Investigation of the Persistence, Toxicological Effects, and Ecological Issues of S-Triazine Herbicides and Their Biodegradation Using Emerging Technologies: A Review
Abstract
1. Introduction
2. Potential Microbial Species Employed for Biodegradation of S-Triazines
| Target S-Triazine Herbicide | Microorganisms | Source of Isolation | Degradation (%) | References |
|---|---|---|---|---|
| Atrazine | Acinetobacter sp. Strain A6 | Polluted soil | 80 | [37] |
| Rhodococcus sp. BCH2 | Polluted soil | 75 | [38] | |
| Bacillus badius ABP6 | Maize field | 89.7 | [39] | |
| Arthrobacter sp. C3 | Corn field | 100 | [40] | |
| Cryptococcus laurentii | Agricultural soil | 100 | [41] | |
| Klebsiella variicola FH-1 Arthrobacter sp. NJ-1 | Polluted soil | 97.4 | [42] | |
| Ochrobactrum oryzae | Wastewater | 83.5 | [43] | |
| Rhodobacter sphaeroides W16 | Agricultural soil | 96.86 | [44] | |
| Penicillium sp. | Corn field | 50 | [45] | |
| Pseudomonas sp. ADP | Commercial | 99 | [46] | |
| Citricoccus sp. strain TT3 | Wastewater | 100 | [47] | |
| Alcaligenes sp. S3 | Agricultural field | 90.56 | [48] | |
| Arthrobacter sp. DNS10 Enterobacter sp. P1 | Polluted soil | 99.18 | [49] | |
| Pseudomonas sp. | Agricultural soil | 99 | [50] | |
| Pleurotus ostreatus INCQS 40310 | Commercial | 82 | [51] | |
| Atraton | Leucobacter sp. JW-1 | Polluted soil | 98 | [52] |
| Leucobacter sp. JW-1 | Polluted soil | 13 | [53] | |
| Pseudomonas sp. V1 | Wastewater | 10 | [54] | |
| Ametryn | Chryseobacterium sp. Variovorax sp., Aeromonas sp. and Xanthobacter sp. | Agricultural soil | 97 | [55] |
| Rhodococcus sp. FJ1117YT | Agricultural soil | 50 | [56] | |
| Scenedesmus vacuolatus | Wastewater | 94.7 | [57] | |
| Acidithiobacillu ferrooxidans | Acid mine drainage | 84.9 | [58] | |
| Arthrobacter sp. MCM B-436 | Rhizosphere soil | 92 | [59] | |
| Acidithiobcillus ferrooxidans BMSNITK17 | Soil | 94.24 | [60] | |
| Pseudomonas sp. YAYA6 | Soil | 50 | [61] | |
| Pseudomonas sp. DY-1 | Paddy field | 87.8 | [62] | |
| Rhodococcus sp. FJ1117YT | Soil | 48.9 | [56] | |
| Nocardioides sp. DN36 | Paddy field | 100 | [18] | |
| Leucobacter sp. JW-1 | Soil | 99.9 | [52] | |
| Cyanazine | Agrobacterium radiobacte M91-3 | Commercial | 100 | [63] |
| Arthrobacter nicotinovorans HIM | Agricultural soil | 100 | [64] | |
| Rhodococcus corallinus, Pseudomonas sp. D | Soil | 99 | [65] | |
| Rhodococcus TE1 | Soil | 100 | [66] | |
| Acinetobacter sp. A6 | Agricultural soil | 100 | [67] | |
| Arthrobacter sp. MCM B-436 | Rhizosphere soil | 97 | [59] | |
| Desmetryn | Pseudomonas sp. DY-1 | Paddy field | 93.6 | [62] |
| Rhodococcus sp. FJ1117YT | Soil | 63.9 | [56] | |
| Dimethametryn | Rhodococcus sp. FJ1117YT | Agricultural soil | 100 | [68] |
| Bacillus cereus JUN7 | Soil | 100 | [69] | |
| Rhodococcus sp. FJ1117YT | Soil | 81.1 | [56] | |
| Nocardioides sp. DN36 | Paddy field | 100 | [18] | |
| Pleurotus mutilus | Commercial | 70 | [70] | |
| Burkholderia cepacia CH-9 | Soil | 86 | [71] | |
| Botrytis cinerea, Sordaria superba, Absidia fusca | Wastewater | 50 | [72] | |
| Paracoccus sp. QCT6 | Polluted soil | 86.4 | [73] | |
| Bacillus subtilis | Agricultural soil | 98 | [74] | |
| Pseudomonas sp. DY-1 | Paddy field | 38.2 | [62] | |
| Rhodococcus sp. FJ1117YT | Soil | 75.5 | [56] | |
| Nocardioides sp. DN36 | Paddy field | 100 | [18] | |
| Prometryn | Microbacterium sp., Enterobacter sp., Acinetobacter sp., and Flavobacterium sp. | Soil | 100 | [75] |
| Rhodococcus sp. FJ1117YT | Soil | 75.5 | [56] | |
| Nocardioides sp. DN36 | Paddy field | 100 | [18] | |
| Leucobacter sp. JW-1 | Soil | 100 | [52] | |
| Aspergillus sp. | Commercial | 61 | [76] | |
| Leucobacter sp. JW-1 | Soil | 100 | [53] | |
| Pseudomonas sp. DY-1 | Agricultural soil | 100 | [62] | |
| Prometon | Leucobacter sp. JW-1 | Soil | 95.2 | [52] |
| Leucobacter sp. JW-1 | Soil | 10 | [53] | |
| Propazine | Leucobacter sp. JW-1 | Soil | 100 | [52] |
| Pseudomonas stutzeri sp. Y2 | Commercial | 96 | [77] | |
| Arthrobacter sp. MCM B-436 | Rhizosphere soil | 87 | [59] | |
| Leucobacter sp. JW-1 | Soil | 41 | [53] | |
| Pleurotus ostreatus INCQS 40310 | Commercial | 90 | [78] | |
| Phanerochaete chrysosporium | Commercial | 100 | [79] | |
| Simazine | Klebsiella pneumoniae | Soil | 100 | [80] |
| Arthrobacter sp. MCM B-436 | Rhizosphere soil | 73 | [59] | |
| β-proteobacterium CDB21 | Bacterial consortium | 100 | [81] | |
| Bradyrhizobium japonicam CSB1, Arthrobacter sp. CD7w and β-Proteobacteria CDB21 | Agricultural soil | 100 | [68] | |
| Leucobacter sp. JW-1 | Soil | 77.9 | [52] | |
| Phanerochaete chrysosporium | Commercial | 100 | [79] | |
| Arthrobacter ureafaciens XMJ-Z01 | Soil | 99.1 | [34] | |
| Leucobacter sp. JW-1 | Soil | 28 | [53] | |
| Pseudomonas stutzeri sp. Y2 | Commercial | 100 | [77] | |
| Simetryn | Pseudomonas sp. DY-1 | Paddy field | 90.2 | [62] |
| Rhodococcus sp. FJ1117YT | Agricultural soil | 100 | [68] | |
| Nocardioides sp. DN36 | Paddy field | 100 | [18] | |
| Rhodococcus sp. FJ1117YT | Soil | 100 | [56] | |
| Leucobacter sp. JW-1 | Soil | 100 | [53] | |
| Bacillus cereus JUN7 | Soil | 100 | [69] | |
| Leucobacter sp. JW-1 | Soil | 77.9 | [52] | |
| Simeton | Nocardioides sp. DN36 | Paddy field | 100 | [18] |
| Terbuthylazine | Leucobacter sp. JW-1 | Soil | 98.9 | [52] |
| Pseudomonas stutzeri sp. Y2 | Commercial | 100 | [77] | |
| Phanerochaete chrysosporium | Commercial | 100 | [79] | |
| Terbumeton | Leucobacter sp. JW-1 | Soil | 31.6 | [52] |
| Leucobacter sp. JW-1 | Soil | 12 | [53] |
2.1. Degradation of S-Triazines Using Microbial Enzymes and Genes
2.2. Microbial Metabolic Pathways of S-Triazine Herbicides
3. Phytoremediation of S-Triazine Herbicides
| Target S-Triazine Herbicide | Name of Plant Species | Statement | References |
|---|---|---|---|
| Atrazine | Potamogeton crispus Myriophyllum spicatum | Both species efficiently degraded atrazine up to 90%, and the half-life of atrazine was recorded at 8.60 and 9.72 days, respectively, for both plants. | [141] |
| Lolium perenne Festuca arundinacea Hordeum vulgare Zea mays | All plant species were able to degrade atrazine into their metabolites. The final degradation by plant species was 88.6–99.6%, while in unplanted plots, the degradation of atrazine was 63.1–78.2%. | [142] | |
| Panicum virgatum | The results revealed that this plant species detoxifies atrazine into its non-toxic metabolites, which are present in the leaf only. | [143] | |
| Lolium perenne L. | In this investigation, the electrokinetic-assisted phytoremediation of atrazine was tested. The results revealed that plant species degraded atrazine 27% faster as compared to natural attenuation. | [144] | |
| Phaseolus vulgaris L. | Through the combination of rhizome microbial species and plants, 76.63% of atrazine was degraded at a concentration of 50 mg/L in soil. | [145] | |
| Eremanthus crotonoides Inga striata | Atrazine was degraded by both plant species, and these plants prohibited the leaching of atrazine residues into the underground water system. | [146] | |
| Pistia stratiotes Eichhornia crassipes | Atrazine was spiked in both plants at a concentration of 10–100 µg/L in 5 L pots. The results demonstrated that atrazine residual concentration was less in the plant system as compared to the water solution. | [147] | |
| Potamogeton crispus Myriophyllum spicatum | Both plants absorbed atrazine and converted it into their metabolites. Additionally, chromatography analysis revealed that an equal concentration of atrazine was adsorbed by roots and shoots. | [148] | |
| Ametryn | Ananas comosus | Gas chromatography and mass spectrometry analysis revealed that the residues of ametryn were degraded effectively, and 0.002 mg/kg of atrazine in the plant and 0.005 mg/kg in the soil were detected. | [149] |
| Digitaria horizontalis | The results revealed that this plant was able to treat ametryn in avocado fields up to 6 L/ha. | [150] | |
| Cyanazine | Panicum dichotomiflorum Setaria viridis L. Zea mays | The results revealed that cyanazine more rapidly metabolizes in Zea mays and a large amount of cyanazine was absorbed by the root system as compared to other plant systems. | [151] |
| Sorghum bicolor L. | In this investigation, cyanazine and atrazine were applied and the degradation efficiency of the plant was tested. The results showed that the half-life of cyanazine was 15 days, and in atrazine, 60 days was observed. | [152] | |
| Zea mays | The breakdown of cyanazine in corn was investigated and the results revealed that it converts into hydrolysis products such as amide and hydroxy acid. | [153] | |
| Bromus inermis Leyss Elymus junceus Ficsch. Agropyron intermedium | All the grasses completely removed cyanazine, and a very small concentration of 0.02 mg/kg was detected via gas chromatography. | [154] | |
| Typha latifolia | This plant was investigated for the removal of s-triazine herbicides (atrazine, ametryn, and dimethametryn). The results revealed that the flower of this plant effectively removed residues of all treated pesticides and this method is cost-effective, easy, and ecofriendly. | [155] | |
| Metribuzin | Glycine max L. | The results revealed that the tetraploid of this plant showed great resistance, while diploid plants are highly susceptible. Moreover, tetraploid plants efficiently degraded metribuzin into non-toxic substances. | [156] |
| Triticum turgidum L. | The residual concentration of metribuzin was tested via gas chromatography. The results showed that a very small quantity of metribuzin is present in plants, and others are degraded into less toxic substances that are further used by the plant system. | [157] | |
| Glycine max L. | The results demonstrated that metribuzin was absorbed by the plant system efficiently. About 97% of metribuzin was absorbed by the root system and transformed into its metabolites. | [158] | |
| Daucus carota L. | The residues of metribuzin and other herbicides were monitored in this plant. The results indicated that the highest uptake was observed by the root system as compared to the leaves and shoots. | [159] | |
| Bromus tectorum Triticum aestivum | The tolerance rate of metribuzin was investigated by both plant species. The results indicated that Bromus tectorum absorbs and transforms a two-times-higher concentration of metribuzin as compared to other plants. | [160] | |
| Prometryn | Phaseolus vulgaris L. | A 10–100 µM concentration of prometryn was applied. The results indicated that the bean plant tolerated prometryn and was a reliable material that could be used for herbicide pollution sensing. | [161] |
| Arachis hypogaea | Various degradation percentages of prometryn were observed in different parts of plants. The results of gas chromatography revealed that the lowest concentration of metribuzin detected was 0.02 mg/kg. | [162] | |
| Angelica acutiloba | The residues of prometryn were degraded more effectively by the root system and the final concentration detected was 0.0368 in dried roots. Meanwhile, after harvesting of the plant, a concentration of prometryn of 0.0464 was observed via gas chromatography and mass spectrometry analysis. | [163] | |
| Pontederia cordata L. Typha latifolia L. and Cyperus alternifolius L. | These three plants degraded s-triazine herbicides (atrazine, prometryn, and simazine) in more than 25% of the wastewater. | [164] | |
| Propazine | Zea mays Triticum aestivum Brassica napus | The results indicated that propazine was effectively degraded by all plants. Six metabolites of propazine were detected in the wheat plant. Through the foliar application of salicylic acid (plant growth regulator), the herbicide rapidly degraded and the growth of the plant increased. | [165] |
| Gossypium hirsutum | The results revealed that a higher concentration of propazine is tolerated by this plant and causes less injury when applied at the pre-emergence stage. This plant uptakes the residue of propazine rapidly. | [166] | |
| Zea mays | The results revealed that 96% of propazine was degraded at an initial concentration of 50 mg/L by maize and bacteria-immobilized beads. | [77] | |
| Simazine | Typha latifolia | The results revealed that after seven days, the residues of 65%simazine were degraded. This study concluded that this plant is an excellent candidate for the removal of simazine from polluted soil. | [167] |
| Oryza sativa | The human cytochrome P450 CYP1A1 gene was injected into rice plants. The results concluded that human genes make it possible to tolerate simazine and atrazine herbicides and rapidly degrade their residues in culture media and polluted soil. | [168] | |
| Canna hybrida | The results revealed that 80% of simazine was degraded after seven days by plants and considered a good candidate for the removal of herbicides. | [169] | |
| Zea mays | In corn plants, the absorption and translocation of atrazine and simazine were studied. The results revealed that both herbicides were metabolized into non-toxic products. | [170] | |
| Zea mays | The results indicated that simazine was rapidly degraded by the root and shoot of corn plants in polluted soil. | [171] | |
| Terbutryn | Myriophyllum aquaticum | The uptake translocation and metabolism of atrazine and terbutryn were investigated. The results revealed that a lower concentration of terbutryn was observed in roots and shoots as compared to atrazine. Moreover, plants metabolized both herbicides into less toxic substances. | [172] |
| Gammarus fossarum Asellus aquaticus | The results indicated that complete degradation of terbutryn was observed in both plant species, and a half-life of terbutryn of 5 h was recorded. | [173] | |
| Gossypium hirsutum Phaseolus vulgaris | Terbutryn was degraded by both plant species and a higher concentration was mineralized by the root system of a plant. | [174] | |
| Terbuthylazine | Lolium multiflorum | An experiment was performed using Lolium multiflorum and terbuthylazine at aqueous concentrations of 0.5, 1.0, and 2.0 mg/L. The plant was able to remove 38%, 42%, and 33% of the initial concentrations, respectively, in 240 h; over this period, the capacity of plants to absorb the herbicide at 0.5 mg L1 increased from 1.58 to 3.50 mg g1 of fresh weight. | [175] |
| Lolium multiflorum L. | The ryegrass efficiently degraded terbuthylazine through the activation of glutathione s-transferase and peroxidase enzymes. | [176] | |
| Lemna minor | This study concluded that duckweed is suitable for cleaning terbuthylazine-polluted water from the environment and degrading toxic residues through the activation of peroxidase and catalase enzymes. | [177] | |
| Typha latifolia L. | The plant degraded terbuthylazine into its metabolites in the wetland system. These results concluded that this plant could be used for the remediation of pollutants from wastewater. | [178] | |
| Typha latifolia L. | This study concluded that the degradation of terbuthylazine showed gradient behavior via the depth of the sediment substrate of wetlands, and its metabolites followed the effect of the biotic and abiotic mechanisms of degradation in the bioreactor substrate. | [179] |
4. Nano-Remediation of S-Triazine Herbicides
| S-Triazine Herbicide | Type of Nanoparticles | Function | References |
|---|---|---|---|
| Atrazine | Fe3O4 | At optimum conditions, nanoparticles performed well and efficiently degraded 80% of atrazine in an aqueous solution with a short retention time of 20 min. Moreover, atrazine degraded into various intermediate products and finally degraded into less toxic substances. | [193] |
| TiO2 modified with (Au, Cu, and Ni) | At an initial concentration of 25 mg/L, titanium nanoparticles were able to degrade 48% of atrazine, while their modification with a gold catalyst performed well and degraded 60% of atrazine at a retention time of 300 min. | [194] | |
| CoFe2O4 | To remove atrazine from wastewater, hybrid cobalt ferrite nanoparticles were synthesized and employed as a highly efficient peroxymonosulfate activator, which rapidly degraded 98.2% of atrazine. | [195] | |
| TiO2 modified with Fe+3 | At an initial concentration of 25 mg/L, modified titanium oxide performs well at pH 11, and following UV radiation, 99% degradation of atrazine was achieved in water samples. | [196] | |
| Carbon nanotubes | Carbon nanotubes with three different catalysts (pure metallic pallidum, oxide, and silver-coated pallidum) completely removed atrazine from water. | [197] | |
| Bi2O3 | At a neutral pH, initial concentrations of 5 mg/L and 1 g/L of Bi2O3 nanoparticles with an average size of 20 nm were able to degrade 92.1% of atrazine within one hour. | [198] | |
| Cu–Cu2O | Cu–Cu2O nanoparticles were optimized and synthesized via a simple route. The results revealed that complete degradation of atrazine was achieved with 30 min of visible light in water samples. | [199] | |
| Fe3O4 | Mesoporous Fe3O4 nanoparticles were synthesized for the removal of atrazine from water. The results revealed that they showed higher catalytic ozonation activity and their activation level increased by increasing pH, which effectively degraded atrazine from the aqueous solution. | [200] | |
| Co3O4/TiO2 | Co3O4/TiO2 nanoparticles were produced through the simple sol-gel method. Modified nanoparticles perform catalytic reactions, which effectively degrade atrazine from water samples. Moreover, sulfate radicals play a crucial role in the remediation process. | [201] | |
| GO-α-γ-Fe2O3 | Graphene oxide and iron oxide nanoparticles were synthesized for the remediation of atrazine from water. Via an adsorption process, atrazine was rapidly degraded. | [202] | |
| Ametryn | Fe | Spherical green iron nanoparticles with a size of 20–70 nm were synthesized by eucalyptus leaf extracts. The results revealed that at a pH range of 2–5 and retention time of 30–240 min, ametryn was effectively degraded from water. | [203] |
| Fe3O4/rGO | The results revealed that at pH 5 and a temperature of 25 °C, using a nanocomposite efficiently degraded 93.61 of prometryn within 70 min in water samples. | [204] | |
| Fe | In this study, iron nanoparticles at a size of 75 nm were synthesized from the leaves of Tectona Grandis and the authors investigated their degradation efficiency in the water. The results revealed that at optimum conditions, complete degradation was achieved with a retention time of 135 min. | [205] | |
| TiO2 | At an initial concentration of 0.4 g/L with a TiO2 catalyst, complete removal of ametryn was observed within one hour. Ametryn was further degraded into its metabolites in water samples. | [206] | |
| GO-Fe3O4 | Reduced graphene oxide sheets were investigated using dopamine and decorated with magnetic Fe3O4 nanoparticles with an average size of 12 nm via a simple co-precipitation method that produced artificial nano-enzymes. The results revealed that nano-enzymes were able to degrade 92% of ametryn. | [207] | |
| Cyanazine | Fe | An eco-friendly, less costly, and feasible iron nanoparticle was synthesized for the removal of cyanazine from water. The results revealed that nanoparticles at pH 7, a temperature of 25 °C, and a retention time of 30 min performed efficiently and removed cyanazine. | [208] |
| TiO2 | The degradation of simazine in polluted water by TiO2 stimulated with solar light was investigated. The results demonstrated that cyanazine and other pesticides rapidly degraded in an aqueous solution. | [209] | |
| TiO2 | For the treatment of organic wastewater, an aggression experiment with TiO2 nanoparticles was carried out. The results indicated that after 12 h, using particles with a size of 84 nm, residues of cyanazine and other organic compounds gradually decreased. | [210] | |
| Metribuzin | Zero-valent iron nanoparticles | The results demonstrated that zero-valent iron nanoparticles depended on pH, and the degradation of metribuzin was also affected by changing the value of pH. At pH 10, 7, and 4, the degradation of 93.22%, 83.74%, and 70.09% of metribuzin was achieved, respectively, in water samples. | [211] |
| Ag-ZnO | The results indicated that upon using 100 mg Ag/ZnO composites, 90% degradation of metribuzin at a concentration of 14 mg/L in 90 min was recorded. | [212] | |
| SnS2 | The results showed that metribuzin was degraded into a deaminometribuzin metabolite by attacking SnS2 nanoparticles. This study concluded that the degradation of metribuzin in water was very slow using this type of nanoparticle. | [213] | |
| H-Ag-BMO/TiO2 | This study concluded that under visible light irradiation, H-Ag-BMO/TiO2 shows excellent photodegradation of 93.7% for metribuzin. | [214] | |
| TiO2 | The mineralization and degradation of metribuzin using titanium oxide nanoparticles was investigated. The results showed that at a concentration of 100 mg/L of metribuzin and 10 mg/L of titanium oxide nanoparticles, 80% degradation was achieved within 300 min of irradiation. | [215] | |
| Prometryn | Pt-TiO2/(Er3+:Y3Al5O12@Ta2O5) | The results indicated that Z-scheme P-TET/(OB or Syn)-HAP sonocatalysts were synthesized. To investigate their sonocatalytic efficiency, various parameters such as ultrasonic irradiation time, inorganic oxidants, used times, and trapping agents on the sonocatalytic degradation of prometryn were studied. The best degradation ratio (80.31% based on N computing and 85.07% based on S atom computing) of prometryn could be achieved for 10 mmol/L K2S2O8, 1 g/L P-TET/OB-HAP sonocatalyst and 150 min ultrasonic irradiation. | [216] |
| Al2Si2O5(OH)4-H2SO4 | A halloysite was modified with H2SO4 for the removal of prometryn from aqueous samples. The results demonstrated that modified halloysite performs excellently at pH 5 and degraded 96% of prometryn. | [217] | |
| Fe3O4-TiO2/rGO | The results indicated that at optimum conditions (nanoparticle catalysts 0.5 g/L, concentration of herbicide 15 mg/L, and pH 5), 94% degradation of prometryn was achieved in water samples. | [218] | |
| TiO2 | Hydrogen peroxide was immobilized with titanium oxide nanoparticles, and their degradation efficiency for prometryn in an aqueous solution was investigated. The results indicated that both herbicides were degraded, and the final product, cyanuric acid, was obtained. | [219] | |
| Fe3O4/rGO | The results revealed that at pH 5 and a temperature of 25 °C, using nanocomposite efficiently degraded 91.34 of prometryn within 70 min in water samples. | [204] | |
| Prometon | TiO2 | The photocatalytic remediation of prometon and other s-triazine herbicides was investigated using titanium oxide nanoparticles under simulated solar light. The results showed that all herbicides were efficiently removed from water samples. | [220] |
| TiO2 | Hydrogen peroxide was immobilized with titanium oxide nanoparticles, and their degradation efficiency for prometryn and prometon in an aqueous solution was investigated. The results indicated that both herbicides were degraded, and the final product, cyanuric acid, was obtained. | [219] | |
| Propazine | FeO | The adsorption of propazine in an aqueous solution using iron oxide nanoparticles with modification of carbon nanoparticles was investigated. The results indicated that the modified nanoparticles performed efficiently at low pH. | [221] |
| TiO2 | The degradation of simazine in polluted water by TiO2 stimulated with solar light was investigated. The results demonstrated that propazine and other s-triazine herbicides rapidly degraded in an aqueous solution. | [209] | |
| Simazine | TiO2-Cu | TiO2-Cu nanoparticles were synthesized by anodic oxidation method for the removal of simazine. Findings showed that at optimum conditions 64% photodegradation of simazine was achieved. | [222] |
| Fe3O4/rGO | Results revealed that at pH 5 and temperature 25 °C using nanocomposite efficiently degraded 88.55 of simazine within 70 min in water samples. | [204] | |
| Au–TiO2 | The sonophotocatalytic removal of simazine based on Au–TiO2 was investigated. Simazine was degraded into its intermediate products and finally mineralized into less toxic substances. | [223] | |
| Fe3O4-TiO2/rGO | The results indicated that at optimum conditions (nanoparticle catalysts 0.5, g/L, concentration of herbicide 15 mg/L, and pH 5) 90% degradation of simazine was achieved in water samples. | [218] | |
| TiO2 | Titanium oxide nanotubes efficiently degraded simazine and converted it into the cyanuric acid final product under UV light. | [222] | |
| GO-Fe3O4 | The findings indicated that the highest removal of simazine, 97%, was achieved in 50 min of sunlight irradiation at optimum conditions (catalyst loading 0.3 g/L, concentration of simazine 0.3 mM, and pH 5). | [207] | |
| Simeton | Fe3O4/rGO | The results revealed that at pH 5 and a temperature of 25 °C, using a nanocomposite efficiently degraded 81.22 of simeton within 70 min in water samples. | [201] |
| Fe3O4-TiO2/rGO | The results indicated that at optimum conditions (nanoparticle catalysts 0.5, g/L, concentration of herbicide 15 mg/L, and pH 5), 92% degradation of simeton was achieved in water samples. | [218] | |
| GO-Fe3O4 | Reduced graphene oxide sheets were investigated using dopamine and decorated with magnetic Fe3O4 nanoparticles with an average size of 12 nm via a simple co-precipitation method, which produced artificial nano-enzymes. The results revealed that nano-enzymes were able to degrade 89% of simeton in water samples. | [207] | |
| Terbutryn | TiO2 | The photodegradation of terbutryn using titanium oxide nanotubes as a photocatalysis was studied. The results indicated that 70% of terbutryn was degraded within one hour. | [224] |
| FeSO4-Fe (0) | The findings revealed that the FeSO4 and Fe (0) catalytic Fenton oxidation process was able to completely remove terbutryn and other pollutants from the wastewater. | [225] | |
| Zero-variant iron nanopowder | At various concentrations of terbutryn (1–1000 µg/L), complete removal of terbutryn was observed at pH 3 and 5 in wastewater using zero-variant iron nanopowder. | [226] | |
| N-TiO2 | The results demonstrated that photocatalytic oxidation and ozonation using the highest tube diameter were efficient in removing almost 100% of terbutryn in aqueous samples. | [227] | |
| Terbuthylazine | TiO2 | Via a photocatalytic oxidation process, TiO2 nanoparticles effectively removed terbuthylazine from wastewater samples. | [228] |
| FeSO4-Fe (0) | The findings revealed that the FeSO4 and Fe (0) catalytic Fenton oxidation process was able to completely remove terbuthylazine and other pollutants from the wastewater. | [225] | |
| TiO2 | Titanium oxide nanoparticles were immobilized in chitosan glass fiber for the removal of terbuthylazine. Under UV irradiation, the satisfactory removal of terbuthylazine was observed in water samples. | [229] | |
| Zero-variant iron nanopowder | At various concentrations of terbutryn (1–1000 µg/L), complete removal of terbutryn was observed at pH 3 and 70% at pH 5 in wastewater using zero-variant iron nanopowder. | [226] | |
| TiO2-H2O2 | The results demonstrated that under visible light and a hydrogen peroxide catalyst, titanium oxide nanoparticles efficiently performed and degraded 100% of terbuthylazine within 180 min. | [230] |
5. In Silico Methods Used for Prediction of Toxicological Effects of S-Triazine Herbicides
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Viegas, C.A.; Silva, V.P.; Varela, V.M.; Correia, V.; Ribeiro, R.; Moreira-Santos, M. Evaluating formulation and storage of Arthrobacter aurescens strain TC1 as a bioremediation tool for terbuthylazine contaminated soils: Efficacy on abatement of aquatic ecotoxicity. Sci. Total Environ. 2019, 668, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Seffernick, J.L.; Wackett, L.P. Ancient evolution and recent evolution converge for the biodegradation of cyanuric acid and related triazines. Appl. Environ. Microbiol. 2016, 82, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, J.; Vela, N.; Navarro, G.; Pérez-Lucas, G.; Navarro, S. Assessment of agro-industrial and composted organic wastes for reducing the potential leaching of triazine herbicide residues through the soil. Sci. Total Environ. 2014, 493, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Bub, S.; Petschick, L.L.; Stehle, S.; Wolfram, J. Applied pesticide toxicity shifts toward plants and invertebrates, even in GM crops. Science 2021, 372, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Mnyandu, H.; Mahlambi, P. Optimization and application of QuEChERS and SPE methods followed by LC-PDA for the determination of triazines residues in fruits and vegetables from Pietermaritzburg local supermarkets. Food Chem. 2021, 360, 129818. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, A.A.; Aboul-Enein, H.Y. Separation and analysis of triazine herbcide residues by capillary electrophoresis. Biomed. Chromatogr. 2015, 29, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, X.; Chen, J.; Li, X.; Jia, G.; Zou, Y.; Zhang, Y.; Cui, Y. Occurrence, distribution and ecological risks of antibiotics and pesticides in coastal waters around Liaodong Peninsula, China. Sci. Total Environ. 2019, 656, 946–951. [Google Scholar] [CrossRef]
- Tian, H.; Fu, H.; Xu, C.; Xu, C. Simultaneous determination of three herbicides in honey samples using an aqueous biphasic system coupled with HPLC–MS/MS. Chromatographia 2019, 82, 1571–1577. [Google Scholar] [CrossRef]
- Graymore, M.; Stagnitti, F.; Allinson, G. Impacts of atrazine in aquatic ecosystems. Environ. Int. 2001, 26, 483–495. [Google Scholar] [CrossRef]
- McKenzie, M.R.; Templeman, M.A.; Kingsford, M. Detecting effects of herbicide runoff: The use of Cassiopea maremetens as a biomonitor to hexazinone. Aquat. Toxicol. 2020, 221, 105442. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhang, Y.; Gu, X.; Tysklind, M.; Lin, C.; Wang, B.; Xin, M. Occurrence, transportation, and distribution difference of typical herbicides from estuary to bay. Environ. Int. 2019, 130, 104858. [Google Scholar] [CrossRef] [PubMed]
- Reindl, A.R.; Falkowska, L.; Grajewska, A. Chlorinated herbicides in fish, birds and mammals in the Baltic Sea. Water Air Soil Pollut. 2015, 226, 276. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, H.W.; Bhatt, P. Microbial adaptation and impact into the pesticide’s degradation. Arch. Microbiol. 2022, 204, 288. [Google Scholar] [CrossRef] [PubMed]
- Neuwirthová, N.; Trojan, M.; Svobodová, M.; Vašíčková, J.; Šimek, Z.; Hofman, J.; Bielská, L. Pesticide residues remaining in soils from previous growing season(s)—Can they accumulate in non-target organisms and contaminate the food web? Sci. Total Environ. 2019, 646, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Chaudhary, P.; Ahmad, S.; Bhatt, K.; Chandra, D.; Chen, S. Recent advances in the application of microbial inoculants in the phytoremediation of xenobiotic compounds. In Unravelling Plant-Microbe Synergy; Chandra, D., Bhatt, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 37–48. [Google Scholar] [CrossRef]
- Korpraditskul, R. Degradation of atrazine by soil bacteria in the stationary phase. J. Pestic. Sci. 1993, 18, 293–298. [Google Scholar] [CrossRef][Green Version]
- Erickson, L.E.; Lee, K.H.; Sumner, D.D. Degradation of atrazine and related s-triazines. Crit. Rev. Environ. Cont. 1989, 19, 1–14. [Google Scholar] [CrossRef]
- Satsuma, K. Mineralization of s-triazine herbicides by a newly isolated Nocardioides species strain DN36. Appl. Microbiol. Biotechnol. 2010, 86, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Chen, M.; Cheng, Z.; Xie, L.; Li, M. Bioaugmentation of strain Methylobacterium sp. C1 towards p-nitrophenol removal with broad spectrum coaggregating bacteria in sequencing batch biofilm reactors. J. Hazard. Mater. 2018, 344, 431–440. [Google Scholar] [CrossRef]
- Ahmad, S.; Dongming, C.; Zhong, G.; Liu, J. Microbial technologies employed for biodegradation of neonicotinoids in the agroecosystem. Front. Microbiol. 2021, 3510, 759439. [Google Scholar] [CrossRef]
- Ahmad, S.; Bhatt, P.; Ahmad, H.W.; Cui, D.; Guo, J.; Zhong, G. Enzymes involved in the bioremediation of pesticides. In Industrial Applications of Microbial Enzymes; Bhatt, P., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 133–168. [Google Scholar] [CrossRef]
- Wirsching, J.; Pagel, H.; Ditterich, F.; Uksa, M.; Werneburg, M.; Zwiener, C.; Berner, D.; Kandeler, E.; Poll, C. Biodegradation of pesticides at the limit: Kinetics and microbial substrate use at low concentrations. Front. Microbiol. 2020, 11, 2107. [Google Scholar] [CrossRef]
- Gangola, S.; Sharma, A.; Joshi, S.; Bhandari, G.; Prakash, O.; Govarthanan, M.; Kim, W.; Bhatt, P. Novel mechanism and degradation kinetics of pesticides mixture using Bacillus sp. strain 3C in contaminated sites. Pestic. Biochem. Physiol. 2022, 181, 104996. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Santhiya, D.; Sharma, J.G. Microbial remediation of micro-nano plastics: Current knowledge and future trends. Environ. Pollut. 2020, 265, 115044. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Tripathi, G.D.; Mishra, M.; Dashora, K. Actinomycetes—The microbial machinery for the organic-cycling, plant growth, and sustainable soil health. Biocatal. Agric. Biotechnol. 2021, 31, 101893. [Google Scholar] [CrossRef]
- Bharadwaj, A. 2018. Bioremediation of Xenobiotics: An Eco-friendly Cleanup Approach. In Green Chemistry in Environmental Sustainability and Chemical Education; Parmar, V., Malhotra, P., Mathur, D., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Ma, F.; Bai, S.; Yang, J.; Qi, S. Pseudomonas sp. ZXY-1, a newly isolated and highly efficient atrazine-degrading bacterium, and optimization of biodegradation using response surface methodology. J. Environ. Sci. 2017, 54, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Lett. 2018, 16, 211–237. [Google Scholar] [CrossRef]
- Karlsson, A.S.; Weihermüller, L.; Tappe, W.; Mukherjee, S.; Spielvogel, S. Field scale boscalid residues and dissipation half-life estimation in a sandy soil. Chemosphere 2016, 145, 163–173. [Google Scholar] [CrossRef]
- Cao, D.; He, S.; Li, X.; Shi, L.; Wang, F.; Yu, S.; Xu, S.; Ju, C.; Fang, H.; Yu, Y. Characterization, genome functional analysis, and detoxification of atrazine by Arthrobacter sp. C2. Chemosphere 2021, 264, 128514. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Y.; Fan, Z.; Liu, F.; Liu, H.; Wang, L.; Wu, H. Soil bacterial community dynamics following bioaugmentation with Paenarthrobacter sp. W11 in atrazine-contaminated soil. Chemosphere 2021, 282, 130976. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, L.; Meng, Z.; Jin, C. Characteristics of an atrazine degrading bacterium and the construction of a microbial agent for effective atrazine degradation. Water Environ. J. 2021, 35, 7–17. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Fu, L.; Liu, Z.; Li, X.; Meng, Z. Application of a simazine degrading bacterium, Arthrobacter ureafaciens XMJ-Z01 for bioremediation of simazine pollution. Water Environ. J. 2020, 34, 561–572. [Google Scholar] [CrossRef]
- Błaszak, M.; Pełech, R.; Graczyk, P. Screening of microorganisms for biodegradation of simazine pollution (Obsolete Pesticide Azotop 50 WP). Water Air Soil Pollut. 2011, 220, 373–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, Q.; Wan, R.; Xie, S. Simazine degradation in bioaugmented soil: Urea impact and response of ammonia-oxidizing bacteria and other soil bacterial communities. Environ. Sci. Pollut. Res. 2014, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Cameotra, S.S. Influence of microbial and synthetic surfactant on the biodegradation of atrazine. Environ. Sci. Pollut. Res. 2014, 21, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Kolekar, P.D.; Phugare, S.S.; Jadhav, J.P. Biodegradation of atrazine by Rhodococcus sp. BCH2 to N-isopropylammelide with subsequent assessment of toxicity of biodegraded metabolites. Environ. Sci. Pollut. Res. 2014, 21, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, H.; Rai, J. Optimization studies on biodegradation of atrazine by Bacillus badius ABP6 strain using response surface methodology. Biotechnol. Rep. 2020, 26, e00459. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Li, J.; Lin, M.; Hu, X. Biodegradation of atrazine by Arthrobacter sp. C3, isolated from the herbicide-contaminated corn field. Int. J. Environ. Sci. Technol. 2016, 13, 257–262. [Google Scholar] [CrossRef]
- Evy, A.; Lakshmi, V.; Nilanjana, D.J.J.o.M.; Research, B. Biodegradation of atrazine by Cryptococcus laurentii isolated from contaminated agricultural soil. J. Microb. Biotech. Res. 2012, 2, 450–457. [Google Scholar]
- Gao, N.; Zhang, J.; Pan, Z.; Zhao, X.; Ma, X.; Zhang, H. Biodegradation of atrazine by mixed bacteria of Klebsiella variicola strain FH-1 and Arthrobacter sp. NJ-1. Bull. Environ. Contam. Toxicol. 2020, 105, 481–489. [Google Scholar] [CrossRef]
- Reyad, A.; Radwan, T.; Ibrahim, W.M.; Essa, A. Biodegradation of atrazine by Ochrobactrum oryzae isolated from the agricultural wastewater. Wulfenia 2014, 21, 286–310. [Google Scholar]
- Du, J.; Zhang, Y.; Ma, Y.; Li, J.; Zhang, Q. Simulation study of atrazine-contaminated soil biodegradation by strain W16. Procedia Environ. Sci. 2011, 11, 1488–1492. [Google Scholar] [CrossRef][Green Version]
- Singh, S.B.; Lal, S.P.; Pant, S.; Kulshrestha, G.J. Degradation of atrazine by an acclimatized soil fungal isolate. J. Environ. Sci. Health B. 2008, 43, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.; Viana, P.; André, S.; Chelinho, S.; Costa, C.; Ribeiro, R.; Sousa, J.; Fialho, A.; Viegas, C. Evaluating a bioremediation tool for atrazine contaminated soils in open soil microcosms: The effectiveness of bioaugmentation and biostimulation approaches. Chemosphere 2009, 74, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, H.; Zhu, C.; Geng, B. Biodegradation of atrazine by the novel Citricoccus sp. strain TT3. Ecotoxicol. Environ. Saf. 2018, 147, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Geed, S.; Prasad, S.; Kureel, M.; Singh, R.; Rai, B. Biodegradation of wastewater in alternating aerobic-anoxic lab scale pilot plant by Alcaligenes sp. S3 isolated from agricultural field. J. Environ. Manag. 2018, 214, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, X.; Wang, Z.; Cao, B.; Deng, S.; Bi, M.; Zhang, Y. Enhanced biodegradation of atrazine by Arthrobacter sp. DNS10 during co-culture with a phosphorus solubilizing bacterium: Enterobacter sp. P1. Ecotoxicol. Environ. Saf. 2019, 172, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.F.T.; Braz, V.S.; Bauermeister, A.; Paschoal, J.A.R.; Lopes, N.P.; Stehling, E.G. Degradation of atrazine by Pseudomonas sp. and Achromobacter sp. isolated from Brazilian agricultural soil. Int. Biodeterior. Biodegrad. 2018, 130, 17–22. [Google Scholar] [CrossRef]
- de Oliveira Lopes, R.; Pereira, P.M.; Pereira, A.R.B.; Fernandes, K.V.; Carvalho, J.F.; da Silva de França, A.; Icon, R.H.V.; da Silva Icon, M.; Ferreira-Leitão, V.S. Ferreira-Leitão. Atrazine, desethylatrazine (DEA) and desisopropylatrazine (DIA) degradation by Pleurotus ostreatus INCQS 40310. Biocatal. Biotransformation 2020, 38, 415–430. [Google Scholar] [CrossRef]
- Liu, J.; Pan, D.; Wu, X.; Chen, H.; Cao, H.; Li, Q.X.; Hua, R. Enhanced degradation of prometryn and other s-triazine herbicides in pure cultures and wastewater by polyvinyl alcohol-sodium alginate immobilized Leucobacter sp. JW-1. Sci. Total Environ. 2018, 615, 78–86. [Google Scholar] [CrossRef]
- Liu, J.; Hua, R.; Lv, P.; Tang, J.; Wang, Y.; Cao, H.; Wu, X.; Li, Q.X. Novel hydrolytic de-methylthiolation of the s-triazine herbicide prometryn by Leucobacter sp. JW-1. Sci. Total Environ. 2017, 579, 115–123. [Google Scholar] [CrossRef]
- Udiković Kolić, N.; Martin-Laurent, F.; Devers, M.; Petrić, I.; Begonja Kolar, A.; Hršak, D. Genetic potential, diversity and activity of an atrazine-degrading community enriched from a herbicide factory effluent. J. Appl. Microbiol. 2008, 105, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Carrasco, C.A.; Ahuatzi-Chacón, D.; Galíndez-Mayer, J.; Ruiz-Ordaz, N.; Juárez-Ramírez, C.; Martínez-Jerónimo, F. Biodegradation of a mixture of the herbicides ametryn, and 2, 4-dichlorophenoxyacetic acid (2, 4-D) in a compartmentalized biofilm reactor. Bioresour. Technol. 2013, 145, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Takagi, K.; Hiradate, S.; Iwasaki, A.; Harada, N. Biodegradation of methylthio-s-triazines by Rhodococcus sp. strain FJ1117YT, and production of the corresponding methylsulfinyl, methylsulfonyl and hydroxy analogues. Pest Manag. Sci. 2007, 63, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Tantawy, M.M.; Seleim, Z.M.; Al-Marsafy, H.T.; Hassanein, A.M. Metabolic detoxification as a viable mechanism for triazine herbicide resistance in ametryn resistant biotype of the fresh water chlorophyte, Scenedesmus vacuolatus. Sci. Res. Essays 2009, 4, 1331–1341. [Google Scholar]
- Bhaskar, S.; Manu, B.; Sreenivasa, M.Y. Bacteriological synthesis of iron hydroxysulfate using an isolated Acidithiobacillus ferrooxidans strain and its application in ametryn degradation by Fenton’s oxidation process. J. Environ. Manag. 2019, 232, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, P.A.; Kanekar, P.P.; Dhakephalkar, P.K. Isolation and characterization of Arthrobacter sp. strain MCM B-436, an atrazine-degrading bacterium, from rhizospheric soil. Int. Biodeterior. Biodegrad. 2007, 60, 273–278. [Google Scholar] [CrossRef]
- Manu, B.; MY, S. Bioleaching of iron from laterite soil using an isolated Acidithiobacillus ferrooxidans strain and application of leached laterite iron as Fenton’s catalyst in selective herbicide degradation. PLoS ONE 2021, 16, e0243444. [Google Scholar] [CrossRef]
- Yanze-Kontchou, C.; Gschwind, N. Mineralization of the herbicide atrazine as a carbon source by a Pseudomonas strain. Appl. Environ. Microbiol. 1994, 60, 4297–4302. [Google Scholar] [CrossRef]
- Liang, D.; Xiao, C.; Song, F.; Li, H.; Liu, R.; Gao, J. Complete Genome Sequence and Function Gene Identify of Prometryne-Degrading Strain Pseudomonas sp. DY-1. Microorgansims 2021, 9, 1261. [Google Scholar] [CrossRef]
- Gebendinger, N.; Radosevich, M. Inhibition of atrazine degradation by cyanazine and exogenous nitrogen in bacterial isolate M91-3. Appl. Microbiol. Biotechnol. 1999, 51, 375–381. [Google Scholar] [CrossRef]
- Aislabie, J.; Bej, A.K.; Ryburn, J.; Lloyd, N.; Wilkins, A. Characterization of Arthrobacter nicotinovorans HIM, an atrazine-degrading bacterium, from agricultural soil New Zealand. FEMS Microbiol. Ecol. 2005, 52, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.M.; Hickey, W.J.; Harris, R.F.; Talaat, R.E. Integrating chemical and biological remediation of atrazine and S-triazine-containing pesticide wastes. Environ. Toxicol. Chem. 1996, 15, 1255–1262. [Google Scholar] [CrossRef]
- Behki, R.; Topp, E.; Dick, W.; Germon, P. Metabolism of the herbicide atrazine by Rhodococcus strains. Appl. Environ. Microbiol. 1993, 59, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Suri, C.; Cameotra, S.S. Isolation of a member of Acinetobacter species involved in atrazine degradation. Biochem. Biophys. Res. Commun. 2004, 317, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.-I.; Takagi, K.; Fujii, K.; Iwasaki, A.; Harada, N.; Uchimura, T. Simultaneous biodegradation of chloro-and methylthio-s-triazines using charcoal enriched with a newly developed bacterial consortium. J. Pestic. Sci. 2008, 33, 266–270. [Google Scholar] [CrossRef]
- Harada, N.; Takagi, K.; Fujii, K.; Iwasaki, A. Transformation of methylthio-s-triazines via sulfur oxidation by strain JUN7, a Bacillus cereus species. Soil Biol. Biochem. 2006, 38, 2952–2957. [Google Scholar] [CrossRef]
- Behloul, M.; Lounici, H.; Abdi, N.; Drouiche, N.; Mameri, N. Adsorption study of metribuzin pesticide on fungus Pleurotus mutilus. Int. Biodeterior. Biodegrad. 2017, 119, 687–695. [Google Scholar] [CrossRef]
- Gopal, M.; Dutta, D.; Jha, S.; Kalra, S.; Bandyopadhyay, S.; Das, S.K. Biodegradation of imidacloprid and metribuzin by Burkholderia cepacia strain CH9. Pestic. Res. J. 2011, 23, 36–40. [Google Scholar]
- Memić, M.; Vrtačnik, M.; Vatrenjak-Velagić, V.; Grm, K.W. Comparative biodegradation studies of pre-emergence broadleaf and grass herbicides in aqueous medium. Int. Biodeterior. Biodegrad. 2005, 55, 109–113. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, H.; Chen, F.; Song, M. Colonization of Paracoccus sp. QCT6 and enhancement of metribuzin degradation in maize rhizosphere soil. Curr. Microbiol. 2018, 75, 156–162. [Google Scholar] [CrossRef]
- Ergüven, G.Ö. Bacillus Subtilis bakterisi ile metribuzin herbisitinin biyoıslahının yapay tarla düzeneğinde araştırılması. Int. J. Pure Appl. Sci. 2019, 5, 46–52. [Google Scholar] [CrossRef]
- Pérez-Bárcena, J.F.; Ahuatzi-Chacón, D.; Castillo-Martínez, K.L.; Ruiz-Ordaz, N.; Galíndez-Mayer, J.; Juárez-Ramírez, C.; Ramos-Monroy, O. Effect of herbicide adjuvants on the biodegradation rate of the methylthiotriazine herbicide prometryn. Biodegradation 2014, 25, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Q.; Liu, F.; Peng, Q.; Bian, Y. Performance and kinetic of pesticide residues removal by microporous starch immobilized laccase in a combined adsorption and biotransformation process. Environ. Technol. Innov. 2021, 21, 101235. [Google Scholar] [CrossRef]
- Zhang, B.; Ni, Y.; Liu, J.; Yan, T.; Zhu, X.; Li, Q.X.; Hua, R.; Pan, D.; Wu, X. Bead-immobilized Pseudomonas stutzeri Y2 prolongs functions to degrade s-triazine herbicides in industrial wastewater and maize fields. Sci. Total Environ. 2020, 731, 139183. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.B.; Pereira, P.M.; da Silva de França, A.; da Silva, M. Propazine degradation by intra-and extracellular enzymes from Pleurotus ostreatus INCQS 40310. Biocatal. Biotransformation 2016, 34, 66–75. [Google Scholar] [CrossRef]
- Mougin, C.; Laugero, C.; Asther, M.; Chaplain, V. Biotransformation of s-Triazine Herbicides and Related Degradation Products in Liquid Cultures by the White Rot Fungus Phanerochaete chrysosporium. Pestic. Sci. 1997, 49, 169–177. [Google Scholar] [CrossRef]
- Chan, C.Y.; Chan, H.S.; Wong, P.K. Integrated photocatalytic-biological treatment of triazine-containing pollutants. Chemosphere 2019, 222, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Takagi, K.; Yoshioka, Y.; Fujii, K.; Kojima, Y.; Harada, N. Isolation and characterization of a novel simazine-degrading β-proteobacterium and detection of genes encoding s-triazine-degrading enzymes. Pest Manag. Sci. 2007, 63, 261–268. [Google Scholar] [CrossRef]
- Wahla, A.Q.; Anwar, S.; Mueller, J.A.; Arslan, M.; Iqbal, S. Immobilization of metribuzin degrading bacterial consortium MB3R on biochar enhances bioremediation of potato vegetated soil and restores bacterial community structure. J. Hazard. Mater. 2020, 390, 121493. [Google Scholar] [CrossRef]
- Takagi, K.; Fujii, K.; Yamazaki, K.-i.; Harada, N.; Iwasaki, A. Biodegradation of melamine and its hydroxy derivatives by a bacterial consortium containing a novel Nocardioides species. Appl. Microbiol. Biotechnol. 2012, 94, 1647–1656. [Google Scholar] [CrossRef]
- Shiomi, N.; Ako, M. Biodegradation of melamine and cyanuric acid by a newly-isolated microbacterium strain. Adv. Microbiol. 2012, 2, 303. [Google Scholar] [CrossRef][Green Version]
- Radosevich, M.; Traina, S.J.; Tuovinen, O.H. Degradation of binary and ternary mixtures of s-triazines by a soil bacterial isolate. J. Environ. Sci. Health B 1995, 30, 457–471. [Google Scholar] [CrossRef]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H. Diverse metabolic capacities of fungi for bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Sagar, V.; Singh, D. Biodegradation of lindane pesticide by non white-rots soil fungus Fusarium sp. World J. Microbiol. Biotechnol. 2011, 27, 1747–1754. [Google Scholar] [CrossRef]
- Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Overview on the biochemical potential of filamentous fungi to degrade pharmaceutical compounds. Front. Microbiol. 2017, 8, 1792. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Naranjo, S.B.; da Silva, G.F.; Duque-Castaño, D.C.; Araújo, W.L.; Peres, C.K.; Boroski, M.; Bonugli-Santos, R.C. Potential for the biodegradation of atrazine using leaf litter fungi from a subtropical protection area. Curr. Microbiol. 2021, 78, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Gallardo, B.E.; Guzmán-Gil, R.; Colín-Luna, J.A.; García-Martínez, J.C.; León-Santiesteban, H.H.; González-Brambila, O.M.; González-Brambila, M.M. Atrazine biodegradation in soil by Aspergillus niger. Can. J. Chem. Eng. 2021, 99, 932–946. [Google Scholar] [CrossRef]
- Pereira, P.M.; Teixeira, R.S.S.; de Oliveira, M.A.L.; da Silva, M.; Ferreira-Leitão, V.S. Optimized atrazine degradation by Pleurotus ostreatus INCQS 40310: An alternative for impact reduction of herbicides used in sugarcane crops. J. Microb. Biochem. Technol. 2013, S12, 1–8. [Google Scholar] [CrossRef]
- Dhiman, N.; Jasrotia, T.; Sharma, P.; Negi, S.; Chaudhary, S.; Kumar, R.; Mahnashi, M.H.; Umar, A.; Kumar, R. Immobilization interaction between xenobiotic and Bjerkandera adusta for the biodegradation of atrazine. Chemosphere 2020, 257, 127060. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Tu, C.; Lin, S.; Xing, D.; Hill, P.; Chadwick, D.; Jones, D.L. Arbuscular mycorrhizal fungi and biochar influence simazine decomposition and leaching. Glob. Chang. Biol. Bioenergy 2021, 13, 708–718. [Google Scholar] [CrossRef]
- Nishimura, K.; Yamamoto, M.; Nakagomi, T.; Takiguchi, Y.; Naganuma, T.; Uzuka, Y. Biodegradation of triazine herbicides on polyvinylalcohol gel plates by the soil yeast Lipomyces starkeyi. Appl. Microbiol. Biotechnol. 2002, 58, 848–852. [Google Scholar] [CrossRef]
- Mollamohammada, S.; Aly Hassan, A.; Dahab, M. Immobilized algae-based treatment of herbicide-contaminated groundwater. Water Environ. Res. 2021, 93, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Wang, J.; Wang, W.; Wu, X. Purification, characterization, and catalytic mechanism of N-Isopropylammelide isopropylaminohydrolase (AtzC) involved in the degradation of s-triazine herbicides. Environ. Pollut. 2021, 268, 115803. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhai, W.; Liu, D.; Wang, P. Coexisting antibiotic changes the persistence and metabolic profile of atrazine in the environment. Chemosphere 2021, 269, 129333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Deng, S.; Wang, L.; Hu, Y.; Cao, B.; Lv, J.; Qu, J.; Wang, L.; Wang, Y.; Zhang, Y. Nicosulfuron inhibits atrazine biodegradation by Arthrobacter sp. DNS10: Influencing mechanisms insight from bacteria viability, gene transcription and reactive oxygen species production. Environ. Pollu. 2021, 273, 116517. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Singh, D.K. Atrazine detoxification by intracellular crude enzyme extracts derived from epiphytic root bacteria associated with emergent hydrophytes. J. Environ. Sci. Health B 2021, 56, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Wackett, L.; Sadowsky, M.; Martinez, B.; Shapir, N. Biodegradation of atrazine and related s-triazine compounds: From enzymes to field studies. Appl. Microbiol. Biotechnol. 2002, 58, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Strong, L.C.; Rosendahl, C.; Johnson, G.; Sadowsky, M.J.; Wackett, L.P. Arthrobacter aurescens TC1 metabolizes diverse s-triazine ring compounds. Appl. Environ. Microbiol. 2002, 68, 5973–5980. [Google Scholar] [CrossRef]
- Balotra, S.; Newman, J.; Cowieson, N.P.; French, N.G.; Campbell, P.M.; Briggs, L.J.; Warden, A.C.; Easton, C.J.; Peat, T.S.; Scott, C. X-ray structure of the amidase domain of AtzF, the allophanate hydrolase from the cyanuric acid-mineralizing multienzyme complex. Appl. Environ. Microbiol. 2015, 81, 470–480. [Google Scholar]
- Devers, M.; El Azhari, N.; Kolic, N.-U.; Martin-Laurent, F. Detection and organization of atrazine-degrading genetic potential of seventeen bacterial isolates belonging to divergent taxa indicate a recent common origin of their catabolic functions. FEMS Microbiol. Lett. 2007, 273, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, S.; Yuan, J.; Yang, P.; Liu, Y.; Stewart, K. Rapid biodegradation of atrazine by Ensifer sp. strain and its degradation genes. Int. Biodeterior. Biodegrad. 2017, 116, 133–140. [Google Scholar] [CrossRef]
- Sajjaphan, K.; Shapir, N.; Wackett, L.P.; Palmer, M.; Blackmon, B.; Tomkins, J.; Sadowsky, M. Arthrobacter aurescens TC1 atrazine catabolism genes trzN, atzB, and atzC are linked on a 160-kilobase region and are functional in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 4402–4407. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, J.; Wan, R.; Xie, S. Impacts of carbon sources on simazine biodegradation by Arthrobacter strain SD3-25 in liquid culture and soil microcosm. Int. Biodeterior. Biodegrad. 2014, 89, 1–6. [Google Scholar] [CrossRef]
- Fajardo, C.; Saccà, M.L.; Gibello, A.; Martinez-Iñigo, M.J.; Nande, M.; Lobo, C.; Martin, M. Assessment of s-triazine catabolic potential in soil bacterial isolates applying atz genes as functional biomarkers. Water Air Soil Pollut. 2012, 223, 3385–3392. [Google Scholar] [CrossRef]
- Li, Z.J.E.; Safety, E. Improving screening model of pesticide risk assessment in surface soils: Considering degradation metabolites. Ecotoxicol. Environ. Saf. 2021, 222, 112490. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Jaffar, S.; Ahmad, S.; Lu, Y. Contribution of insect gut microbiota and their associated enzymes in insect physiology and biodegradation of pesticides. Front. Microbiol. 2022, 13, 979383. [Google Scholar] [CrossRef]
- Szewczyk, R.; Kuśmierska, A.; Bernat, P. Ametryn removal by Metarhizium brunneum: Biodegradation pathway proposal and metabolic background revealed. Chemosphere 2018, 190, 174–183. [Google Scholar] [CrossRef]
- Jurina, T.; Terzić, S.; Ahel, M.; Stipičević, S.; Kontrec, D.; Kurtanjek, Ž.; Udiković-Kolić, N. Catabolism of terbuthylazine by mixed bacterial culture originating from s-triazine-contaminated soil. Appl. Microbiol. Biotechnol. 2014, 98, 7223–7232. [Google Scholar] [CrossRef]
- Jiang, C.; Lu, Y.C.; Xu, J.Y.; Song, Y.; Song, Y.; Zhang, S.H.; Ma, L.Y.; Lu, F.F.; Wang, Y.K.; Yang, H. Activity, biomass and composition of microbial communities and their degradation pathways in exposed propazine soil. Ecotoxicol. Environ. Saf. 2017, 145, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Morgante, V.; Flores, C.; Villalobos, P.; González, M.; Miralles, P.; Dinamarca, A.; Seeger, M. Modern approaches for the study of s-triazine herbicide bioremediation in agricultural soils. J. Soil Sci. Plant Nutr. 2008, 8, 19–30. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, S.; Wang, X.; Lu, Z.; Sun, P.; Zhang, H.; Sun, F. Biodegradation of atrazine by the novel Klebsiella variicola strain FH-1. BioMed Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Gu, J.-G.; Qiao, C.; Duan, S.; Gu, J.-D. Degradability of atrazine, cyanazine, and dicamba in methanogenic enrichment culture microcosms using sediment from the Pearl River of Southern China. Biol. Fertil. Soils 2006, 42, 395–401. [Google Scholar] [CrossRef]
- Gu, J.-G.; Qiao, C.; Gu, J.-D. Biodegradation of the herbicides atrazine, cyanazine, and dicamba by methanogenic enrichment cultures from selective soils of China. Bull. Environ. Contam. Toxicol. 2003, 71, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Q.; Liu, F.; Peng, Q.; Teng, P. Removal of nine pesticide residues from water and soil by biosorption coupled with degradation on biosorbent immobilized laccase. Chemosphere 2019, 233, 49–56. [Google Scholar] [CrossRef]
- Santos, E.; Pires, F.R.; Icon, A.D.F.; Filho, F.B.E.; da Rocha Junior, P.R. Phytoremediation and natural attenuation of sulfentrazone: Mineralogy influence of three highly weathered soils. Int. J. Phytoremediation 2019, 21, 652–662. [Google Scholar] [CrossRef]
- Wei, Z.; Van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef]
- Eevers, N.; White, J.C.; Vangronsveld, J.; Weyens, N. Bio-and phytoremediation of pesticide-contaminated environments: A review. Adv. Bot. Res. 2017, 83, 277–318. [Google Scholar] [CrossRef]
- Rohrbacher, F.; St-Arnaud, M. Root exudation: The ecological driver of hydrocarbon rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y. Role of insect gut microbiota in pesticide degradation: A review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.-X.; Yu, J.; Zhao, H.-M.; Cheng, Y.-T.; Mo, C.-H.; Cai, Q.-Y.; Li, Y.-W.; Li, H.; Wong, M.-H. Efficient phytoremediation of organic contaminants in soils using plant–endophyte partnerships. Sci. Total Environ. 2017, 583, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, J.L.; Light, L.A.; McCutcheon, S.C.; Wolfe, N.L.; Carreia, L.H. Phytoremediation of organic and nutrient contaminants. Environ. Sci. Technol. 1995, 29, 318A–323A. [Google Scholar] [CrossRef] [PubMed]
- Azab, E.; Hegazy, A.K.; El-Sharnouby, M.E.; Abd Elsalam, H.E. Phytoremediation of the organic Xenobiotic simazine by p450-1a2 transgenic Arabidopsis thaliana plants. Int. J. Phytoremediation 2016, 18, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Kawahigashi, H.; Hirose, S.; Ohkawa, H.; Ohkawa, Y.J. Transgenic rice plants expressing human P450 genes involved in xenobiotic metabolism for phytoremediation. J. Mol. Microbiol. Biotechnol. 2008, 15, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, Y.; Zheng, Y.; Hua, Y.; Datta, R.; Dan, Y.; Lv, P.; Sarkar, D. Uptake of 2,4-bis (Isopropylamino)-6-methylthio-s-triazine by vetiver grass (Chrysopogon zizanioides L.) from hydroponic media. Bull. Environ. Contam. Toxicol. 2016, 96, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lv, P.; Datta, R.; Ni, J.; Su, Y.; Sarkar, D.; Zheng, Y. Uptake of 2, 4-bis (Isopropylamino)-6-methylthio-s-triazine by Canna indica. J. Environ. Biol. 2019, 40, 577–583. [Google Scholar] [CrossRef]
- Buono, D.D.; Pannacci, E.; Bartucca, M.L.; Nasini, L.; Proietti, P.; Tei, F. Use of two grasses for the phytoremediation of aqueous solutions polluted with terbuthylazine. Int. J. Phytoremediation 2016, 18, 885–891. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.Y.; Lu, Y.C.; Jiang, S.S.; Wu, H.J.; Yang, H. Comprehensive analysis of degradation and accumulation of ametryn in soils and in wheat, maize, ryegrass and alfalfa plants. Ecotoxicol. Environ. Saf. 2017, 140, 264–270. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Izadi-Darbandi, E.; Naseri Pour Yazdi, M.T.; Rastgoo, M.; Malaekeh-Nikouei, B.; Nassirli, H. Impacts of different organic amendments on soil degradation and phytotoxicity of metribuzin. Int. J. Recycl. Org. Waste Agric. 2019, 8, 113–121. [Google Scholar] [CrossRef]
- Lamoureux, G.L.; Shimabukuro, R.; Swanson, H.; Frear, D. Metabolism of 2-chloro-4-ethylamino-6-isopropylamino-s-triazine (atrazine) in excised sorghum leaf sections. J. Agric. Food Chem. 1970, 18, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.H.; Moreland, D.E. Simazine: Degradation by corn seedlings. Science 1962, 135, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhen, Z.; Chen, C.; Li, Y.; Luo, C.; Zhong, L.; Hu, H.; Li, J.; Zhang, Y.; Liang, Y. Rhizospheric effects on atrazine speciation and degradation in laterite soils of Pennisetum alopecuroides (L.) Spreng. Environ. Sci. Pollut. Res. 2018, 25, 12407–12418. [Google Scholar] [CrossRef]
- Pérez, D.J.; Doucette, W.J.; Moore, M.T. Atrazine uptake, translocation, bioaccumulation and biodegradation in cattail (Typha latifolia) as a function of exposure time. Chemosphere 2022, 287, 132104. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, M.; Lu, X.; Chen, Z.; Guan, S.; Du, H.; Zhu, D. Evaluation of the potential of Potamogeton crispus and Myriophyllum spicatum on phytoremediation of atrazine. Int. J. Environ. Anal. Chem. 2019, 99, 243–257. [Google Scholar] [CrossRef]
- Yu, Q.Q.; Lu, F.F.; Ma, L.Y.; Yang, H.; Song, N.H. Residues of Reduced Herbicides Terbuthylazine, Ametryn, and Atrazine and Toxicology to Maize and the Environment through Salicylic Acid. ACS Omega 2021, 6, 27396–27404. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Li, N.; Li, H.; Yang, T.; Liu, W.; Yan, Y.; Feng, X.; Zhu, D. Phytoextraction and biodegradation of atrazine by Myriophyllum spicatum and evaluation of bacterial communities involved in atrazine degradation in lake sediment. Chemosphere 2018, 209, 439–448. [Google Scholar] [CrossRef]
- Sánchez, V.; López-Bellido, F.J.; Cañizares, P.; Rodríguez, L. Assessing the phytoremediation potential of crop and grass plants for atrazine-spiked soils. Chemosphere 2017, 185, 119–126. [Google Scholar] [CrossRef]
- Murphy, I.J.; Coats, J.R. The capacity of switchgrass (Panicum virgatum) to degrade atrazine in a phytoremediation setting. Environ. Toxicol. Chem. 2011, 30, 715–722. [Google Scholar] [CrossRef]
- Sánchez, V.; López-Bellido, J.; Rodrigo, M.A.; Rodríguez, L. Enhancing the removal of atrazine from soils by electrokinetic-assisted phytoremediation using ryegrass (Lolium perenne L.). Chemosphere 2019, 232, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Madariaga-Navarrete, A.; Rodríguez-Pastrana, B.R.; Villagómez-Ibarra, J.R.; Acevedo-Sandoval, O.A.; Perry, G.; Islas-Pelcastre, M.J. Bioremediation model for atrazine contaminated agricultural soils using phytoremediation (using Phaseolus vulgaris L.) and a locally adapted microbial consortium. J. Environ. Sci. Health B 2017, 52, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, L.M.; Dos Santos, J.B.; Barroso, G.M.; Ferreira, E.A.; Cabral, C.M.; Costa, M.R.; Vieira, E.R.D.; Zanuncio, J.C. Phytoremediation by Eremanthus crotonoides and Inga striata decay atrazine and clomazone residues in the soil. Int. J. Phytoremediation 2020, 22, 827–833. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, N.M.C.; Monteiro, P.G.; Ferreira, E.A.; Alencar, B.T.B.; Cabral, C.M.; dos Santos, J.B. Use of Eichhornia crassipes and Pistia stratiotes for environmental services: Decontamination of aquatic environments with atrazine residues. Aquat. Bot. 2022, 176, 103470. [Google Scholar] [CrossRef]
- Qu, M.; Mei, Y.; Liu, G.; Zhao, J.; Liu, W.; Li, S.; Huang, F.; Zhu, D. Transcriptomic profiling of atrazine phytotoxicity and comparative study of atrazine uptake, movement, and metabolism in Potamogeton crispus and Myriophyllum spicatum. Environ. Res. 2021, 194, 110724. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-S.; Lu, Z.-X.; Lin, M.-Z. Ametryn residues and its degradation dynamics in pineapple and soil. Chin. J. Eco. Agric. 2006, 14, 138–140. [Google Scholar]
- Machi, A.; Arthur, P.; Ferrari, L.; Arthur, V. Efficiency of ametryn herbicide irradiated on different weeds in an orchard of avocado. Aust. J. Basic Appl. Sci. 2015, 9, 205–210. [Google Scholar]
- Kern, A.; Meggitt, W.; Penner, D. Uptake, movement, and metabolism of cyanazine in fall panicum, green foxtail, and corn. Weed Sci. 1975, 23, 277–282. [Google Scholar] [CrossRef]
- De Billot, M.I.C.; Nel, P. Soil, Recovery of grain sorghum [Sorghum bicolar (L.) Moench] from atrazine and cyanazine phytotoxicity. S. Afr. J. Plant Soil 1985, 2, 146–150. [Google Scholar] [CrossRef]
- Beynon, K.; Stoydin, G.; Wright, A.N. The breakdown of the triazine herbicide cyanazine in soils and maize. Pestic. Sci. 1972, 3, 293–305. [Google Scholar] [CrossRef]
- Cessna, A.J.; Darwent, A.L.; Moyer, J.R.; Cole, D.E. Dissipation of residues of MCPA and cyanazine in three forage grasses following post-emergence application as a mixture in the establishment year. Pestic. Sci. 1994, 40, 127–132. [Google Scholar] [CrossRef]
- Tolcha, T.; Gemechu, T.; Megersa, N. Flower of Typha latifolia as a low-cost adsorbent for quantitative uptake of multiclass pesticide residues from contaminated waters. S. Afr. J. Chem. 2020, 73, 22–29. [Google Scholar]
- Abusteit, E.O.; Corbin, F.T.; Schmitt, D.P.; Burton, J.W.; Worsham, A.D.; Thompson, L.J. Absorption, translocation, and metabolism of metribuzin in diploid and tetraploid soybean (Glycine max) plants and cell cultures. Weed Sci. 1985, 33, 618–628. [Google Scholar] [CrossRef]
- Garcia-Baudin, J.; Villarroya, M.; Chueca, M.; Tadeo, J. Different tolerance of two cultivars of Triticum turgidum L. to metribuzin. Chemosphere 1990, 21, 223–230. [Google Scholar] [CrossRef]
- Frear, D.; Swanson, H.; Mansager, E. Alternate pathways of metribuzin metabolism in soybean: Formation of N-glucoside and homoglutathione conjugates. Pestic. Biochem. Physiol. 1985, 23, 56–65. [Google Scholar] [CrossRef]
- Šuk, J.; Hamouzová, K.; Hajšlová, J.; Jursík, M. Dynamics of herbicides degradation in carrot (Daucus carota L.) roots and leaves. Plant Soil Environ. 2021, 67, 353–359. [Google Scholar] [CrossRef]
- Devlin, D.L.; Gealy, D.R.; Morrow, L.A. Differential absorption and translocation of metribuzin by downy brome (Bromus tectorum) and winter wheat (Triticum aestivum). Weed Sci. 1987, 35, 1–5. [Google Scholar] [CrossRef]
- Boulahia, K.; Carol, P.; Planchais, S.; Abrous-Belbachir, O. Phaseolus vulgaris L. seedlings exposed to prometryn herbicide contaminated soil trigger an oxidative stress response. J. Agric. Food Chem. 2016, 64, 3150–3160. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K. Degradation dynamics and implementation advice of prometryn in peanut. J. Food Saf. Qual. 2018, 9, 3226–3230. [Google Scholar]
- Wu, M.; Wang, L.; Fu, M. Weeding effect of prometryn and its residue analysis in root of Angelica acutiloba (Sieb. et Zucc.) Kitag. J. Fujian Agric. For. Univ. 2010, 39, 347–350. [Google Scholar]
- Kirumba, G.; Ge, L.; Wei, D.; Xu, C.; He, Y.; Zhang, B.; Jiang, C.; Mao, F. The role of a hybrid phytosystem in landscape water purification and herbicides removal. Water Sci. Technol. 2015, 72, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Wang, Y.K.; Zhou, J.H.; Xie, F.; Guo, Q.N.; Lu, F.F.; Jin, S.F.; Zhu, H.M.; Yang, H. Reduced phytotoxicity of propazine on wheat, maize and rapeseed by salicylic acid. Ecotoxicol. Environ. Saf. 2018, 162, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kendig, J.; Nichols, R.; Ohmes, G.J.P.H.P. Tolerance of cotton (Gossypium hirsutum) seedlings to preemergence and postemergence herbicides with four modes of action. Plant Health Prog. 2007, 8, 4. [Google Scholar] [CrossRef]
- Wilson, P.; Whitwell, T.; Klaine, S. Metalaxyl and simazine toxicity to and uptake by Typha latifolia. Arch. Environ. Contam. Toxicol. 2000, 39, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Kawahigashi, H.; Hirose, S.; Ohkawa, H.; Ohkawa, Y.J. Transgenic rice plants expressing human CYP1A1 remediate the triazine herbicides atrazine and simazine. J. Agric. Food Chem. 2005, 53, 8557–8564. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.C.; Whitwell, T.; Klaine, S. Phytotoxicity, uptake, and distribution of [14C] simazine in Canna hybrida ‘Yellow King Humbert’. Environ. Toxicol. Chem. 1999, 18, 1462–1468. [Google Scholar] [CrossRef]
- Montgomery, M.; Freed, V.H. The uptake, translocation and metabolism of simazine and atrazine by corn plants. Weeds 1961, 9, 231–237. [Google Scholar] [CrossRef]
- Burauel, B.; Führ, F. The enhanced mineralization of simazine and bentazon in soil after plant uptake. Zeitschrift für Pflanzenernahrung und Bodenkunde 1988, 151, 311–314. [Google Scholar] [CrossRef]
- Turgut, C. Uptake and modeling of pesticides by roots and shoots of parrotfeather (Myriophyllum aquaticum). Environ. Sci. Poll. Res. Int. 2005, 12, 342–346. [Google Scholar] [CrossRef]
- Richter, S.; Nagel, R. Bioconcentration, biomagnification and metabolism of 14C-terbutryn and 14C-benzo [a] pyrene in Gammarus fossarum and Asellus aquaticus. Chemosphere 2007, 66, 603–610. [Google Scholar] [CrossRef]
- Rubin, B.; Eshel, Y. Absorption and translocation of terbutryn and fluometuron in cotton (Gossypium hirsutum) and snapbeans (Phaseolus vulgaris). Weed Sci. 1977, 25, 499–505. [Google Scholar] [CrossRef]
- Bravin, M.N.; Michaud, A.M.; Larabi, B.; Hinsinger, P. RHIZO test: A plant-based biotest to account for rhizosphere processes when assessing copper bioavailability. Environ. Pollut. 2010, 158, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Bartucca, M.L.; Del Buono, D.; Cesco, S. Italian ryegrass for the phytoremediation of solutions polluted with terbuthylazine. Chemosphere 2015, 119, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Panfili, I.; Bartucca, M.L.; Del Buono, D. The treatment of duckweed with a plant biostimulant or a safener improves the plant capacity to clean water polluted by terbuthylazine. Sci. Total Environ. 2019, 646, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Zalidis, G. The use of Typha Latifolia L. in constructed wetland microcosms for the remediation of herbicide Terbuthylazine. Environ. Process. 2019, 6, 985–1003. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Takavakoglou, V.; Gikas, E.; Tsarbopoulos, A.; Zalidis, G. Transport and dissipation study of the herbicide terbuthylazine and its major metabolites in wetland sediment substrates planted with Typha latifolia L. Desalination Water Treat. 2012, 39, 209–214. [Google Scholar] [CrossRef]
- Hussain, A.; Rehman, F.; Rafeeq, H.; Waqas, M.; Asghar, A.; Afsheen, N.; Rahdar, A.; Bilal, M.; Iqbal, H.M. In-situ, Ex-situ, and nano-remediation strategies to treat polluted soil, water, and air—A review. Chemosphere 2022, 289, 133252. [Google Scholar] [CrossRef] [PubMed]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-valent iron nanoparticles for soil and groundwater remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef]
- Galdames, A.; Mendoza, A.; Orueta, M.; de Soto García, I.; Sánchez, M.; Virto, I.; Vilas, J. Development of new remediation technologies for contaminated soils based on the application of zero-valent iron nanoparticles and bioremediation with compost. Resour. Effic. Technol. 2017, 3, 166–176. [Google Scholar] [CrossRef]
- Bhavya, G.; Belorkar, S.A.; Mythili, R.; Geetha, N.; Shetty, H.S.; Udikeri, S.S.; Jogaiah, S. Remediation of emerging environmental pollutants: A review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 2021, 275, 129975. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; AL-Othman, Z.A.; Alwarthan, A. Green synthesis of functionalized iron nano particles and molecular liquid phase adsorption of ametryn from water. J. Mol. Liq. 2016, 221, 1168–1174. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, R.; Chen, G. Sorption of four s-triazine herbicides on natural zeolite and clay mineral materials with microporosity. Fundam. Res. 2021, 1, 285–295. [Google Scholar] [CrossRef]
- Ali, I.; Alothman, Z.; Al-Warthan, A. Sorption, kinetics and thermodynamics studies of atrazine herbicide removal from water using iron nano-composite material. Int. J. Environ. Sci. Technol. 2016, 13, 733–742. [Google Scholar] [CrossRef]
- Addorisio, V.; Pirozzi, D.; Esposito, S.; Sannino, F. Decontamination of waters polluted with simazine by sorption on mesoporous metal oxides. J. Hazard. Mater. 2011, 196, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Flores, K.; Valdes, C.; Ramirez, D.; Eubanks, T.; Lopez, J.; Hernandez, C.; Alcoutlabi, M.; Parsons, J. The effect of hybrid zinc oxide/graphene oxide (ZnO/GO) nano-catalysts on the photocatalytic degradation of simazine. Chemosphere 2020, 259, 127414. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.L.; Eren, T.; Atar, N. A novel efficient photocatalyst based on TiO2 nanoparticles involved boron enrichment waste for photocatalytic degradation of atrazine. Chem. Eng. J. 2014, 250, 288–294. [Google Scholar] [CrossRef]
- Ali, I.; Al-Othman, Z.A.; Al-Warthan, A. Removal of secbumeton herbicide from water on composite nanoadsorbent. Desalination Water Treat. 2016, 57, 10409–10421. [Google Scholar] [CrossRef]
- Khodayar, M.J.; Namdar, F.; Hojati, S.; Landi, A.; Nazarikhorasgani, Z.; Alamolhoda, S. Removal of ametryn from aqueous solutions with zeolite nanoparticles optimized using the Box-Behnken design. Jundishapur J. Nat. Pharm. Prod. 2016, 11, 2. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Karatas, O.; Khataee, A.; Kobya, M.; Can, O.T.; Soltani, R.D.C.; Isleyen, M. Peroxydisulfate activation by in-situ synthesized Fe3O4 nanoparticles for degradation of atrazine: Performance and mechanism. Sep. Purif. Technol. 2020, 247, 116925. [Google Scholar] [CrossRef]
- Santacruz-Chávez, J.A.; Oros-Ruiz, S.; Prado, B.; Zanella, R. Photocatalytic degradation of atrazine using TiO2 superficially modified with metallic nanoparticles. J. Environ. Chem. Eng. 2015, 3, 3055–3061. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lin, C.; Zhang, H.; Zhou, Z.; Fan, G.; Ma, J. Cobalt ferrite nanoparticles supported on drinking water treatment residuals: An efficient magnetic heterogeneous catalyst to activate peroxymonosulfate for the degradation of atrazine. Chem. Eng. J. 2019, 367, 208–218. [Google Scholar] [CrossRef]
- Shamsedini, N.; Dehghani, M.; Nasseri, S.; Baghapour, M.A. Photocatalytic degradation of atrazine herbicide with Illuminated Fe+3-TiO2 Nanoparticles. J. Environ. Health Sci. Eng. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Vijwani, H.; Nadagouda, M.; Mukhopadhyay, S. Robust nanocatalyst membranes for degradation of atrazine in water. J. Water Process. Eng. 2018, 25, 15–21. [Google Scholar] [CrossRef]
- Sudrajat, H.; Sujaridworakun, P. Correlation between particle size of Bi2O3 nanoparticles and their photocatalytic activity for degradation and mineralization of atrazine. J. Mol. Liq. 2017, 242, 433–440. [Google Scholar] [CrossRef]
- Mkhalid, I.; Shawky, A. Cu-supported Cu2O nanoparticles: Optimized photodeposition enhances the visible light photodestruction of atrazine. J. Alloys Compd. 2021, 853, 157040. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, B.; Yu, Y.; Bu, L.; Deng, J.; Zhou, S. Heterogeneous catalysis of ozone using ordered mesoporous Fe3O4 for degradation of atrazine. Chem. Eng. J. 2017, 328, 527–535. [Google Scholar] [CrossRef]
- Cai, H.; Li, J.; Yin, H.; Yao, G.; Lai, B. Degradation of atrazine in aqueous solution through peroxymonosulfate activated by Co-modified nano-titanium dioxide. Water Environ. Res. 2020, 92, 1363–1375. [Google Scholar] [CrossRef]
- Andrade, M.B.; Santos, T.R.; Fernandes Silva, M.; Vieira, M.F.; Bergamasco, R.; Hamoudi, S. Graphene oxide impregnated with iron oxide nanoparticles for the removal of atrazine from the aqueous medium. Sep. Sci. Technol. 2019, 54, 2653–2670. [Google Scholar] [CrossRef]
- Sangami, S.; Manu, B. Synthesis of Green Iron Nanoparticles using Laterite and their application as a Fenton-like catalyst for the degradation of herbicide Ametryn in water. Environ. Technol. Innov. 2017, 8, 150–163. [Google Scholar] [CrossRef]
- Boruah, P.K.; Sharma, B.; Hussain, N.; Das, M.R. Magnetically recoverable Fe3O4/graphene nanocomposite towards efficient removal of triazine pesticides from aqueous solution: Investigation of the adsorption phenomenon and specific ion effect. Chemopshere 2017, 168, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Sangami, S.; Manu, B. Catalytic efficiency of laterite-based FeNPs for the mineralization of mixture of herbicides in water. Environ. Technol. 2018, 40, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.P.; de Oliveira, D.M.; da Silva, L.d.M.; Gimenez, J.; Esplugas, S.; de Oliveira, S.C.; Dantas, R.F.; Sans, C.; Junior, A.M. Evaluation of the main active species involved in the TiO2 photocatalytic degradation of ametryn herbicide and its by-products. J. Environ. Chem. Eng. 2021, 9, 105109. [Google Scholar] [CrossRef]
- Boruah, P.K.; Darabdhara, G.; Das, M.R. Polydopamine functionalized graphene sheets decorated with magnetic metal oxide nanoparticles as efficient nanozyme for the detection and degradation of harmful triazine pesticides. Chemosphere 2021, 268, 129328. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Alharbi, O.M.; Alothman, Z.A.; Alwarthan, A. Facile and eco-friendly synthesis of functionalized iron nanoparticles for cyanazine removal in water. Colloids Surf. B 2018, 171, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Sakellarides, T.M.; Sakkas, V.A.; Albanis, T.A. Photocatalytic degradation of selected s-triazine herbicides and organophosphorus insecticides over aqueous TiO2 suspensions. Environ. Sci. Technol. 2001, 35, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bartelt-Hunt, S.L.; Li, Y. Development of Quantitative Structure–Activity Relationships for Nanoparticle Titanium Dioxide Aggregation in the Presence of Organic Contaminants. Environ. Eng. Sci. 2018, 35, 918–926. [Google Scholar] [CrossRef]
- K’Owino Isaac, O.; Masika, K.; Okello, V.A. Kinetics of degradation of metribuzin in aqueous solution using zero valent iron nanoparticles. Al-Nahrain J. Sci. 2018, 21, 1–9. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, S.; Li, X.; Meng, H.; Zhang, X.; Wang, Z.; Han, Y. Ag nanoparticle-functionalized ZnO micro-flowers for enhanced photodegradation of herbicide derivatives. Chem. Phys. Lett. 2017, 679, 119–126. [Google Scholar] [CrossRef]
- Oreggioni, D.; Pérez Parada, A.; Aguiar, I.; Colazzo, M.; Pareja, L.; De León, M.A.; Pereira, H.B.; Pérez Barthaburu, M.E. Sulfur precursor and citric acid effect on SnS2 nanoparticles and their influence on the photodegradation activity of selected organic compounds. Environ. Sci. Pollut. Res. 2021, 28, 18234–18245. [Google Scholar] [CrossRef]
- Yin, J.; Xing, Z.; Kuang, J.; Li, Z.; Zhu, Q.; Zhou, W. Dual oxygen vacancy defects-mediated efficient electron-hole separation via surface engineering of Ag/Bi2MoO6 nanosheets/TiO2 nanobelts ternary heterostructures. J. Ind. Eng. Chem. 2019, 78, 155–163. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Konstantinou, I. Photocatalytic treatment of metribuzin herbicide over TiO2 aqueous suspensions: Removal efficiency, identification of transformation products, reaction pathways and ecotoxicity evaluation. J. Photochem. Photobiol. A Chem. 2014, 294, 110–120. [Google Scholar] [CrossRef]
- Li, G.; Cao, Y.; Yi, L.; Wang, J.; Song, Y. Construction of Z-scheme Pt-TiO2/(Er3+:Y3Al5O12@Ta2O5)/HAP and its application in sonocatalytic degradation of prometryn and disinfection of Escherichia coli. J. Chem. Technol. Biotechnol. 2020, 95, 1756–1772. [Google Scholar] [CrossRef]
- Grabka, D.; Raczyńska-Żak, M.; Czech, K.; Słomkiewicz, P.M.; Jóźwiak, M.A. Modified halloysite as an adsorbent for prometryn from aqueous solutions. Appl. Clay Sci. 2015, 114, 321–329. [Google Scholar] [CrossRef]
- Boruah, P.K.; Das, M.R. Dual responsive magnetic Fe3O4-TiO2/graphene nanocomposite as an artificial nanozyme for the colorimetric detection and photodegradation of pesticide in an aqueous medium. J. Hazard. Mater. 2020, 385, 121516. [Google Scholar] [CrossRef] [PubMed]
- Borio, O.; Gawlik, B.; Bellobono, I.; Muntau, H. Photooxidation of prometryn and prometon in aqueous solution by hydrogen peroxide on photocatalytic membranes immobilising titanium dioxide. Chemosphere 1998, 37, 975–989. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Maurino, V.; Minero, C.; Carlin, V.; Tosato, M.L.; Pramauro, E.; Zerbinati, O. Photocatalytic degradation of atrazine and other s-triazine herbicides. Environ. Sci. Technol. 1990, 24, 1559–1565. [Google Scholar] [CrossRef]
- Eshete, M.; Bowleg, J.; Perales, S.G.; Okunrobo, M.; Watkins, D.; Spencer, H. Adsorption of propazine, simazine and bisphenol A on the surface of nanoparticles of iron oxide nanoparticles of carbon and metallic oxides. J. Environ. Prot. 2018, 9, 13. [Google Scholar] [CrossRef][Green Version]
- Meriam Suhaimy, S.H.; Abd Hamid, S.B.; Lai, C.W.; Hasan, M.; Johan, M.R. TiO2 nanotubes supported Cu nanoparticles for improving photocatalytic degradation of simazine under UV illumination. Catalysts 2016, 6, 167. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mangalaraja, R.V.; Mansilla, H.D.; Gracia-Pinilla, M.; Anandan, S. Sonophotocatalytic (42 kHz) degradation of Simazine in the presence of Au–TiO2 nanocatalysts. Appl. Catal. B 2014, 160, 692–700. [Google Scholar] [CrossRef]
- Salmerón, I.; Sharma, P.; Polo-López, M.; Tolosana, A.; McMichael, S.; Oller, I.; Byrne, J.; Fernández-Ibáñez, P. Electrochemically assisted photocatalysis for the simultaneous degradation of organic micro-contaminants and inactivation of microorganisms in water. Process Saf. Environ. Prot. 2021, 147, 488–496. [Google Scholar] [CrossRef]
- Goswami, A.; Jiang, J.-Q. Comparative performance of catalytic fenton oxidation with zero-valent iron (Fe (0)) in comparison with ferrous sulphate for the removal of micropollutants. Appl. Sci. 2019, 9, 2181. [Google Scholar] [CrossRef]
- Goswami, A.; Jiang, J.-Q.; Petri, M. Treatability of five micro-pollutants using modified Fenton reaction catalysed by zero-valent iron powder (Fe (0)). J. Environ. Chem. Eng. 2021, 9, 105393. [Google Scholar] [CrossRef]
- Gomes, J.; Roccamante, M.; Contreras, S.; Medina, F.; Oller, I.; Martins, R.C. Scale-up impact over solar photocatalytic ozonation with benchmark-P25 and N-TiO2 for insecticides abatement in water. J. Environ. Chem. Eng. 2021, 9, 104915. [Google Scholar] [CrossRef]
- Kanan, S.; Moyet, M.A.; Arthur, R.B.; Patterson, H.H. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal. Rev. 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Le Cunff, J.; Tomašić, V.; Wittine, O. Photocatalytic degradation of the herbicide terbuthylazine: Preparation, characterization and photoactivity of the immobilized thin layer of TiO2/chitosan. J. Photochem. Photobiol. A Chem. 2015, 309, 22–29. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Y.; Jiang, T.; Yang, S. Degradation of terbuthylazine by TiO2 visible photocatalysis with electro-generated Fenton reagent assistance. China Environ. Sci. 2016, 36, 3304–3310. [Google Scholar]
- Rakhshan, N.; Pakizeh, M. Removal of triazines from water using a novel OA modified SiO2/PA/PSf nanocomposite membrane. Sep. Purif. Technol. 2015, 147, 245–256. [Google Scholar] [CrossRef]
- Chang, C.M.; Chang, C.W.; Wu, F.W.; Chang, L.; Liu, T.C. In Silico Ecotoxicological Modeling of Pesticide Metabolites and Mixtures. In Ecotoxicological QSARs; Springer: Berlin/Heidelberg, Germany, 2020; pp. 561–589. [Google Scholar]
- Sasikala, S.; Minu Jenifer, M.; Velavan, K.; Sakthivel, M.; Sivasamy, R.; Fenwick Antony, E.R. Predicting the relationship between pesticide genotoxicity and breast cancer risk in South Indian women in in vitro and in vivo experiments. Sci. Rep. 2023, 13, 9712. [Google Scholar] [CrossRef]
- Qiao, K.; Fu, W.; Jiang, Y.; Chen, L.; Li, S.; Ye, Q.; Gui, W. QSAR models for the acute toxicity of 1,2,4-triazole fungicides to zebrafish (Danio rerio) embryos. Environ. Pollut. 2020, 265, 114837. [Google Scholar] [CrossRef]
- Buglak, A.A.; Zherdev, A.V.; Lei, H.T.; Dzantiev, B.B. QSAR analysis of immune recognition for triazine herbicides based on immunoassay data for polyclonal and monoclonal antibodies. PLoS ONE 2019, 14, e0214879. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; He, Q.; Zhang, Y.; Hu, Y.; Wang, Y.; Lin, Z. In silico rational design and virtual screening of antixoidant tripeptides based on 3D-QSAR modeling. J. Mol. Struct. 2019, 1193, 223–230. [Google Scholar] [CrossRef]
- Salaković, B.; Kovačević, S.; Karadžić Banjac, M.; Podunavac-Kuzmanović, S.; Jevrić, L.; Pajčin, I.; Grahovac, J. New Perspective on Comparative Chemometric and Molecular Modeling of Antifungal Activity and Herbicidal Potential of Alkyl and Cycloalkyl s-Triazine Derivatives. Processes 2023, 11, 358. [Google Scholar] [CrossRef]
- Jevrić, L.R.; Podunavac-Kuzmanović, S.O.; Švarc-Gajić, J.V.; Kovačević, S.Z.; Jovanović, B.Ž. RP-HPTLC Retention Data in Correlation with the In-silico ADME Properties of a Series of s-triazine Derivatives. Iran. J. Pharm. Res. IJPR 2014, 13, 1203. [Google Scholar] [PubMed]
- Türkmenoğlu, B.; Güzel, Y.; Su, E.M.; Kızılcan, D.Ş. Investigation of inhibitory activity of monoamine oxidase A with 4D-QSAR using Fukui indices identifier. Mater. Today Commun. 2020, 25, 101583. [Google Scholar] [CrossRef]
- Zain-Alabdeen, A.I.; El-Moselhy, T.F.; Sharafeldin, N.; Angeli, A.; Supuran, C.T.; El-Hamamsy, M.H. Synthesis and anticancer activity of new benzensulfonamides incorporating s-triazines as cyclic linkers for inhibition of carbonic anhydrase IX. Sci. Rep. 2022, 12, 16756. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Z.; Ahmad, S.; Ngegba, P.M.; Zhong, G. Role of Endocrine System in the Regulation of Female Insect Reproduction. Biology 2021, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Wilson, B.; Ishaque, A.; Ransome, R.; Huang, M.-J.; Leszczynski, J. Toxicity Assessment of Atrazine and Related Triazine Compounds in the Microtox Assay, and Computational Modeling for Their Structure-Activity Relationship. Int. J. Mol. Sci. 2000, 1, 63–74. [Google Scholar] [CrossRef]
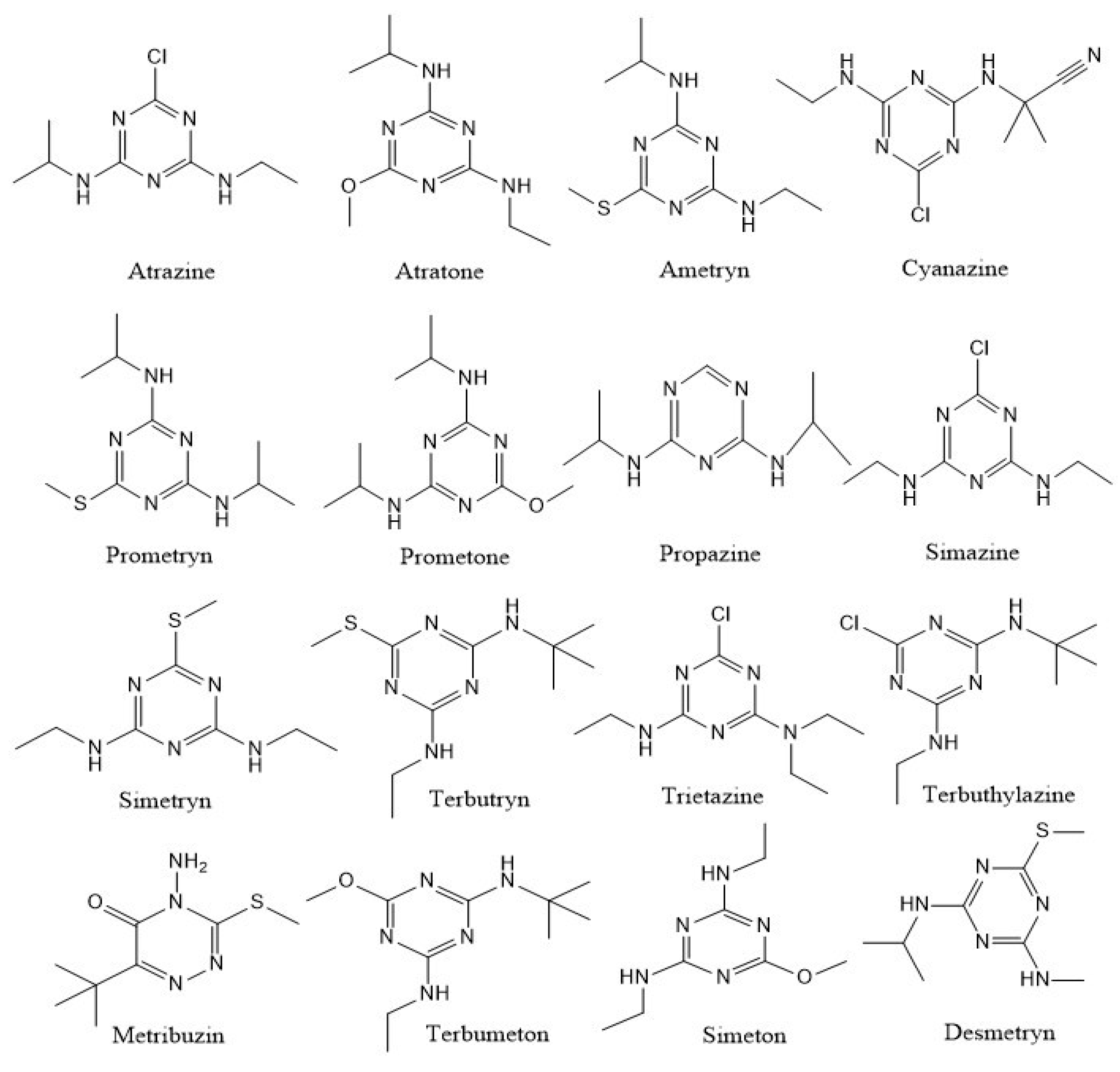
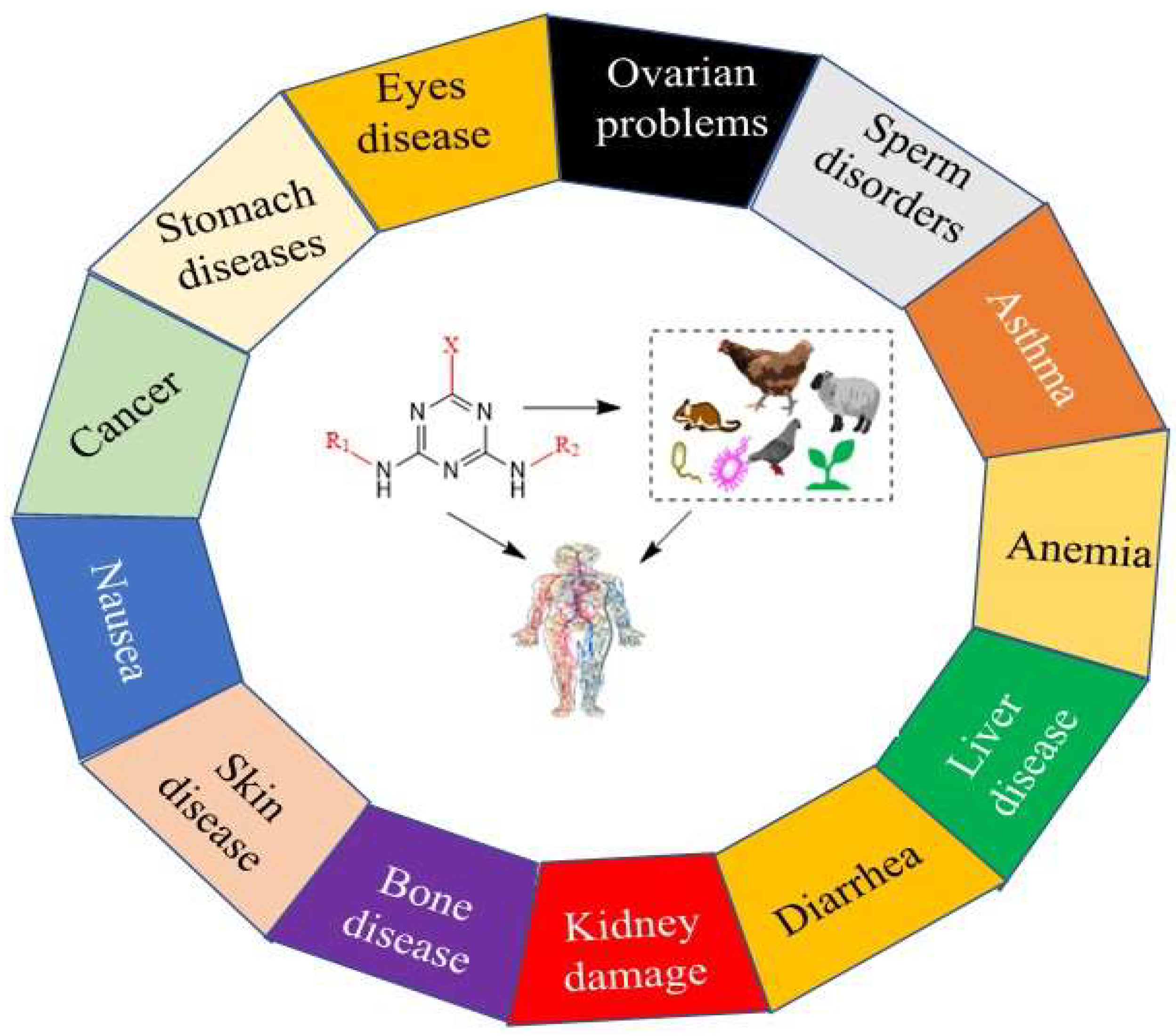
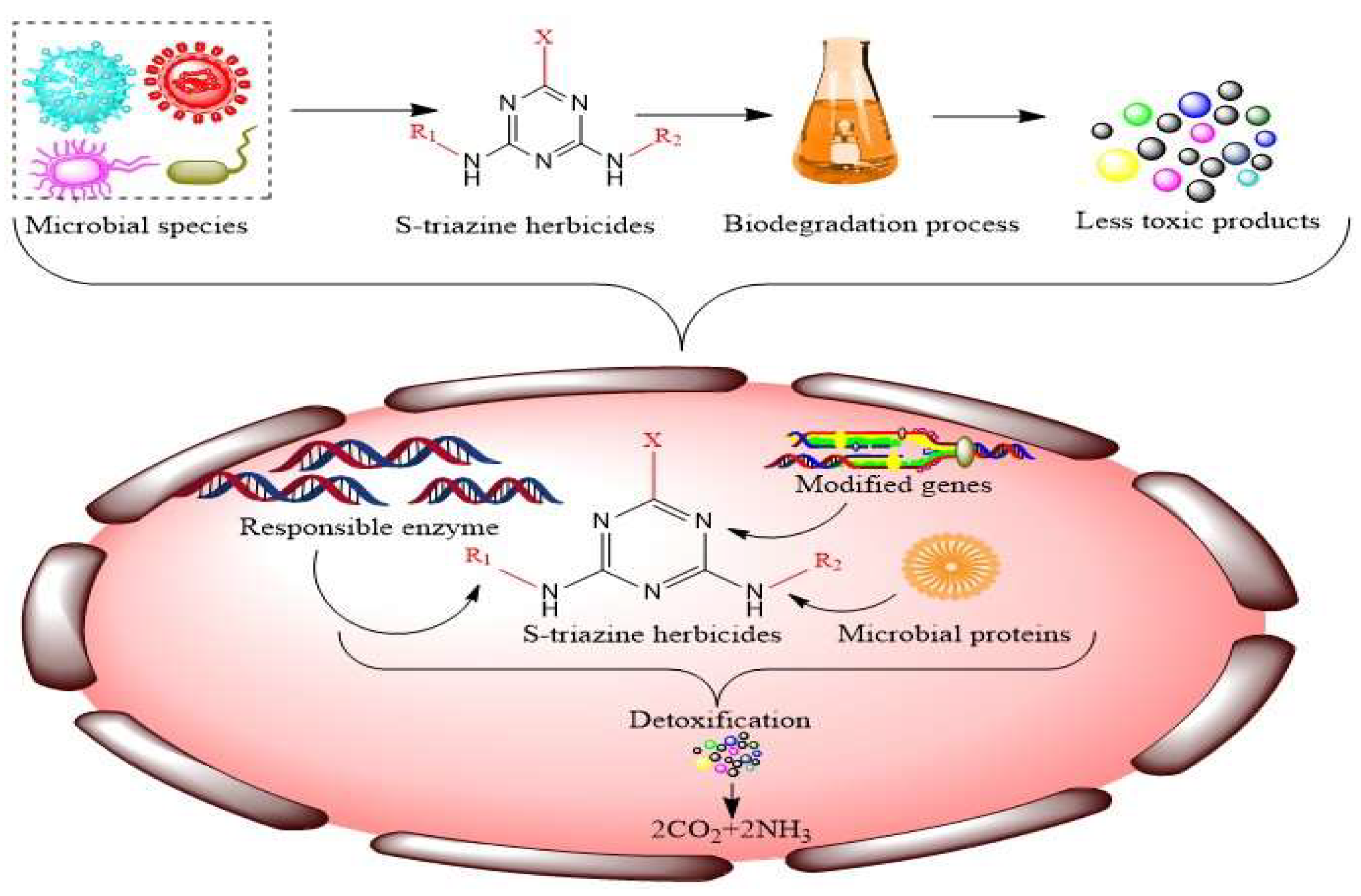
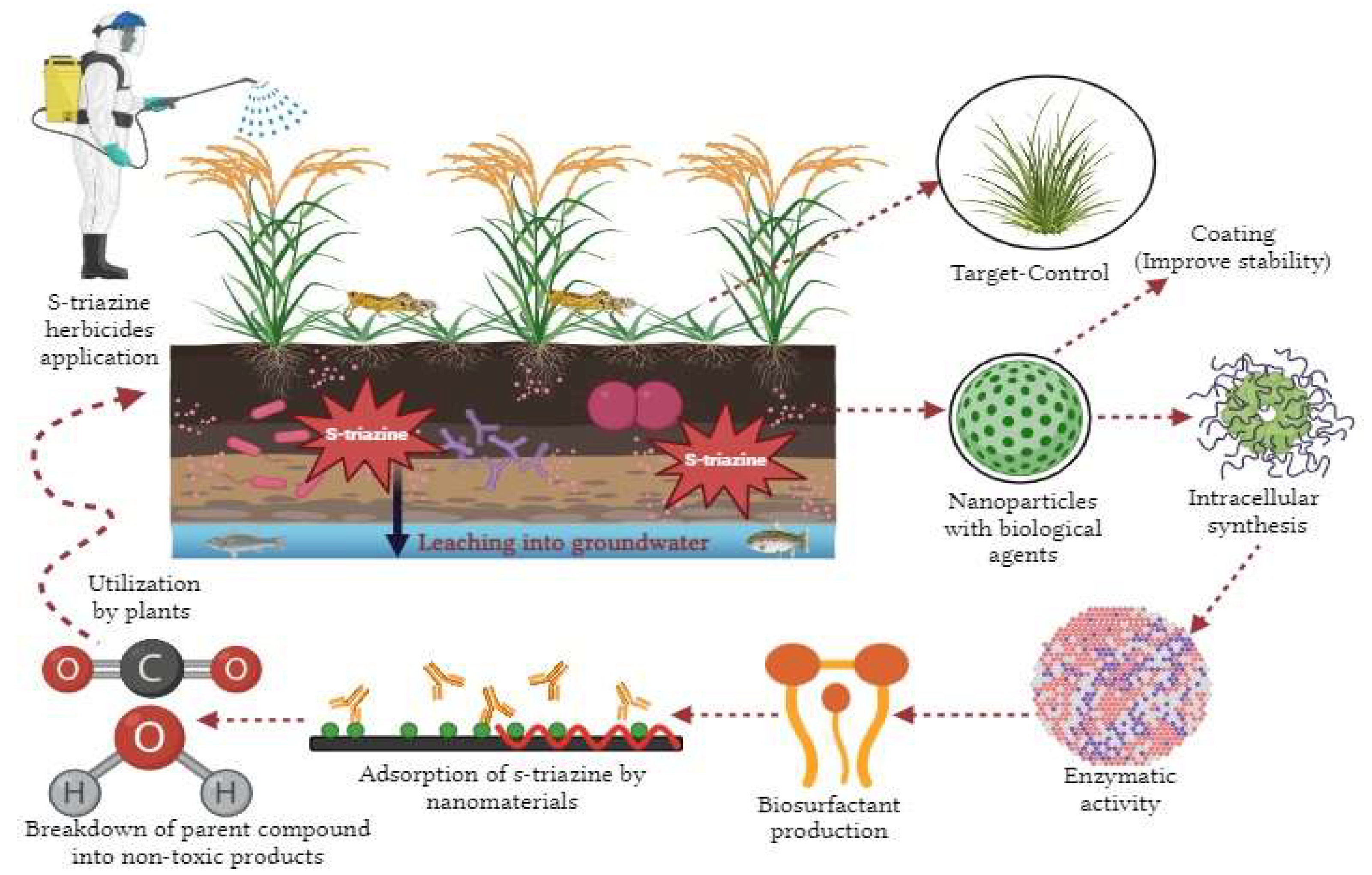
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Chandrasekaran, M.; Ahmad, H.W. Investigation of the Persistence, Toxicological Effects, and Ecological Issues of S-Triazine Herbicides and Their Biodegradation Using Emerging Technologies: A Review. Microorganisms 2023, 11, 2558. https://doi.org/10.3390/microorganisms11102558
Ahmad S, Chandrasekaran M, Ahmad HW. Investigation of the Persistence, Toxicological Effects, and Ecological Issues of S-Triazine Herbicides and Their Biodegradation Using Emerging Technologies: A Review. Microorganisms. 2023; 11(10):2558. https://doi.org/10.3390/microorganisms11102558
Chicago/Turabian StyleAhmad, Sajjad, Murugesan Chandrasekaran, and Hafiz Waqas Ahmad. 2023. "Investigation of the Persistence, Toxicological Effects, and Ecological Issues of S-Triazine Herbicides and Their Biodegradation Using Emerging Technologies: A Review" Microorganisms 11, no. 10: 2558. https://doi.org/10.3390/microorganisms11102558
APA StyleAhmad, S., Chandrasekaran, M., & Ahmad, H. W. (2023). Investigation of the Persistence, Toxicological Effects, and Ecological Issues of S-Triazine Herbicides and Their Biodegradation Using Emerging Technologies: A Review. Microorganisms, 11(10), 2558. https://doi.org/10.3390/microorganisms11102558







