Gastrointestinal Pathogens in Multi-Infected Individuals: A Cluster Analysis of Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Laboratory Techniques
2.3. Statistical Methods
2.4. Descriptive Analysis
2.5. Inferential Analysis
2.6. Datasets
- Dataset I
- Dataset II
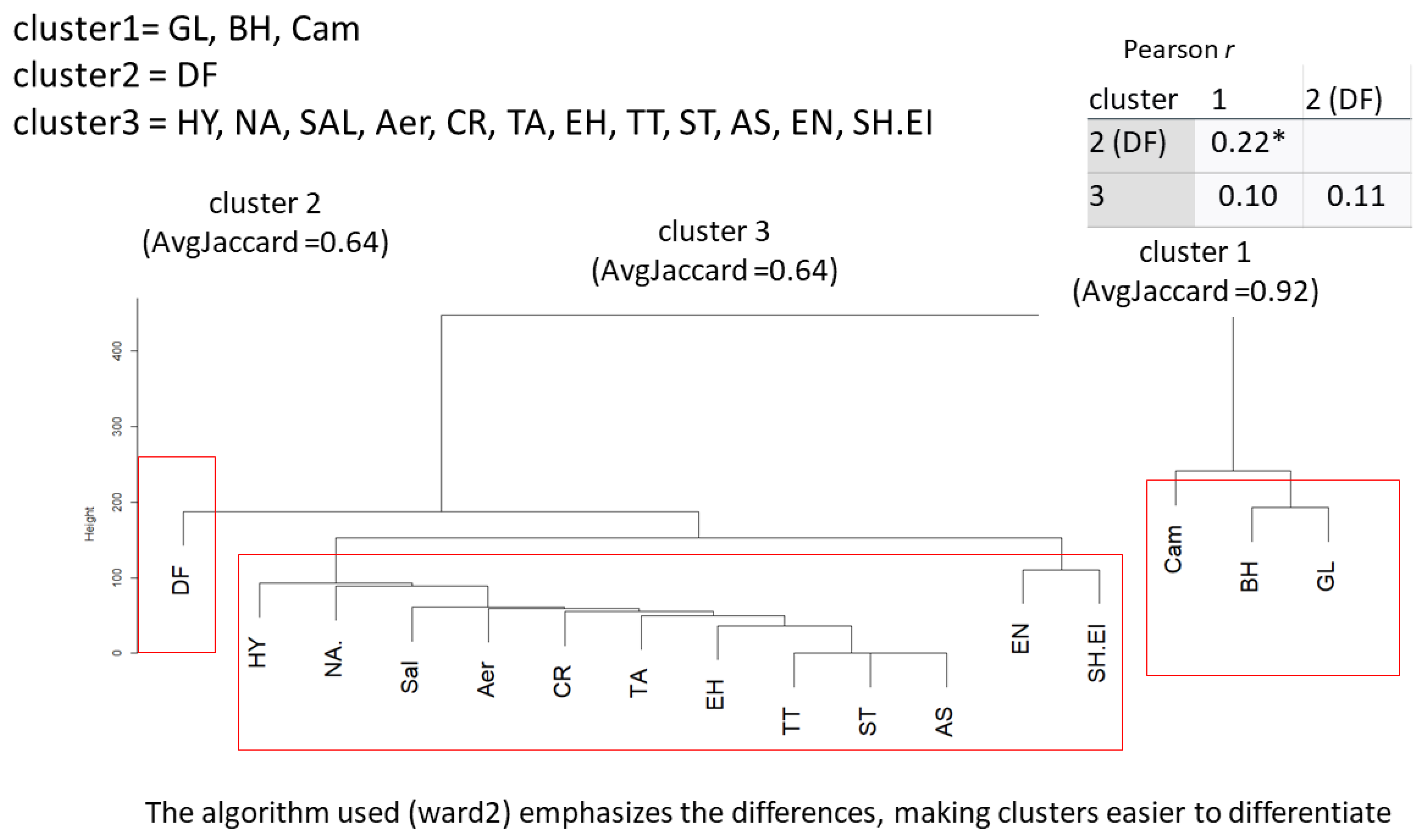
3. Results
Differences between Datasets
- Dataset I
- Dataset II
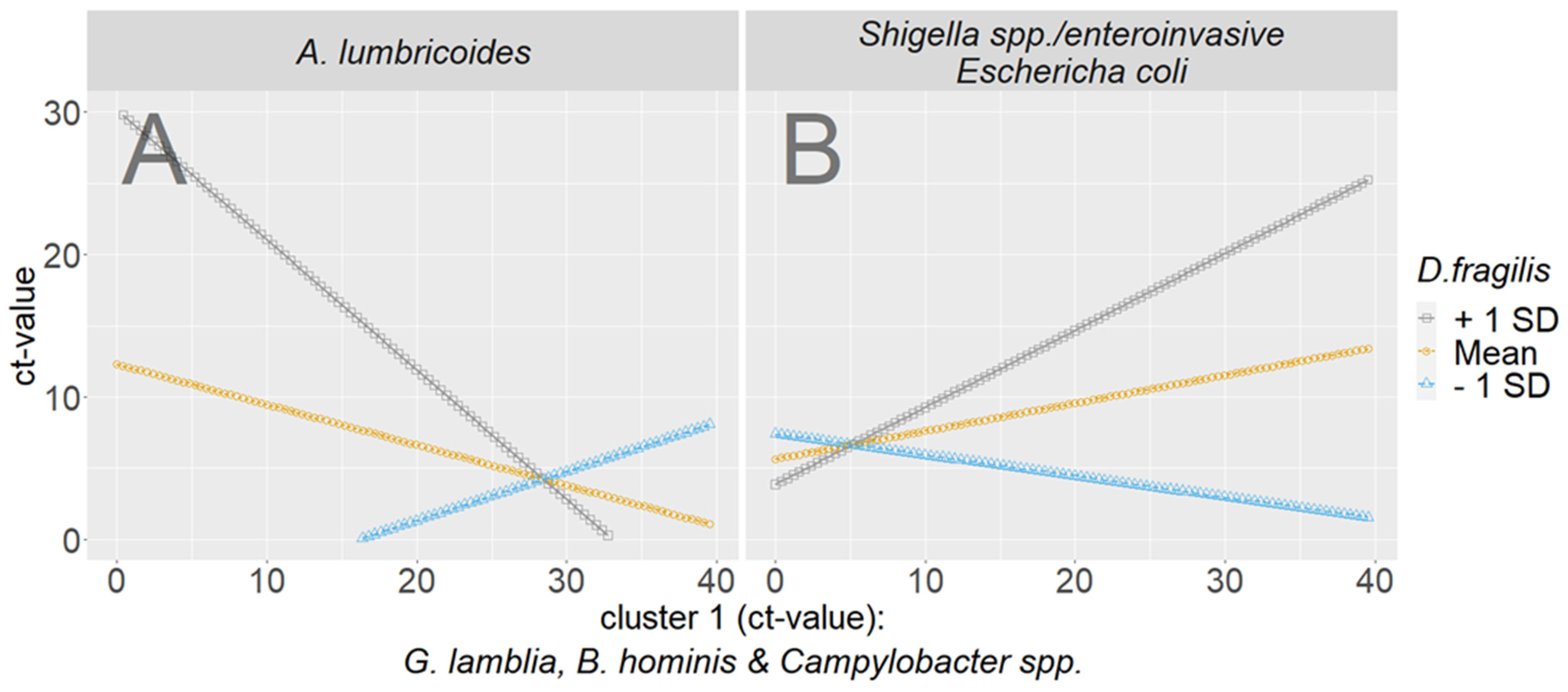
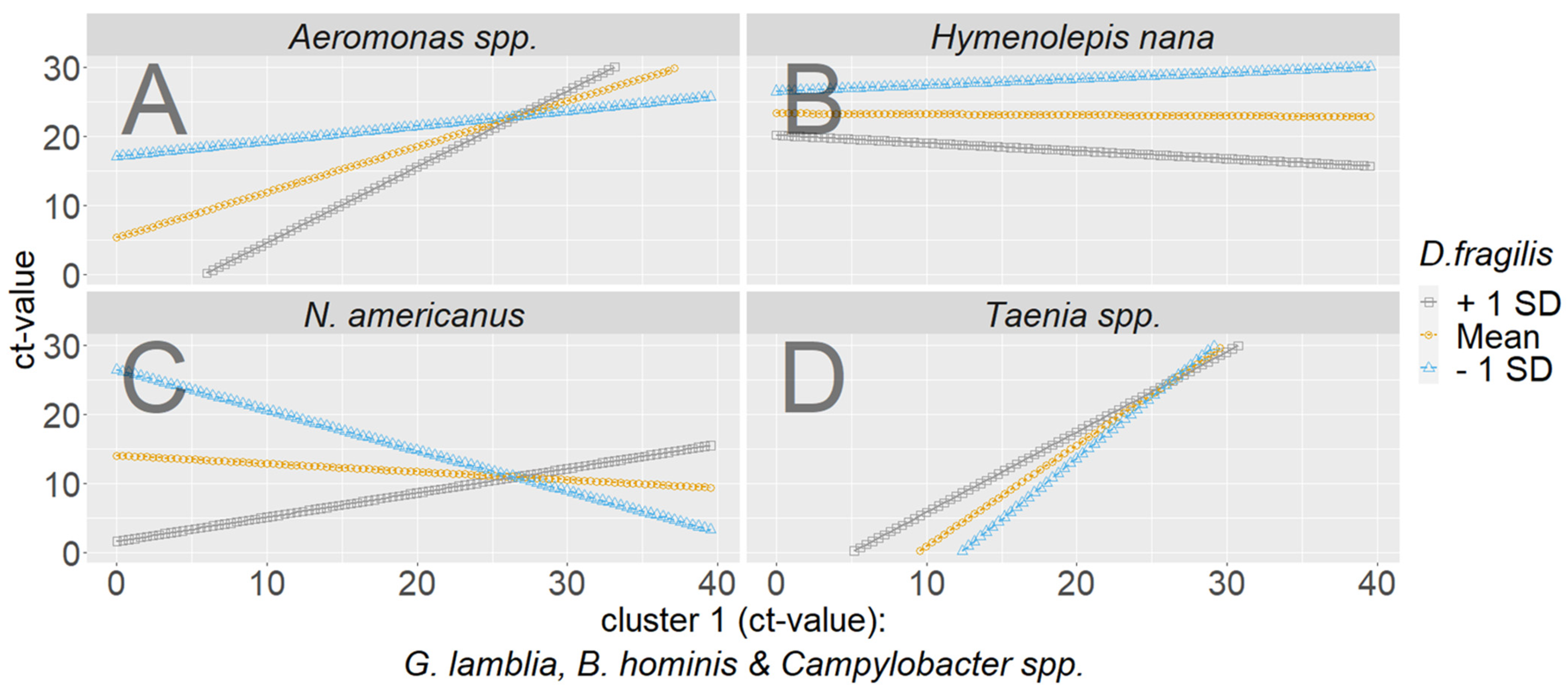
- Exploratory moderated regression to scrutinize the interplay of the clusters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kann, S.; Bruennert, D.; Hansen, J.; Mendoza, G.A.C.; Gonzalez, J.J.C.; Quintero, C.L.A.; Hanke, M.; Hagen, R.M.; Backhaus, J.; Frickmann, H. High Prevalence of Intestinal Pathogens in Indigenous in Colombia. J. Clin. Med. 2020, 9, 2786. [Google Scholar] [CrossRef]
- Kann, S.; Eberhardt, K.; Hinz, R.; Schwarz, N.G.; Dib, J.C.; Aristizabal, A.; Mendoza, G.A.C.; Hagen, R.M.; Frickmann, H.; Barrantes, I.; et al. The Gut Microbiome of an Indigenous Agropastoralist Population in a Remote Area of Colombia with High Rates of Gastrointestinal Infections and Dysbiosis. Microorganisms 2023, 11, 625. [Google Scholar]
- Frickmann, H.; Alker, J.; Hansen, J.; Dib, J.C.; Aristizabal, A.; Concha, G.; Schotte, U.; Kann, S. Seasonal Differences in Cyclospora cayetanensis Prevalence in Colombian Indigenous People. Microorganisms 2021, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Kann, S.; Hartmann, M.; Alker, J.; Hansen, J.; Dib, J.C.; Aristizabal, A.; Concha, G.; Schotte, U.; Kreienbrock, L.; Frickmann, H. Seasonal Patterns of Enteric Pathogens in Colombian Indigenous People—A More Pronounced Effect on Bacteria Than on Parasites. Pathogens 2022, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Kann, S.; Concha, G.; Hartmann, M.; Köller, T.; Alker, J.; Schotte, U.; Kreienbrock, L.; Frickmann, H.; Warnke, P. Only Low Effects of Water Filters on the Enteric Carriage of Gastrointestinal Pathogen DNA in Colombian Indigenous People. Microorganisms 2022, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Kann, S.; Concha, G.; Köller, T.; Alker, J.; Schotte, U.; Hahn, A.; Frickmann, H.; Warnke, P. Enteric Bacteria and Parasites with Pathogenic Potential in Individuals of the Colombian Indigenous Tribe Kogui. Microorganisms 2022, 10, 1862. [Google Scholar] [CrossRef]
- Köller, T.; Hahn, A.; Altangerel, E.; Verweij, J.J.; Landt, O.; Kann, S.; Dekker, D.; May, J.; Loderstädt, U.; Podbielski, A.; et al. Comparison of commercial and in-house real-time PCR platforms for 15 parasites and microsporidia in human stool samples without a gold standard. Acta Trop. 2020, 207, 105516. [Google Scholar] [CrossRef]
- Weinreich, F.; Hahn, A.; Eberhardt, K.A.; Kann, S.; Köller, T.; Warnke, P.; Dupke, S.; Dekker, D.; May, J.; Frickmann, H.; et al. Multicentric Evaluation of SeeGene Allplex Real-Time PCR Assays Targeting 28 Bacterial, Microsporidal and Parasitic Nucleic Acid Sequences in Human Stool Samples. Diagnostics 2022, 12, 1007. [Google Scholar] [CrossRef]
- Frickmann, H.; Hoffmann, T.; Köller, T.; Hahn, A.; Podbielski, A.; Landt, O.; Loderstädt, U.; Tannich, E. Comparison of five commercial real-time PCRs for in-vitro diagnosis of Entamoeba histolytica, Giardia duodenalis, Cryptosporidium spp., Cyclospora cayetanensis, and Dientamoeba fragilis in human stool samples. Travel Med. Infect. Dis. 2021, 41, 102042. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 30 August 2023).
- Rasch, D.; Guiard, V. The robustness of parametric statistical methods. Psych. Sci. 2004, 46, 175–208. [Google Scholar]
- Knief, U.; Forstmeier, W. Violating the normality assumption may be the lesser of two evils. Behav. Res. Methods 2021, 53, 2576–2590. [Google Scholar] [PubMed]
- Tang, M.; Kaymaz, Y.; Logeman, B.L.; Eichhorn, S.; Liang, Z.S.; Dulac, C.; Sackton, T.B. Evaluating single-cell cluster stability using the Jacacrd similarity index. Bioinformatics 2021, 37, 2212–2214. [Google Scholar] [CrossRef] [PubMed]
- Cobuccio, L.G.; Laurent, M.; Gardiol, C.; Wampfler, R.; Poppert, S.; Senn, N.; Eperon, G.; Genton, B.; Locatelli, I.; de Vallière, S. Should we treat Blastocystis sp.? A double-blind placebo-controlled randomized pilot trial. J. Travel Med. 2023, 30, taac143. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, D.; Schwarz, N.G.; Burchard, G.D.; Frickmann, H.; Loderstaedt, U.; Hagen, R.M. Surveillance of enteropathogenic bacteria, protozoa and helminths in travellers returning from the tropics. Eur. J. Microbiol. Immunol. 2020, 10, 147–155. [Google Scholar]
- Shah, M.; Tan, C.B.; Rajan, D.; Ahmed, S.; Subramani, K.; Rizvon, K.; Mustacchia, P. Blastocystis hominis and Endolimax nana Co-Infection Resulting in Chronic Diarrhea in an Immunocompetent Male. Case Rep. Gastroenterol. 2012, 6, 358–364. [Google Scholar] [CrossRef]
- Aykur, M.; Calıskan Kurt, C.; Dirim Erdogan, D.; Biray Avcı, C.; Vardar, R.; Aydemir, S.; Girginkardeşler, N.; Gündüz, C.; Dagci, H. Investigation of Dientamoeba fragilis Prevalence and Evaluation of Sociodemographic and Clinical Features in Patients with Gastrointestinal Symptoms. Acta Parasitol. 2019, 64, 162–170. [Google Scholar]
- Rajamanikam, A.; Isa, M.N.M.; Samudi, C.; Devaraj, S.; Govind, S.K. Gut bacteria influence Blastocystis sp. phenotypes and may trigger pathogenicity. PLoS Negl. Trop. Dis. 2023, 17, e0011170. [Google Scholar]
- Cinek, O.; Polackova, K.; Odeh, R.; Alassaf, A.; Kramná, L.; Ibekwe, M.U.; Majaliwa, E.S.; Ahmadov, G.; Elmahi, B.M.E.; Mekki, H.; et al. Blastocystis in the faeces of children from six distant countries: Prevalence, quantity, subtypes and the relation to the gut bacteriome. Parasit. Vectors 2021, 14, 399. [Google Scholar]
- Even, G.; Lokmer, A.; Rodrigues, J.; Audebert, C.; Viscogliosi, E.; Ségurel, L.; Chabé, M. Changes in the Human Gut Microbiota Associated with Colonization by Blastocystis sp. and Entamoeba spp. in Non-Industrialized Populations. Front. Cell Infect. Microbiol. 2021, 11, 533528. [Google Scholar]
- Veja, L.; Herrera, G.; Muñoz, M.; Patarroyo, M.A.; Maloney, J.G.; Santín, M.; Ramírez, J.D. Gut microbiota profiles in diarrheic patients with co-occurrence of Clostridioides difficile and Blastocystis. PLoS ONE 2021, 16, e0248185. [Google Scholar]
- Veja, L.; Herrera, G.; Muñoz, M.; Patarroyo, M.A.; Ramírez, J.D. Occurrence of Blastocystis in Patients with Clostridioides difficile Infection. Pathogens 2020, 9, 283. [Google Scholar]
- Deng, L.; Tay, H.; Peng, G.; Lee, J.W.J.; Tan, K.S.W. Prevalence and molecular subtyping of Blastocystis in patients with Clostridium difficile infection, Singapore. Parasit. Vectors 2021, 14, 277. [Google Scholar]
- Naceanceno, K.S.; Matamoros, G.; Gabrie, J.A.; Bottazzi, M.E.; Sanchez, A.; Mejia, R. Use of Multi-Parallel Real-Time Quantitative PCR to Determine Blastocystis Prevalence and Association with Other Gastrointestinal Parasite Infection in a Rural Honduran Location. Am. J. Trop. Med. Hyg. 2020, 102, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Singha, B.; Dhar, D.; Roychoudhury, S. Prevalence of Giardia intestinalis with other co-infecting parasites in Barak Valley, Assam, India: A molecular approach. J. Parasit. Dis. 2019, 43, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Taghipour, A.; Bakhshi, B.; Shams, S.; Pirestani, M. Prevalence of intestinal parasitic infections and Campylobacter spp. among children with gastrointestinal disorders in Tehran, Iran. Parasite Epidemiol. Control. 2021, 13, e00207. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montoya, G.M.; Galvan-Diaz, A.L.; Alzate, J.F. Metataxomics reveals Blastocystis subtypes mixed infections in Colombian children. Infect. Genet. Evol. 2023, 113, 105478. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, Y.G.; Liu, Z.L.; Guo, A.J.; Li, X.L.; Shi, Z.Q.; Zhu, X.Q.; Han, X.M.; Wang, S. Echinococcosis Is Associated with the Increased Prevalence of Intestinal Blastocystis Infection in Tibetans and Host Susceptibility to the Blastocystis in Mice. Biology 2022, 11, 773. [Google Scholar] [CrossRef]
- Hamdy, D.A.; Abd El Wahab, W.M.; Senosy, S.A.; Mabrouk, A.G. Blastocystis spp. and Giardia intestinalis co-infection profile in children suffering from acute diarrhea. J. Parasit. Dis. 2020, 44, 88–98. [Google Scholar]
- Khorshidvand, Z.; Khazaei, S.; Amiri, M.; Taherkhani, H.; Mirzaei, A. Worldwide prevalence of emerging parasite Blastocystis in immunocompromised patients: A systematic review and meta-analysis. Microb. Pathog. 2021, 152, 104615. [Google Scholar]
- Simsek, C.; Bloemen, M.; Jansen, D.; Beller, L.; Descheemaeker, P.; Reynders, M.; Van Ranst, M.; Matthijnssens, J. High Prevalence of Coinfecting Enteropathogens in Suspected Rotavirus Vaccine Breakthrough Cases. J. Clin. Microbiol. 2021, 59, e0123621. [Google Scholar] [CrossRef]
- Pijnacker, R.; van Pelt, W.; Vennema, H.; Kortbeek, L.M.; Notermans, D.W.; Franz, E.; Mughini-Gras, L. Clinical relevance of enteropathogen co-infections in preschool children—A population-based repeated cross-sectional study. Clin. Microbiol. Infect. 2019, 25, 1039.e7–1039.e13. [Google Scholar] [CrossRef] [PubMed]
- Boga, J.A.; Rojo, S.; Fernández, J.; Rodríguez, M.; Iglesias, C.; Martínez-Camblor, P.; Vázquez, F.; Rodríguez-Guardado, A. Is the treatment of Enterobius vermicularis co-infection necessary to eradicate Dientamoeba fragilis infection? Int. J. Infect. Dis. 2016, 49, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Röser, D.; Nejsum, P.; Carlsgart, A.J.; Nielsen, H.V.; Stensvold, C.R. DNA of Dientamoeba fragilis detected within surface-sterilized eggs of Enterobius vermicularis. Exp. Parasitol. 2013, 133, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Sukanahaketu, S. The presence of Dientamoeba fragilis in the Ascaris lumbricoides ova: The first report from Thailand. J. Med. Assoc. Thai. 1977, 60, 256–258. [Google Scholar]
- Little, R.J. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 1988, 83, 1198–1202. [Google Scholar] [CrossRef]
- Miley, D.; Machado, L.B.; Condo, C.; Jergens, A.E.; Yoon, K.J.; Pandey, S. Video capsule endoscopy and ingestible electronics: Emerging trends in sensors, circuits, materials, telemetry, optics, and rapid reading software. Adv. Devices Instrum. 2021, 2021, 9854040. [Google Scholar] [CrossRef]

| Dataset | ||||
|---|---|---|---|---|
| N = 244 | 1 (Kogui) n = 150 | 2 (Wiwa) n = 94 | Both | Differ Significantly? |
| Age M (SD) | 23.35 (18.59) | 21.01 (14.48) | 22.45 (17.13) | No t (230.7) = 1.1, p = 0.27 |
| Persons infected with at least one pathogen | ||||
| helminths n (%column) | 35 (23%) | 14 (15%) | 49 (20%) | No X2 (1, N = 244) = 2.07, p = 0.15 |
| protozoa n (%column) | 138 (92%) | 93 (99%) | 231 (95%) | Yes X2 (1, N = 244) = 4.22, p = 0.04 |
| bacteria n (%column) | 83 (55%) | 34 (36%) | 117 (48%) | Yes X2 (1, N = 244) = 7.75, p = 0.005 |
| Average infections per person (M, SD) | ||||
| helminths n (%column) | 0.28 (0.56) | 0.15 (0.36) | 0.23 (0.49) | Yes t (241.83) = 2.24, p = 0.026 |
| protozoa n (%column) | 1.53 (0.73) | 1.77 (0.72) | 1.62 (0.74) | Yes t (198.7) = −2.50, p = 0.013 |
| bacteria n (%column) | 0.62 (0.62) | 0.40 (0.57) | 0.54 (0.61) | Yes t (209.07) = 2.77, p = 0.006 |
| Maximum number of infections in one person | ||||
| helminths n (occurrence) | 3.00 (1) | 1.00 (14) | 3.00 (1) | |
| protozoa n (occurrence) | 3.00 (9) | 4.00 (1) [3 pathogens found 12 times] | 4.00 (1) | |
| bacteria n (occurrence) | 3.00 (1) | 2.00 (4) | 3.00 (1) | |
| Number of pathogens | ||||
| helminths n (%column) | 42 (28%) | 14 (15%) | 54 (22%) | |
| protozoa n (%column) | 229 (152%) | 166 (176%) | 395 (161%) | |
| bacteria n (%column) | 93 (62%) | 38 (40%) | 131 (54%) | |
| 1 (Kogui) n = 150 | 2 (Wiwa) n = 94 | ||
|---|---|---|---|
| Microsporidia | Encephalitozoon spp. | 0 | 7 |
| Helminths | Strongyloides spp. | 2 | 0 |
| Hymenolepis spp. | 11 | 3 | |
| Ascaris spp. | 12 | 0 | |
| Taenia spp. | 4 | 1 | |
| Trichuris trichiura | 9 | 0 | |
| Necator americanus | 4 | 3 | |
| Protozoa | Blastocystis hominis | 132 | 90 |
| Giardia lamblia | 55 | 59 | |
| Dientamoeba fragilis | 41 | 15 | |
| Entamoeba histolytica | 0 | 1 | |
| Cryptosporidium spp. | 1 | 1 | |
| Bacteria | Shigella spp./EIEC | 11 | 7 |
| Campylobacter spp. | 78 | 29 | |
| Aeromonas spp. | 4 | 1 | |
| Salmonella spp. | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Backhaus, J.; Frickmann, H.; Hagen, R.M.; Concha, G.; Molitor, E.; Hoerauf, A.; Kann, S. Gastrointestinal Pathogens in Multi-Infected Individuals: A Cluster Analysis of Interaction. Microorganisms 2023, 11, 2642. https://doi.org/10.3390/microorganisms11112642
Backhaus J, Frickmann H, Hagen RM, Concha G, Molitor E, Hoerauf A, Kann S. Gastrointestinal Pathogens in Multi-Infected Individuals: A Cluster Analysis of Interaction. Microorganisms. 2023; 11(11):2642. https://doi.org/10.3390/microorganisms11112642
Chicago/Turabian StyleBackhaus, Joy, Hagen Frickmann, Ralf Matthias Hagen, Gustavo Concha, Ernst Molitor, Achim Hoerauf, and Simone Kann. 2023. "Gastrointestinal Pathogens in Multi-Infected Individuals: A Cluster Analysis of Interaction" Microorganisms 11, no. 11: 2642. https://doi.org/10.3390/microorganisms11112642
APA StyleBackhaus, J., Frickmann, H., Hagen, R. M., Concha, G., Molitor, E., Hoerauf, A., & Kann, S. (2023). Gastrointestinal Pathogens in Multi-Infected Individuals: A Cluster Analysis of Interaction. Microorganisms, 11(11), 2642. https://doi.org/10.3390/microorganisms11112642







