Res. 2021, 34, 181–186. [Google Scholar] [CrossRef]
- Gao, X.; Bao, S.; Zhou, Z.; Zhang, J.; Wang, C.; Long, J.; Wang, X.; Miao, Y. Study on germination characteristics of four forage seeds under different stresses. Pratacultural Sci. 2022, 39, 1823–1831. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Wang, Z.; Liu, K.; Wang, P. Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J. Exp. Bot. 2006, 57, 149–160. [Google Scholar] [CrossRef]
- Wang, W.N.; Lan, Z.Y.; Yu, W.L.; Sun, Q.P.; Li, P.Q.; Fan, Y.H. Screening and identification of salt-tolerant and ACC deaminase-producing strains in Halostachys caspica rhizosphere. Soils Fertil. Sci. China 2021, 2, 270–275. [Google Scholar]
- Qing-Pei, S.; Yong-Hong, F.; Li, P.-Q.; Xin-Zheng, Q. Optimization of enzyme-producing conditions and probiotic effect of three strains of ACC deaminase producing plant growth promoting rhizobacteria. Soil Fertil. Sci. China 2023, 2, 234–241. [Google Scholar]
- Tavares, M.J.; Nascimento, F.X.; Glick, B.R.; Rossi, M.J. The expression of an exogenous ACC deaminase by the endophyte Serratia grimesii BXF1 promotes the early nodulation and growth of common bean. Lett. Appl. Microbiol. 2018, 66, 252–259. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Soares, C.R.; McConkey, B.J.; Glick, B.R. New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny. evolution and ecological significance. PLoS ONE 2014, 9, e99168. [Google Scholar] [CrossRef]
- Etesami, H.; Mirseyed, H.H.; Alikhani, H.A. Bacterial biosynthesis of 1-aminocyclopropane-1-caboxylate (ACC) deaminase. a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions. Physiol. Mol. Biol. Plants 2014, 20, 425–434. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Gupta, A.; Rai, S.; Bano, A.; Sharma, S.; Kumar, M.; Binsuwaidan, R.; Suhail, K.M.; Upadhyay, T.K.; Alshammari, N.; Saeed, M.; et al. ACC Deaminase Produced by PGPR Mitigates the Adverse Effect of Osmotic and Salinity Stresses in Pisum sativum through Modulating the Antioxidants Activities. Plants 2022, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Singh, A.V. Multifarious effect of ACC deaminase and EPS producing Pseudomonas sp. and Serratia marcescens to augment drought stress tolerance and nutrient status of wheat. World J. Microbiol. Biotechnol. 2021, 37, 198. [Google Scholar] [CrossRef]
- Gebauer, S.; Grenfell, J.L.; Lammer, H.; de Vera, J.P.; Spross, L.; Airapetian, V.S.; Sinnhuber, M.; Rauer, H. Atmospheric Nitrogen When Life Evolved on Earth. Astrobiology 2020, 20, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Reinprecht, Y.; Schram, L.; Marsolais, F.; Smith, T.H.; Hill, B.; Pauls, K.P. Effects of Nitrogen Application on Nitrogen Fixation in Common Bean Production. Front. Plant Sci. 2020, 11, 1172. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, L.; Zhou, X.; Lin, B.; Zou, C. Effects of sugarcane variety and nitrogen application level on the quality and aerobic stability of sugarcane tops silage. Front. Plant Sci. 2023, 14, 1148884. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.K.; Quoreshi, A.M.; Bhat, N.R.; Manuvel, A.J.; Sivadasan, M.T. Divulging diazotrophic bacterial community structure in Kuwait desert ecosystems and their N2-fixation potential. PLoS ONE 2019, 14, e220679. [Google Scholar] [CrossRef]
- He, J.Q.; Zhang, G.J.; Zhao, W.J.; Wang, X.X. Screening and identification of superior associative nitrogen fixing bacteria in rhizosphere of Hordeum vulgare. Agric. Res. Arid. Areas 2019, 37, 182–186+192. [Google Scholar]
- Men, J.; Tang, B.; Deng, B.; Li, J.-H.; He, Y.-J.; Zhang, J.-L. Isolation.screening and beneficial effects of plant growth-promoting rhizobacteria (PGPR) in the rhizosphere of Leymus chinensis. Acta Prataculturae Sin. 2021, 30, 59–71. [Google Scholar]
- Pan, Y.; Zhang, H.; Li, X.; Cao, M.; Xu, X.; Song, T.; Xie, J. Effect of Salt-tolerant and Growth-promoting Bacteria and Composite Microbial Agent on Growth and Physicoch-emical of Pennisetum alopecuroides Under Salt Stress. Guizhou Agric. Sci. 2023, 51, 39–49. [Google Scholar]
- Lucas, J.A.; Garcia-Villaraco, A.; Ramos, B.; Garcia-Cristobal, J.; Algar, E.; Gutierrez-Manero, J. Structural and functional study in the rhizosphere of Oryza sativa L. plants growing under biotic and abiotic stress. J. Appl. Microbiol. 2013, 115, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Leaungvutiviroj, C.; Ruangphisarn, P.; Hansanimitkul, P.; Shinkawa, H.; Sasaki, K. Development of a new biofertilizer with a high capacity for N2 fixation, phosphate and potassium solubilization and auxin production. Biosci. Biotechnol. Biochem. 2010, 74, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Molina, C.I.; Martinez-Romero, E.; Aguirre-Noyola, J.L.; Manzano-Gomez, L.A.; Zenteno-Rojas, A.; Rogel, M.A.; Rincon-Molina, F.A.; Ruiz-Valdiviezo, V.M.; Rincon-Rosales, R. Bacterial Community with Plant Growth-Promoting Potential Associated to Pioneer Plants from an Active Mexican Volcanic Complex. Microorganisms 2022, 10, 1568. [Google Scholar] [CrossRef]
- Mao, C.; He, J.; Liu, L.; Deng, Q.; Yao, X.; Liu, C.; Qiao, Y.; Li, P.; Ming, F. OsNAC2 integrates auxin and cytokinin pathways to modulate rice root development. Plant Biotechnol. J. 2020, 18, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B. Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant Biol. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.S.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.C. Perspectives and Challenges of Microbial Application for Crop Improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tripti Maleva, M.; Bruno, L.B.; Rajkumar, M. Synergistic effect of ACC deaminase producing Pseudomonas sp. TR15a and siderophore producing Bacillus aerophilus TR15c for enhanced growth and copper accumulation in Helianthus annuus L. Chemosphere 2021, 276, 130038. [Google Scholar] [CrossRef]
- Zheng, R.; Lv, D.; Luo, H.-B. Effect of light intensity on seed germination and physiologic index of maize. Southwest China J. Agric. Sci. 2023, 36, 497–504. [Google Scholar] [CrossRef]
- Huang, Z.H.; Wang, X.H.; Wu, G.L. Effects of Microbial Agents Treatment on Germination and Growth of Corn Seeds. J. Anhui Agric. Sci. 2022, 50, 28–30. [Google Scholar]
- Bing, Z.; Naixin, L.; Fushun, Z.; Acharya, K. Study on Water Absorption Laws of Sugar Beet Seed Germination. China Beet Sugar 2022, 2, 8–11+36. [Google Scholar]
- Dasgupta, D.; Paul, A.; Acharya, K.; Minkina, T.; Mandzhieva, S.; Gorovtsov, A.V.; Chakraborty, N.; Keswani, C. Bioinoculant mediated regulation of signalling cascades in various stress responses in plants. Heliyon 2023, 9, e12953. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R. Modulation of Ethylene and Ascorbic Acid on Reactive Oxygen Species Scavenging in Plant Salt Response. Front. Plant Sci. 2019, 10, 319. [Google Scholar] [CrossRef]
- Chaudhary, S.; Devi, P.; Bhardwaj, A.; Jha, U.C.; Sharma, K.D.; Prasad, P.; Siddique, K.; Bindumadhava, H.; Kumar, S.; Nayyar, H. Identification and Characterization of Contrasting Genotypes/Cultivars for Developing Heat Tolerance in Agricultural Crops: Current Status and Prospects. Front. Plant Sci. 2020, 11, 587264. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Totzke, C.; Kaestner, A.; Ahmed, M.A. Quantification of root water uptake and redistribution using neutron imaging: A review and future directions. Plant J. 2022, 111, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-T.; Wang, P.-L.; He, X.; Wei, J.-D.; Zhang, L.; Wang, M.-C.; Wang, Y.-F.; Yang, P.-W.; Yu, Y.-C.; Zheng, S.-J.; et al. Effects of cover plant on soil properties and microbial diversity under banana cropping system. J. South. Agric. 2020, 51, 496–504. [Google Scholar]
- Azeem, M.A.; Shah, F.H.; Ullah, A.; Ali, K.; Jones, D.A.; Khan, M.; Ashraf, A. Biochemical Characterization of Halotolerant Bacillus safensis PM22 and Its Potential to Enhance Growth of Maize under Salinity Stress. Plants 2022, 11, 1721. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Arshad, M. Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Can. J. Microbiol. 2009, 55, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Zorb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.F.; Singh, G.; Farooq, M.; Abd, A.E. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef]
- Polko, J.K.; Kieber, J.J. 1-Aminocyclopropane 1-Carboxylic Acid and Its Emerging Role as an Ethylene-Independent Growth Regulator. Front. Plant Sci. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Ali, S.; Baloch, A.M. Overview of Sustainable Plant Growth and Differentiation and the Role of Hormones in Controlling Growth and Development of Plants under Various Stresses. Recent. Pat. Food Nutr. Agric. 2020, 11, 105–114. [Google Scholar] [CrossRef]
- Barigah, T.S.; Bonhomme, M.; Lopez, D.; Traore, A.; Douris, M.; Venisse, J.S.; Cochard, H.; Badel, E. Modulation of bud survival in Populus nigra sprouts in response to water stress-induced embolism. Tree Physiol. 2013, 33, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef]
- Murali, M.; Singh, S.B.; Gowtham, H.G.; Shilpa, N.; Prasad, M.; Aiyaz, M.; Amruthesh, K.N. Induction of drought tolerance in Pennisetum glaucum by ACC deaminase producing PGPR- Bacillus amyloliquefaciens through Antioxidant defense system. Microbiol. Res. 2021, 253, 126891. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Munir, A.; Asghar, H.N.; Shaharoona, B.; Arshad, M. Effectiveness of rhizobacteria containing ACC deaminase for growth promotion of peas (Pisum sativum) under drought conditions. J. Microbiol. Biotechnol. 2008, 18, 958–963. [Google Scholar]
- Cui, Z.-Y.; Xu, H.; Tian, A.-M. Effects of Salt Stress on Growth and Physiological Characteristics of Salvia miltiorrhiza Bunge. J. Anhui Agric. Sci. 2021, 49, 70–74. [Google Scholar]
- Chai, C.; Wang, Z.; Zhang, Y.; Qi, J. Spatial Distribution Characteristics of Soil Nutrients in Semi-Arid Loess Hilly Area of Longzhong. For. Resour. Manag. 2021, 4, 114–120. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Li, Q.-Y.; Zhu, X.-W.; Jiang, S.; Yin, Y.-W. Spatial and temporal distribution characteristics of drought and its relationship with land types in Hengshao basin. Hubei Agric. Sci. 2020, 59, 56–62. [Google Scholar] [CrossRef]
- Liu, M.; Gao, H.; Gao, Y.; Xue, X. Study on the physiological and biochemical changes of Phoebe sheareri seed during its dormancy breaking. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2023, 47, 9–17. [Google Scholar]
- Ogawa, T.; Misumi, M.; Sonoike, K. Estimation of photosynthesis in cyanobacteria by pulse-amplitude modulation chlorophyll fluorescence: Problems and solutions. Photosynth. Res. 2017, 133, 63–73. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Habib, S.; Lwin, Y.Y.; Li, N. Down-Regulation of SlGRAS10 in Tomato Confers Abiotic Stress Tolerance. Genes 2021, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, A.; Chuang, H.W. Rhizobacterial Bacillus mycoides functions in stimulating the antioxidant defence system and multiple phytohormone signalling pathways to regulate plant growth and stress tolerance. J. Appl. Microbiol. 2022, 132, 1260–1274. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species. Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Bomle, D.V.; Kiran, A.; Kumar, J.K.; Nagaraj, L.S.; Pradeep, C.K.; Ansari, M.A.; Alghamdi, S.; Kabrah, A.; Assaggaf, H.; Dablool, A.S.; et al. Plants Saline Environment in Perception with Rhizosphere Bacteria Containing 1-Aminocyclopropane-1-Carboxylate Deaminase. Int. J. Mol. Sci. 2021, 22, 11461. [Google Scholar] [CrossRef]
- AAli, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; Amaral Júnior, A.T.d.; et al. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.F.; Liu, J.K.; Yang, F.M.; Zhang, G.Y.; Wang, D.; Zhang, L.; Ou, Y.B.; Yao, Y.A. The WRKY transcription factor WRKY8 promotes resistance to pathogen infection and mediates drought and salt stress tolerance in Solanum lycopersicum. Physiol. Plant 2020, 168, 98–117. [Google Scholar] [CrossRef]
- Li, H.; Zhai, B.; Sun, J.; Fan, Y.; Zou, J.; Cheng, J.; Zhang, X.; Shi, Y.; Guo, D. Antioxidant, Anti-Aging and Organ Protective Effects of Total Saponins from Aralia taibaiensis. Drug Des. Dev. Ther. 2021, 15, 4025–4042. [Google Scholar] [CrossRef]
- Wang, B.; Fang, Y.; Han, X.; Jiang, R.; Zhao, L.; Yang, X.; Jin, J.; Han, A.; Liu, J. Atomization-Induced High Intrinsic Activity of a Biocompatible MgAl-LDH Supported Ru Single-Atom Nanozyme for Efficient Radicals Scavenging. Angew. Chem. Int. Ed. Engl. 2023, 62, e202307133. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Shi, Y.; Liu, Z.; Fan, Y.; Liu, M.; Ningjing, M.; Li, Y. Promotional Properties of ACC Deaminase-Producing Bacterial Strain DY1-3 and Its Enhancement of Maize Resistance to Salt and Drought Stresses. Microorganisms 2023, 11, 2654. https://doi.org/10.3390/microorganisms11112654
Yuan Y, Shi Y, Liu Z, Fan Y, Liu M, Ningjing M, Li Y. Promotional Properties of ACC Deaminase-Producing Bacterial Strain DY1-3 and Its Enhancement of Maize Resistance to Salt and Drought Stresses. Microorganisms. 2023; 11(11):2654. https://doi.org/10.3390/microorganisms11112654
Chicago/Turabian StyleYuan, Ye, Yanlei Shi, Zhenzhen Liu, Yonghong Fan, Min Liu, Mengkedala Ningjing, and Yifei Li. 2023. "Promotional Properties of ACC Deaminase-Producing Bacterial Strain DY1-3 and Its Enhancement of Maize Resistance to Salt and Drought Stresses" Microorganisms 11, no. 11: 2654. https://doi.org/10.3390/microorganisms11112654
APA StyleYuan, Y., Shi, Y., Liu, Z., Fan, Y., Liu, M., Ningjing, M., & Li, Y. (2023). Promotional Properties of ACC Deaminase-Producing Bacterial Strain DY1-3 and Its Enhancement of Maize Resistance to Salt and Drought Stresses. Microorganisms, 11(11), 2654. https://doi.org/10.3390/microorganisms11112654






