SARS-CoV-2 and Brain Health: New Challenges in the Era of the Pandemic
Abstract
:1. Introduction
| Respiratory Viruses | Virus Overview | Neurological Complications | Ref. |
|---|---|---|---|
| Human Coronaviruses (SARS-CoV1, MERS-CoV, SARS-CoV2) |
|
| [24,25,26,27,28,29,30,31,32,33] |
| Influenza A Viruses (H1N1, H3N2, H5N1, H7N7) |
|
| [34,35,36,37,38,39] |
| Human Respiratory Syncytial Virus/Orthopneumovirus |
|
| [37,40] |
| Human Metapneumovirus |
|
| [41,42,43] |
2. Respiratory Virus Transmission Routes to the Nervous System
3. Coronavirus
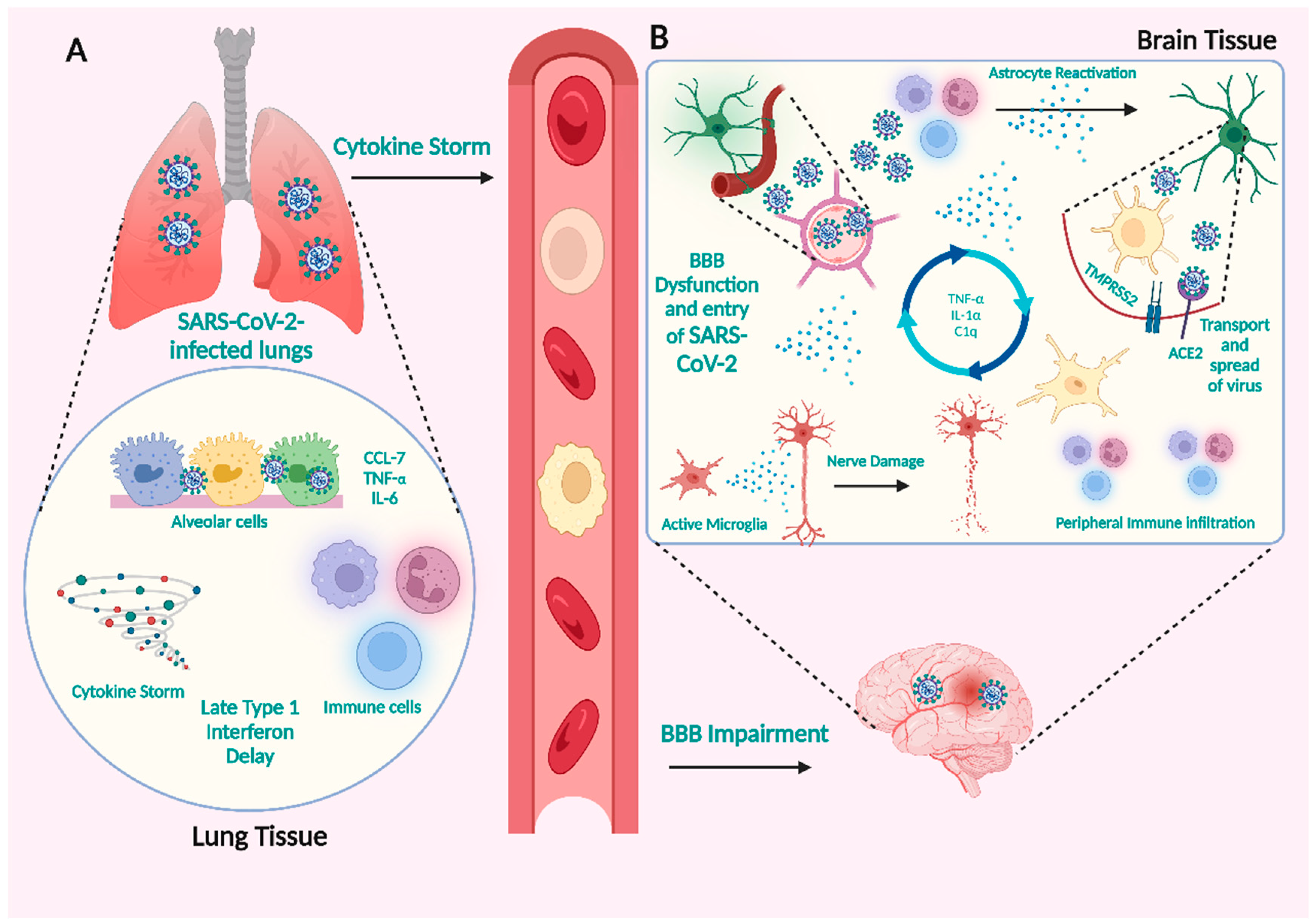
3.1. How SARS-CoV-2 Infections Affect the CNS
The Neuronal Pathway
3.2. Hematogenous Route
3.2.1. SARS-CoV-2 Infection of Vascular Endothelial Cells and Crossing the BBB
3.2.2. Immune Cells Initiate Cytokine Secretion in Response to SARS-CoV-2
3.3. Expression of Essential Viral Infection Factors in the Nervous System
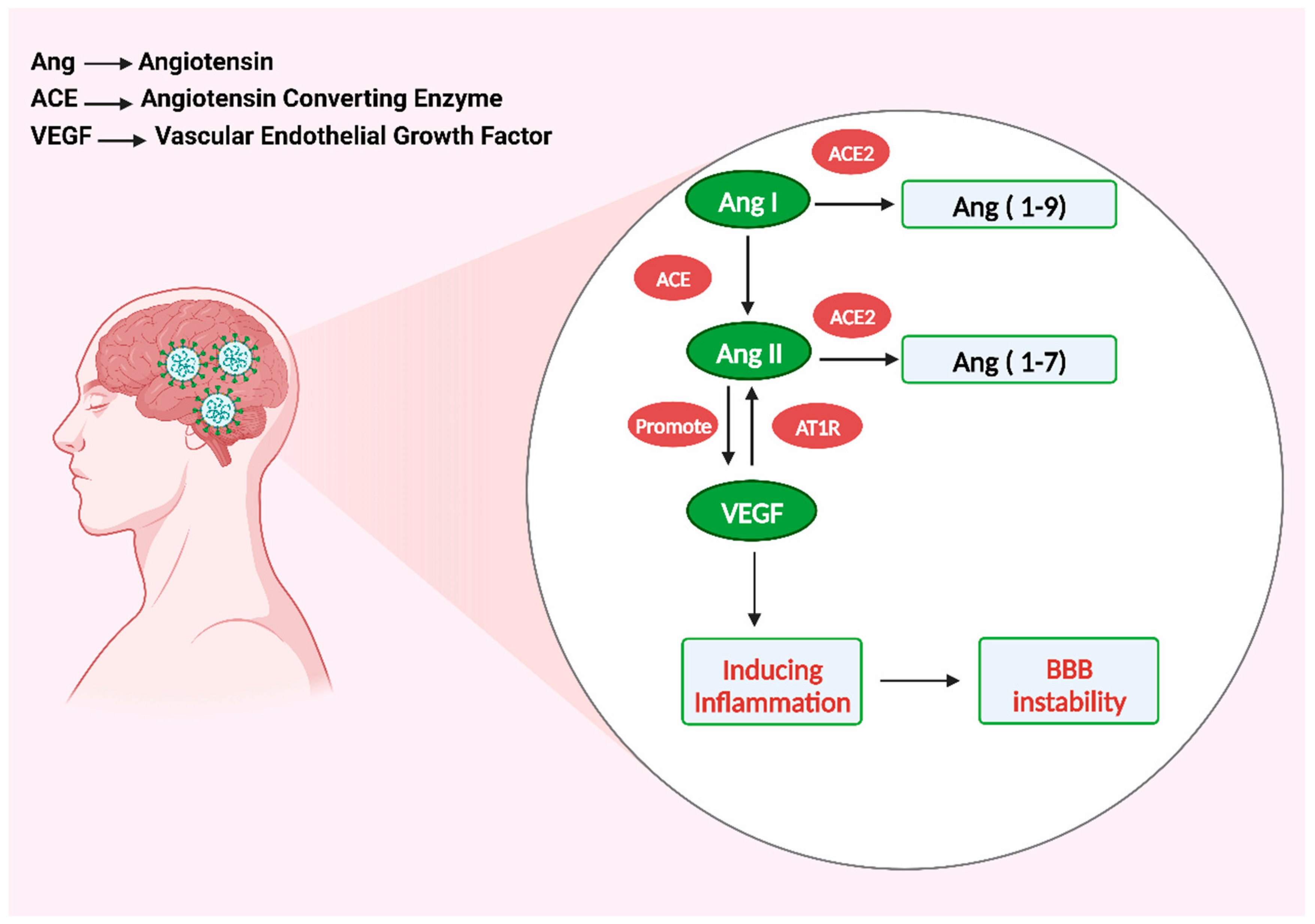
4. Vascular Endothelial Growth Factor Cause Inflammation
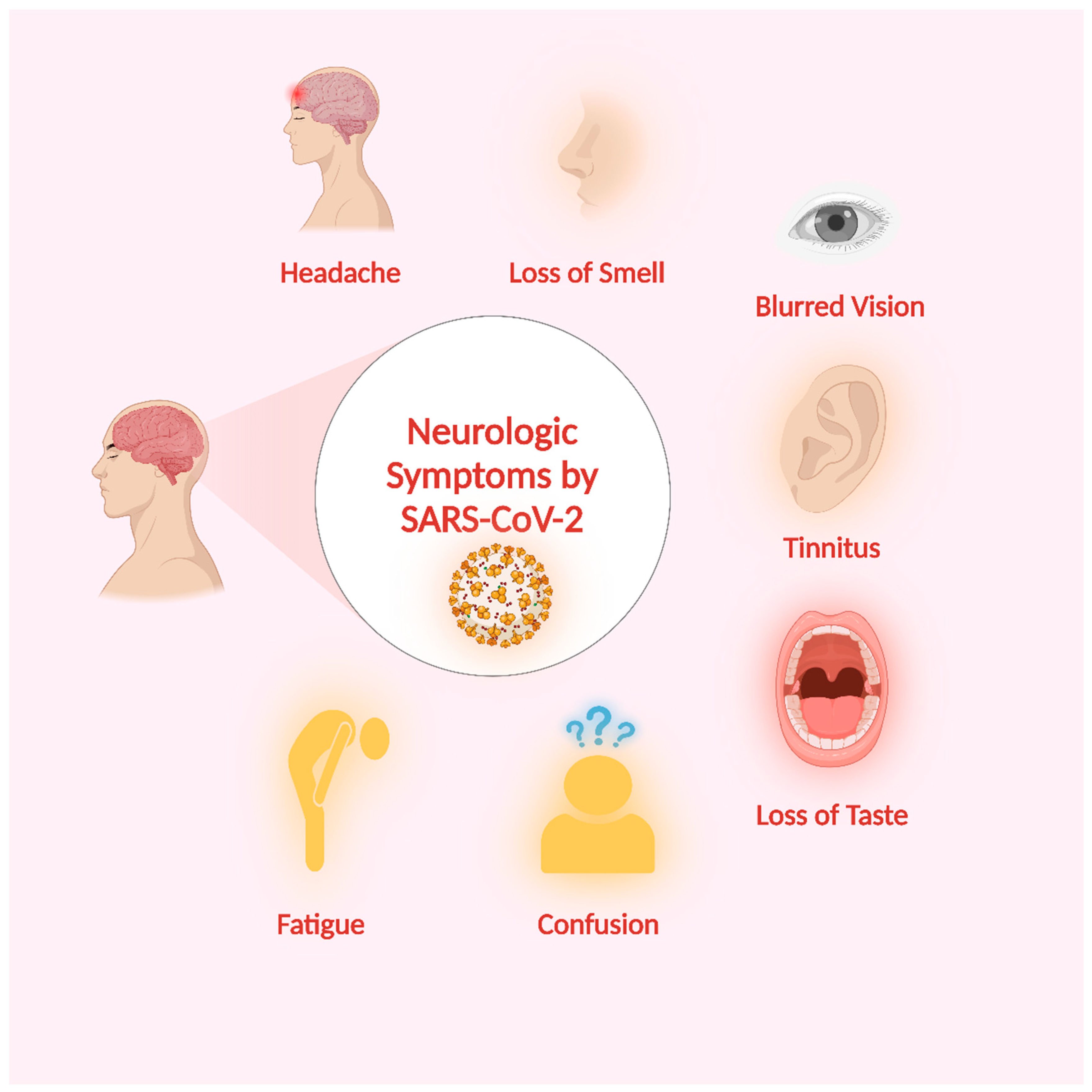
5. Neurologic Symptoms of SARS-CoV-2 Infection
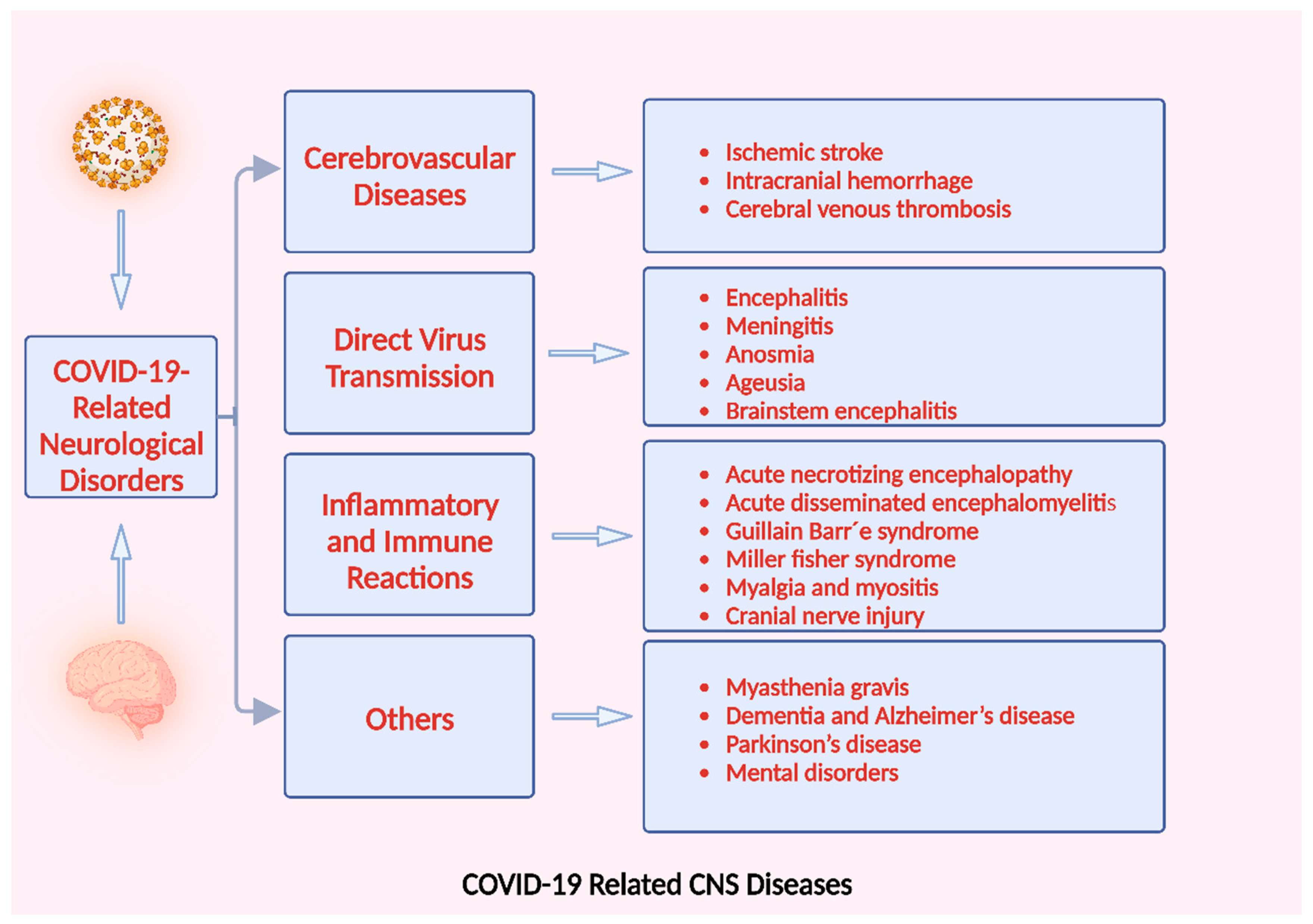
6. SARS-CoV-2-Related Disorders of the Nervous System
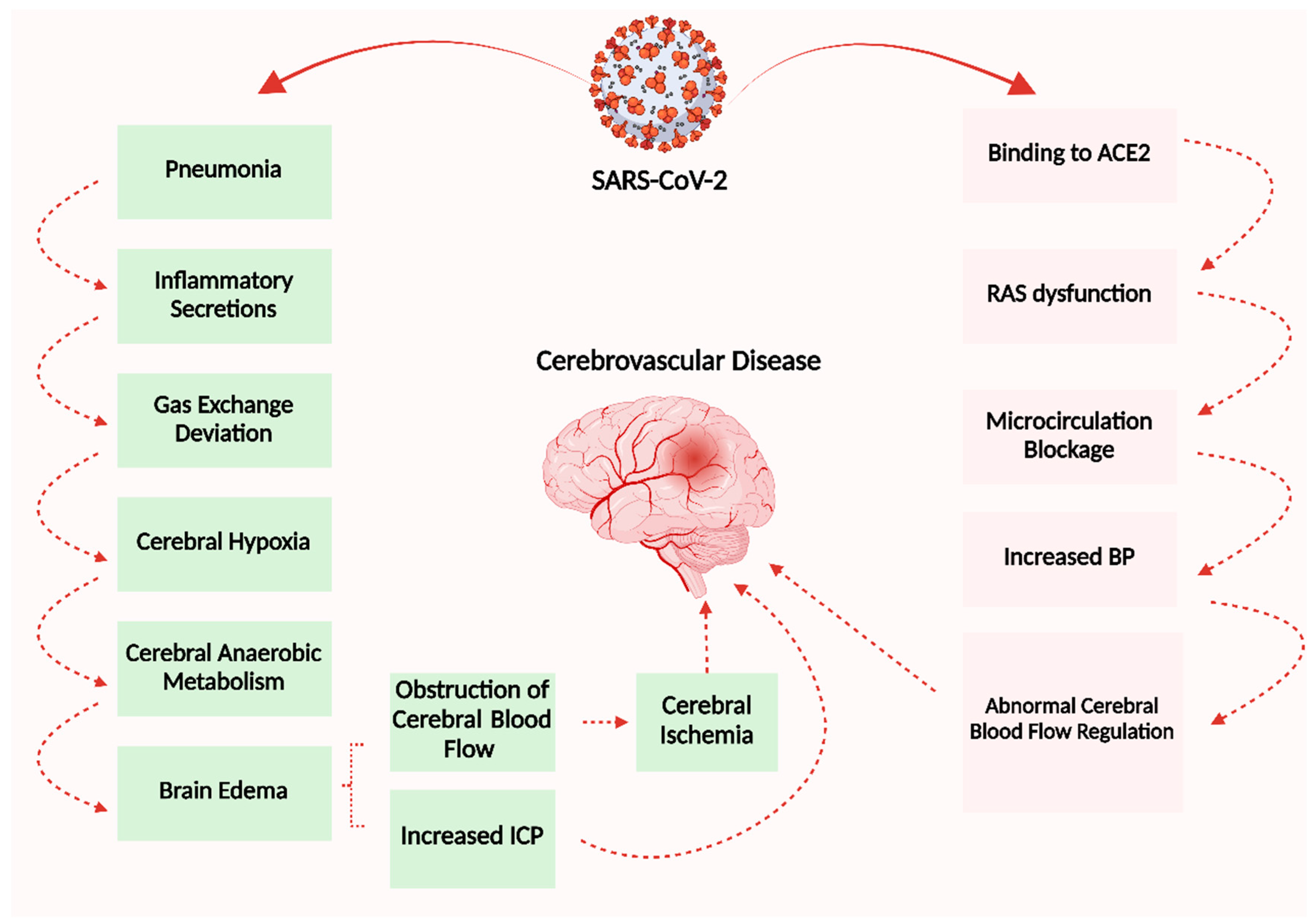
6.1. Cerebrovascular Disease
6.2. CNS Diseases by Direct Virus Transmission
6.3. Nervous System Damage Induced by Abnormal Immune and Inflammatory Reactions
6.4. Other COVID-19-Related Neurological Disorders
7. Conclusions and Future Perspectives
- ✔
- Entry via the olfactory nerve;
- ✔
- Direct infection of vascular endothelial cells;
- ✔
- The initiation of inflammatory reactions that breach the BBB facilitates invasion.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohmwald, K.; Gálvez, N.M.; Ríos, M.; Kalergis, A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses 2019, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, D.A. Transmission of viral respiratory infections in the home. Pediatr. Infect. Dis. J. 2000, 19, S97–S102. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.G.; Peck Campbell, A.J.; Boeckh, M. Respiratory viruses other than influenza virus: Impact and therapeutic advances. Clin. Microbiol. Rev. 2008, 21, 274–290. [Google Scholar] [CrossRef]
- Talbot, H.K.; Falsey, A.R. The diagnosis of viral respiratory disease in older adults. Clin. Infect. Dis. 2010, 50, 747–751. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Schwarze, J.R. Respiratory viral infections in infants: Causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010, 23, 74–98. [Google Scholar] [CrossRef]
- Berry, M.; Gamieldien, J.; Fielding, B.C. Identification of new respiratory viruses in the new millennium. Viruses 2015, 7, 996–1019. [Google Scholar] [CrossRef]
- Koralnik, I.J.; Tyler, K.L. COVID-19: A global threat to the nervous system. Ann. Neurol. 2020, 88, 1–11. [Google Scholar] [CrossRef]
- Masters, P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar]
- Nakagawa, S.; Miyazawa, T. Genome evolution of SARS-CoV-2 and its virological characteristics. Inflamm. Regen. 2020, 40, 17. [Google Scholar] [CrossRef]
- Lin, Y.; Shen, X.; Yang, R.F.; Li, Y.X.; Ji, Y.Y.; He, Y.Y.; Shi, M.D.; Lu, W.; Shi, T.L.; Wang, J. Identification of an epitope of SARS-coronavirus nucleocapsid protein. Cell Res. 2003, 13, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Sanclemente-Alaman, I.; Moreno-Jiménez, L.; Benito-Martín, M.S.; Canales-Aguirre, A.; Matías-Guiu, J.A.; Matías-Guiu, J.; Gómez-Pinedo, U. Experimental models for the study of central nervous system infection by SARS-CoV-2. Front. Immunol. 2020, 11, 2163. [Google Scholar] [CrossRef]
- Algahtani, H.; Subahi, A.; Shirah, B. Neurological complications of Middle East respiratory syndrome coronavirus: A report of two cases and review of the literature. Case Rep. Neurol. Med. 2016, 2016, 3502683. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Fan, R.; Wen, B.; Zhang, J.; Cao, X.; Wang, C.; Song, Z.; Li, S.; Li, X. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology 2017, 59, 163–169. [Google Scholar] [CrossRef]
- Meijer, W.J.; Linn, F.H.; Wensing, A.M.; Leavis, H.L.; van Riel, D.; GeurtsvanKessel, C.H.; Wattjes, M.P.; Murk, J.-L. Acute influenza virus-associated encephalitis and encephalopathy in adults: A challenging diagnosis. JMM Case Rep. 2016, 3. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L. COVID-19 in critically ill patients in the Seattle region—Case series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Acharya, A.; Kevadiya, B.D.; Gendelman, H.E.; Byrareddy, S.N. SARS-CoV-2 infection leads to neurological dysfunction. J. Neuroimmune Pharmacol. 2020, 15, 167–173. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Taquet, M. Neuropsychiatric disorders following SARS-CoV-2 infection. Brain 2023, 146, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.; Lanza, G.; Falzone, L.; Fisicaro, F.; Ferri, R.; Bella, R. SARS-CoV-2 and the nervous system: From clinical features to molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 5475. [Google Scholar] [CrossRef] [PubMed]
- Alshebri, M.S.; Alshouimi, R.A.; Alhumidi, H.A.; Alshaya, A.I. Neurological complications of SARS-CoV, MERS-CoV, and COVID-19. SN Compr. Clin. Med. 2020, 2, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.C.N.K.; Mehta, P.R.; Shukla, G.; Mehta, A.R. COVID-19, SARS and MERS: A neurological perspective. J. Clin. Neurosci. 2020, 77, 13–16. [Google Scholar] [CrossRef]
- Verstrepen, K.; Baisier, L.; De Cauwer, H. Neurological manifestations of COVID-19, SARS and MERS. Acta Neurol. Belg. 2020, 120, 1051–1060. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Poyiadji, N.; Shahin, G.; Noujaim, D.; Stone, M.; Patel, S.; Griffith, B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology 2020, 296, E119–E120. [Google Scholar] [CrossRef]
- Llansó, L.; Urra, X. Posterior reversible encephalopathy syndrome in COVID-19 disease: A case-report. SN Compr. Clin. Med. 2020, 2, 1900–1902. [Google Scholar] [CrossRef]
- Klok, F.; Kruip, M.; Van der Meer, N.; Arbous, M.; Gommers, D.; Kant, K.; Kaptein, F.; van Paassen, J.; Stals, M.; Huisman, M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Waqas, A.; Zhu, Z.; Chen, L. Exosomes: Applications in respiratory infectious diseases and prospects for coronavirus disease 2019 (COVID-19). J. Biomed. Nanotechnol. 2020, 16, 399–418. [Google Scholar] [CrossRef]
- Tyagi, K.; Rai, P.; Gautam, A.; Kaur, H.; Kapoor, S.; Suttee, A.; Jaiswal, P.K.; Sharma, A.; Singh, G.; Barnwal, R.P. Neurological manifestations of SARS-CoV-2: Complexity, mechanism and associated disorders. Eur. J. Med. Res. 2023, 28, 307. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, J.J. Neurologic complications of influenza. In Seminars in Pediatric Neurology; WB Saunders: Philadelphia, PA, USA, 2012; pp. 96–100. [Google Scholar]
- Khandaker, G.; Zurynski, Y.; Buttery, J.; Marshall, H.; Richmond, P.C.; Dale, R.C.; Royle, J.; Gold, M.; Snelling, T.; Whitehead, B. Neurologic complications of influenza A (H1N1) pdm09: Surveillance in 6 pediatric hospitals. Neurology 2012, 79, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Mylonaki, E.; Harrer, A.; Pilz, G.; Stalzer, P.; Otto, F.; Trinka, E.; Wipfler, P. Neurological complications associated with influenza in season 2017/18 in Austria-a retrospective single center study. J. Clin. Virol. 2020, 127, 104340. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.P.; Busl, K.M. Neurologic manifestations of severe respiratory viral contagions. Crit. Care Explor. 2020, 2, e0107. [Google Scholar] [CrossRef]
- Sellers, S.A.; Hagan, R.S.; Hayden, F.G.; Fischer, W.A. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir. Viruses 2017, 11, 372–393. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M., Jr.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin. Infect. Dis. 2019, 68, e1–e47. [Google Scholar] [CrossRef]
- Kho, N.; Kerrigan, J.F.; Tong, T.; Browne, R.; Knilans, J. Respiratory syncytial virus infection and neurologic abnormalities: Retrospective cohort study. J. Child Neurol. 2004, 19, 859–864. [Google Scholar] [CrossRef]
- Vehapoglu, A.; Turel, O.; Uygur Sahin, T.; Kutlu, N.O.; Iscan, A. Clinical significance of human metapneumovirus in refractory status epilepticus and encephalitis: Case report and review of the literature. Case Rep. Neurol. Med. 2015, 2015, 131780. [Google Scholar] [CrossRef]
- Mergeay, M.; Coeckelbergh, E.; De Cauwer, H.; Viaene, M.; Van der Mieren, G. An adult case of metapneumovirus-induced acute encephalitis. Acta Neurol. Belg. 2019, 119, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Jeannet, N.; van den Hoogen, B.G.; Schefold, J.C.; Suter-Riniker, F.; Sommerstein, R. Cerebrospinal Fluid Findings in an Adult with Human Metapneumovirus-Associated Encephalitis. Emerg. Infect. Dis. 2017, 23, 370. [Google Scholar] [CrossRef]
- Huang, H.; Chen, L.; Sanberg, P.R.; Dimitrijevic, M.; Shetty, A.K.; Sharma, H.S.; Wu, P.; Bryukhovetskiy, A.; Al-Zoubi, Z.M.; Chopp, M.; et al. Beijing Declaration of International Association of Neurorestoratology (2023 Xi’an version). J. Neurorestoratol. 2023, 11, 100055. [Google Scholar] [CrossRef]
- Huang, H.; Bach, J.R.; Sharma, H.S.; Saberi, H.; Jeon, S.R.; Guo, X.; Shetty, A.; Hawamdeh, Z.; Sharma, A.; Wild, K.v.; et al. The 2022 yearbook of Neurorestoratology. J. Neurorestoratol. 2023, 11, 100054. [Google Scholar] [CrossRef]
- Sharma, H.S.; Chopp, M.; Chen, L.; Sarnowska, A.; Xue, M.; Ao, Q.; Siniscalco, D.; Chen, L.; Hawamdeh, Z.; Huang, H. The 2021 yearbook of Neurorestoratology. J. Neurorestoratol. 2022, 10, 100008. [Google Scholar] [CrossRef]
- Huang, H.; Chen, L.; Chopp, M.; Young, W.; Robert Bach, J.; He, X.; Sarnowaska, A.; Xue, M.; Chunhua Zhao, R.; Shetty, A.; et al. The 2020 Yearbook of Neurorestoratology. J. Neurorestoratol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiu, Y. The regulation of host cytoskeleton during SARS-CoV-2 infection in the nervous system. Brain Sci. Adv. 2023, 9, 43–52. [Google Scholar] [CrossRef]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef]
- Takao, M.; Ohira, M. Neurological post-acute sequelae of SARS-CoV-2 infection. Psychiatry Clin. Neurosci. 2023, 77, 72–83. [Google Scholar] [CrossRef]
- Kaplan, L.; Chow, B.W.; Gu, C. Neuronal regulation of the blood–brain barrier and neurovascular coupling. Nat. Rev. Neurosci. 2020, 21, 416–432. [Google Scholar] [CrossRef]
- McGavern, D.B.; Kang, S.S. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 2011, 11, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.A.; McGavern, D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015, 11, 44–54. [Google Scholar] [CrossRef]
- Tyrrell, D.; Bynoe, M. Cultivation of a novel type of common-cold virus in organ cultures. Br. Med. J. 1965, 1, 1467. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Swain, B.; Verma, R.; Gunthe, S.S. SARS-CoV, MERS-CoV, and 2019-nCoV viruses: An overview of origin, evolution, and genetic variations. VirusDisease 2020, 31, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zheng, B.; He, Y.; Liu, X.; Zhuang, Z.; Cheung, C.; Luo, S.; Li, P.H.; Zhang, L.; Guan, Y. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 2003, 302, 276–278. [Google Scholar] [PubMed]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef]
- Nassar, M.; Bakhrebah, M.; Meo, S.A.; Alsuabeyl, M.; Zaher, W. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: Epidemiology, pathogenesis and clinical characteristics. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4956–4961. [Google Scholar]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef]
- Huang, H. Why is olfactory neuroepithelium? J. Neurorestoratol. 2021, 9, 211–218. [Google Scholar] [CrossRef]
- DosSantos, M.F.; Devalle, S.; Aran, V.; Capra, D.; Roque, N.R.; Coelho-Aguiar, J.d.M.; Spohr, T.C.L.d.S.e.; Subilhaga, J.G.; Pereira, C.M.; D’Andrea Meira, I. Neuromechanisms of SARS-CoV-2: A review. Front. Neuroanat. 2020, 14, 37. [Google Scholar] [CrossRef]
- Butowt, R.; Bilinska, K. SARS-CoV-2: Olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem. Neurosci. 2020, 11, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- García-García, S.; Cepeda, S.; Arrese, I.; Sarabia, R. Hemorrhagic conditions affecting the central nervous system in COVID-19 patients. Neurosurgery 2020, 87, E394–E396. [Google Scholar] [CrossRef] [PubMed]
- Saleki, K.; Banazadeh, M.; Saghazadeh, A.; Rezaei, N. The involvement of the central nervous system in patients with COVID-19. Rev. Neurosci. 2020, 31, 453–456. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Proposed intranasal route for drug administration in the management of central nervous system manifestations of COVID-19. ACS Chem. Neurosci. 2020, 11, 1523–1524. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.-Y.; Jin, W.-L. The COVID-19 pandemic: Consideration for brain infection. Neuroscience 2020, 437, 130. [Google Scholar] [CrossRef]
- Liang, F. Sustentacular cell enwrapment of olfactory receptor neuronal dendrites: An update. Genes 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Chaiyasut, C. Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging—A Review. Microorganisms 2022, 10, 1405. [Google Scholar]
- Chen, M.; Shen, W.; Rowan, N.R.; Kulaga, H.; Hillel, A.; Ramanathan, M.; Lane, A.P. Elevated ACE-2 expression in the olfactory neuroepithelium: Implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: Identification of cell types and trends with age. ACS Chem. Neurosci. 2020, 11, 1555–1562. [Google Scholar] [CrossRef]
- Bryche, B.; St Albin, A.; Murri, S.; Lacôte, S.; Pulido, C.; Gouilh, M.A.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020, 89, 579–586. [Google Scholar] [CrossRef]
- Deffner, F.; Scharr, M.; Klingenstein, S.; Klingenstein, M.; Milazzo, A.; Scherer, S.; Wagner, A.; Hirt, B.; Mack, A.F.; Neckel, P.H. Histological evidence for the enteric nervous system and the choroid plexus as alternative routes of neuroinvasion by SARS-CoV2. Front. Neuroanat. 2020, 14, 596439. [Google Scholar] [CrossRef] [PubMed]
- de Mattos Coelho-Aguiar, J.; Veríssimo, C.P.; da Silva Costa, D.V.; de Moraes Thomasi, B.B.; Frauches, A.C.B.; Ribeiro, F.P.; Gomes, A.L.T.; de Castro Brito, G.A.; Moura-Neto, V. The enteric glial network acts in the maintenance of intestinal homeostasis and in intestinal disorders. In Glia in Health and Disease; IntechOpen: London, UK, 2019; pp. 1–29. [Google Scholar]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Sarnelli, G. Can the enteric nervous system be an alternative entrance door in SARS-CoV2 neuroinvasion? Brain Behav. Immun. 2020, 87, 93. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Mangalam, A.K.; Guo, Y.; LaFrance-Corey, R.G.; Gamez, J.D.; Atanga, P.A.; Clarkson, B.D.; Zhang, Y.; Wang, E. Neuropilin-1 modulates interferon-γ-stimulated signaling in brain microvascular endothelial cells. J. Cell Sci. 2016, 129, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913.e907. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell 2020, 27, 951–961.e955. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Raikwar, S.P.; Thangavel, R.; Khan, A.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of theCytokine Storm’in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; Wang, J. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020, 53, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Ge, Y.; Wu, B.; Zhang, W.; Wu, T.; Wen, T.; Liu, J.; Guo, X.; Huang, C.; Jiao, Y. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J. Infect. Dis. 2020, 222, 746–754. [Google Scholar] [CrossRef]
- Sfera, A.; Rahman, L.; Zapata-Martín del Campo, C.M.; Kozlakidis, Z. Long COVID as a Tauopathy: Of “Brain Fog” and “Fusogen Storms”. Int. J. Mol. Sci. 2023, 24, 12648. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the nervous system. Cell 2020, 183, 16–27.e1. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Yang, N.; Han, D.; Mi, X.; Li, Y.; Liu, K.; Vuylsteke, A.; Xiang, H.; Guo, X. Neurological manifestations of patients with COVID-19: Potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 2020, 14, 533–541. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Qiu, J.; Li, L.; Jia, X.; Kai, G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020, 53, 66–70. [Google Scholar] [CrossRef]
- Al-Obaidi, M.J.; Bahadoran, A.; Wang, S.; Manikam, R.; Raju, C.S.; Sekaran, S. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol. 2018, 62, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Diamond, M.S. Mechanisms of restriction of viral neuroinvasion at the blood–brain barrier. Curr. Opin. Immunol. 2016, 38, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Doobay, M.F.; Talman, L.S.; Obr, T.D.; Tian, X.; Davisson, R.L.; Lazartigues, E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R373–R381. [Google Scholar] [PubMed]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.-E.; Liu, F.; Dai, Y. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, Y.; Nilsson-Payant, B.E.; Gupta, V.; Wang, P.; Duan, X.; Tang, X.; Zhu, J.; Zhao, Z.; Jaffré, F. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell 2020, 27, 125–136.e7. [Google Scholar] [PubMed]
- Brasso, C.; Bellino, S.; Blua, C.; Bozzatello, P.; Rocca, P. The Impact of SARS-CoV-2 Infection on Youth Mental Health: A Narrative Review. Biomedicines 2022, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host–Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef]
- MacLean, M.; Kamintsky, L.; Leck, E.; Friedman, A. The potential role of microvascular pathology in the neurological manifestations of coronavirus infection. Fluids Barriers CNS 2020, 17, 55. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Stodola, J.K.; Meessen-Pinard, M.; Talbot, P.J. Human coronaviruses: Viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014, 194, 145–158. [Google Scholar] [CrossRef]
- Tremblay, M.-E.; Madore, C.; Bordeleau, M.; Tian, L.; Verkhratsky, A. Neuropathobiology of COVID-19: The role for glia. Front. Cell. Neurosci. 2020, 14, 592214. [Google Scholar] [CrossRef]
- Vazana, U.; Veksler, R.; Pell, G.S.; Prager, O.; Fassler, M.; Chassidim, Y.; Roth, Y.; Shahar, H.; Zangen, A.; Raccah, R. Glutamate-mediated blood–brain barrier opening: Implications for neuroprotection and drug delivery. J. Neurosci. 2016, 36, 7727–7739. [Google Scholar] [CrossRef] [PubMed]
- Merkler, A.E.; Parikh, N.S.; Mir, S.; Gupta, A.; Kamel, H.; Lin, E.; Lantos, J.; Schenck, E.J.; Goyal, P.; Bruce, S.S. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020, 77, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, P.N.; Schaal, H. Human brain organoids to explore SARS-CoV-2-induced effects on the central nervous system. Rev. Med. Virol. 2023, 33, e2430. [Google Scholar] [CrossRef] [PubMed]
- Mollica, V.; Rizzo, A.; Massari, F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Med. 2020, 16, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Li, W.; Bao, J.; Peng, Q.; Wen, D.; Wang, J.; Sun, B. The expression of SARS-CoV-2 receptor ACE2 and CD147, and protease TMPRSS2 in human and mouse brain cells and mouse brain tissues. Biochem. Biophys. Res. Commun. 2020, 533, 867–871. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Chen, Z.; Mi, L.; Xu, J.; Yu, J.; Wang, X.; Jiang, J.; Xing, J.; Shang, P.; Qian, A.; Li, Y. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005, 191, 755–760. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhou, Y.-S.; Lian, J.-Q.; Zhang, Z.; Du, P.; Gong, L.; Zhang, Y.; Cui, H.-Y.; Geng, J.-J. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv 2020. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.-C.; Bosch, B.J.; Li, F.; Li, W.; Lee, K.H.; Ghiran, S.; Vasilieva, N.; Dermody, T.S.; Harrison, S.C.; Dormitzer, P.R. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006, 281, 3198–3203. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.-X.; Zheng, X.-R.; Peng, W.; Wu, M.-L.; Mao, X.-Y. Vascular endothelial growth factor (VEGF) as a vital target for brain inflammation during the COVID-19 outbreak. ACS Chem. Neurosci. 2020, 11, 1704–1705. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Han, J.; Wu, X.; Zeng, H.; Liu, J.; Zhang, H. VEGF-D: A novel biomarker for detection of COVID-19 progression. Crit. Care 2020, 24, 373. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.; Pfeffer, M.A.; Solomon, S.D. Renin–angiotensin–aldosterone system inhibitors in patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Rhoades, R.; Solomon, S.; Johnson, C.; Teng, S. Impact of SARS-CoV-2 on host factors involved in mental disorders. Front. Microbiol. 2022, 13, 845559. [Google Scholar] [CrossRef]
- Polidoro, R.B.; Hagan, R.S.; de Santis Santiago, R.; Schmidt, N.W. Overview: Systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front. Immunol. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Méndez-García, L.A.; Solleiro-Villavicencio, H.; Guartazaca-Guerrero, S.; Rodríguez-Morales, J.; Carrillo-Ruiz, J.D. Neurological Diseases Define the Cytokine Profile in CFS during SARS-CoV-2 Infection in Highly Ill Patients. Trop. Med. Infect. Dis. 2023, 8, 290. [Google Scholar] [CrossRef]
- Picone, P.; Sanfilippo, T.; Guggino, R.; Scalisi, L.; Monastero, R.; Baschi, R.; Mandalà, V.; San Biagio, L.; Rizzo, M.; Giacomazza, D. Neurological consequences, mental health, physical care, and appropriate nutrition in long-COVID-19. Cell. Mol. Neurobiol. 2023, 43, 1685–1695. [Google Scholar] [CrossRef]
- Tsukahara, T.; Brann, D.H.; Datta, S.R. Mechanisms of SARS-CoV-2-associated anosmia. Physiol. Rev. 2023, 103, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.; Sabadia, S.; La Ichan, R. A prospective study of neurological disorders in hospitalized COVID-19 patients in New York city. Neurology 2020. [Google Scholar] [CrossRef]
- Peron, J.P.S. Direct and indirect impact of SARS-CoV-2 on the brain. Hum. Genet. 2023, 142, 1317–1326. [Google Scholar] [CrossRef]
- Ahmadi, S.; Khaledi, S. Brain Renin–Angiotensin System: From Physiology to Pathology in Neuronal Complications Induced by SARS-CoV-2. Anal. Cell. Pathol. 2023, 2023, 8883492. [Google Scholar] [CrossRef]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. In International Forum of Allergy & Rhinology; John and Wiley and Sons: Hoboken, NJ, USA, 2020; pp. 806–813. [Google Scholar]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Granholm, A.-C. Long-term effects of SARS-CoV-2 in the brain: Clinical consequences and molecular mechanisms. J. Clin. Med. 2023, 12, 3190. [Google Scholar] [CrossRef]
- Ahmed, W.; Kuniyan, M.S.; Jawed, A.M.; Chen, L. Engineered Extracellular Vesicles for Drug Delivery in Therapy of Stroke. Pharmaceutics 2023, 15, 2173. [Google Scholar] [CrossRef]
- Abboud, H.; Abboud, F.Z.; Kharbouch, H.; Arkha, Y.; El Abbadi, N.; El Ouahabi, A. COVID-19 and SARS-CoV-2 infection: Pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020, 140, 49–53. [Google Scholar] [CrossRef]
- Zhu, Z.-H.; Jia, F.; Ahmed, W.; Zhang, G.-L.; Wang, H.; Lin, C.-Q.; Chen, W.-H.; Chen, L.-K. Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regen. Res. 2023, 18, 404. [Google Scholar] [PubMed]
- Forero, K.; Buqaileh, R.; Sunderman, C.; AbouAlaiwi, W. COVID-19 and Neurological Manifestations. Brain Sci. 2023, 13, 1137. [Google Scholar] [CrossRef] [PubMed]
- Fugate, J.E.; Lyons, J.L.; Thakur, K.T.; Smith, B.R.; Hedley-Whyte, E.T.; Mateen, F.J. Infectious causes of stroke. Lancet Infect. Dis. 2014, 14, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.; Cohrs, R.; Mahalingam, R.; Wellish, M.C.; Forghani, B.; Schiller, A.; Safdieh, J.E.; Kamenkovich, E.; Ostrow, L.W.; Levy, M. The varicella zoster virus vasculopathies: Clinical, CSF, imaging, and virologic features. Neurology 2008, 70, 853–860. [Google Scholar] [CrossRef]
- Ghotbi, Z.; Estakhr, M.; Hosseini, M.; Shahripour, R.B. Cerebral Vasomotor Reactivity in COVID-19: A Narrative Review. Life 2023, 13, 1614. [Google Scholar] [CrossRef]
- Stein, L.K.; Mayman, N.A.; Dhamoon, M.S.; Fifi, J.T. The emerging association between COVID-19 and acute stroke. Trends Neurosci. 2021, 44, 527–537. [Google Scholar] [CrossRef]
- Maury, A.; Lyoubi, A.; Peiffer-Smadja, N.; de Broucker, T.; Meppiel, E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians. Rev. Neurol. 2021, 177, 51–64. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Complicaciones neurológicas por coronavirus y COVID-19. Rev. Neurol. 2020, 70, 311–322. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A. Large-vessel stroke as a presenting feature of COVID-19 in the young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef]
- Wang, L.; Ren, Z.; Ma, L.; Han, Y.; Wei, W.; Jiang, E.; Ji, X.-Y. Progress in research on SARS-CoV-2 infection causing neurological diseases and its infection mechanism. Front. Neurol. 2021, 11, 592888. [Google Scholar] [CrossRef] [PubMed]
- Morassi, M.; Bagatto, D.; Cobelli, M.; D’Agostini, S.; Gigli, G.L.; Bnà, C.; Vogrig, A. Stroke in patients with SARS-CoV-2 infection: Case series. J. Neurol. 2020, 267, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Wang, D.; Mao, L.; Jin, H.; Hu, B. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc. Neurol. 2020, 5. [Google Scholar] [CrossRef]
- Sharifi-Razavi, A.; Karimi, N.; Rouhani, N. COVID-19 and intracerebral haemorrhage: Causative or coincidental? New Microbes New Infect. 2020, 35, 100669. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.E.; Cardona, P.; Arenillas, J.F.; Talavera, B.; Guillen, A.N.; Chavarría-Miranda, A.; de Lera, M.; Khandelwal, P.; Bach, I.; Patel, P. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: The SVIN COVID-19 multinational registry. Int. J. Stroke 2021, 16, 437–447. [Google Scholar] [CrossRef]
- Sharifian-Dorche, M.; Huot, P.; Osherov, M.; Wen, D.; Saveriano, A.; Giacomini, P.S.; Antel, J.P.; Mowla, A. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J. Neurol. Sci. 2020, 417, 117085. [Google Scholar] [CrossRef]
- Favas, T.T.; Dev, P.; Chaurasia, R.N.; Chakravarty, K.; Mishra, R.; Joshi, D.; Mishra, V.N.; Kumar, A.; Singh, V.K.; Pandey, M.; et al. Neurological manifestations of COVID-19: A systematic review and meta-analysis of proportions. Neurol. Sci. 2020, 41, 3437–3470. [Google Scholar] [CrossRef]
- Rigamonti, A.; Mantero, V.; Piamarta, F.; Spena, G.; Salmaggi, A. Cerebral venous thrombosis associated with coronavirus infection: An underestimated entity? Neurol. Sci. 2021, 42, 317–318. [Google Scholar] [CrossRef]
- Chougar, L.; Mathon, B.; Weiss, N.; Degos, V.; Shor, N. Atypical Deep Cerebral Vein Thrombosis with Hemorrhagic Venous Infarction in a Patient Positive for COVID-19. Am. J. Neuroradiol. 2020, 41, 1377–1379. [Google Scholar] [CrossRef]
- Huang, Y.H.; Jiang, D.; Huang, J.T. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav. Immun. 2020, 87, 149. [Google Scholar] [CrossRef]
- Efe, I.E.; Aydin, O.U.; Alabulut, A.; Celik, O.; Aydin, K. COVID-19—Associated encephalitis mimicking glial tumor. World Neurosurg. 2020, 140, 46–48. [Google Scholar] [CrossRef] [PubMed]
- McAbee, G.N.; Brosgol, Y.; Pavlakis, S.; Agha, R.; Gaffoor, M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr. Neurol. 2020, 109, 94. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Khan, A.; Sundar, W.H.; Naseem, H.; Chen, W.; Feng, J.; Durrani, S.; Chen, L. Neurological diseases caused by coronavirus infection of the respiratory airways. Brain Sci. Adv. 2020, 6, 324–343. [Google Scholar] [CrossRef]
- Reiss, A.B.; Greene, C.; Dayaramani, C.; Rauchman, S.H.; Stecker, M.M.; De Leon, J.; Pinkhasov, A. Long COVID, the Brain, Nerves, and Cognitive Function. Neurol. Int. 2023, 15, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinedo, U.; Matias-Guiu, J.; Sanclemente-Alaman, I.; Moreno-Jimenez, L.; Montero-Escribano, P.; Matias-Guiu, J.A. SARS-CoV-2 as a potential trigger of neurodegenerative diseases. Mov. Disord. 2020, 35, 1104. [Google Scholar] [CrossRef]
- von Weyhern, C.H.; Kaufmann, I.; Neff, F.; Kremer, M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 2020, 395, e109. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Solomon, I.H.; Normandin, E.; Bhattacharyya, S.; Mukerji, S.S.; Keller, K.; Ali, A.S.; Adams, G.; Hornick, J.L.; Padera, R.F.; Sabeti, P. Neuropathological Features of COVID-19. N. Engl. J. Med. 2020, 383, 989–992. [Google Scholar] [CrossRef]
- Welge-Lüssen, A.; Wolfensberger, M. Olfactory disorders following upper respiratory tract infections. Tast. Smell 2006, 63, 125–132. [Google Scholar]
- Boscolo-Rizzo, P.; Borsetto, D.; Fabbris, C.; Spinato, G.; Frezza, D.; Menegaldo, A.; Mularoni, F.; Gaudioso, P.; Cazzador, D.; Marciani, S.; et al. Evolution of Altered Sense of Smell or Taste in Patients With Mildly Symptomatic COVID-19. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 729–732. [Google Scholar] [CrossRef]
- Zubair, A.S.; McAlpine, L.S.; Gardin, T.; Farhadian, S.; Kuruvilla, D.E.; Spudich, S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 2020, 77, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Ermilov, V.; Barkanov, V.; Barkanova, O.; Dorofeev, N.; Filatov, V. Clinical and anatomical features of SARS-CoV-2 with acute hemorrhagic necrotizing encephalopathy. Arkhiv Patol. 2021, 83, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Keyhanian, K.; Umeton, R.P.; Mohit, B.; Davoudi, V.; Hajighasemi, F.; Ghasemi, M. SARS-CoV-2 and nervous system: From pathogenesis to clinical manifestation. J. Neuroimmunol. 2021, 350, 577436. [Google Scholar] [CrossRef] [PubMed]
- Alberti, P.; Beretta, S.; Piatti, M.; Karantzoulis, A.; Piatti, M.L.; Santoro, P.; Viganò, M.; Giovannelli, G.; Pirro, F.; Montisano, D.A. Guillain-Barré syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7. [Google Scholar] [CrossRef]
- Fantini, J.; Di Scala, C.; Chahinian, H.; Yahi, N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents 2020, 55, 105960. [Google Scholar] [CrossRef]
- Tiet, M.Y.; AlShaikh, N. Guillain-Barré syndrome associated with COVID-19 infection: A case from the UK. BMJ Case Rep. CP 2020, 13, e236536. [Google Scholar] [CrossRef]
- Jin, M.; Tong, Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg. Infect. Dis. 2020, 26, 1618. [Google Scholar] [CrossRef]
- Jiang, X.; Coffee, M.; Bari, A.; Wang, J.; Jiang, X.; Huang, J.; Shi, J.; Dai, J.; Cai, J.; Zhang, T.; et al. Towards an Artificial Intelligence Framework for Data-Driven Prediction of Coronavirus Clinical Severity. Comput. Mater. Contin. 2020, 63, 537–551. [Google Scholar] [CrossRef]
- Yuksel, H.; Gursoy, G.T.; Dirik, E.B.; Kenar, S.G.; Bektas, H.; Yamanel, L.; Guner, H.R. Neurological manifestations of COVID-19 in confirmed and probable cases: A descriptive study from a large tertiary care center. J. Clin. Neurosci. 2021, 86, 97–102. [Google Scholar] [CrossRef]
- Galassi, G.; Marchioni, A. Myasthenia gravis at the crossroad of COVID-19: Focus on immunological and respiratory interplay. Acta Neurol. Belg. 2021, 121, 633–642. [Google Scholar] [CrossRef]
- Solé, G.; Mathis, S.; Friedman, D.; Salort-Campana, E.; Tard, C.; Bouhour, F.; Magot, A.; Annane, D.; Clair, B.; Le Masson, G. Impact of coronavirus disease 2019 in a French cohort of myasthenia gravis. Neurology 2021, 96, e2109–e2120. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Filho, A.E.; Silva, A.M.; Estephan, E.P.; Zambon, A.A.; Mendonça, R.H.; Souza, P.V.; Pinto, W.B.; Oliveira, A.S.; Dangoni-Filho, I.; Pouza, A.F. Myasthenia gravis and COVID-19: Clinical characteristics and outcomes. Front. Neurol. 2020, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62,354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.E.; Kumar, S.; Rajji, T.K.; Pollock, B.G.; Mulsant, B.H. Anticipating and Mitigating the Impact of the COVID-19 Pandemic on Alzheimer’s Disease and Related Dementias. Am. J. Geriatr. Psychiatry 2020, 28, 712–721. [Google Scholar] [CrossRef]
- Suzuki, M.; Hotta, M.; Nagase, A.; Yamamoto, Y.; Hirakawa, N.; Satake, Y.; Nagata, Y.; Suehiro, T.; Kanemoto, H.; Yoshiyama, K.; et al. The behavioral pattern of patients with frontotemporal dementia during the COVID-19 pandemic. Int. Psychogeriatr. 2020, 32, 1231–1234. [Google Scholar] [CrossRef]



| Receptors/Protein | Primary Expansion Area | Reference |
|---|---|---|
| ACE2 | Hypothalamus, Pituitary Gland | [100,101,102,103,104,105] |
| TMPRSS2 | Cerebellum, Hypothalamus, Pituitary Gland | [70,106,107] |
| NRP1 | Olfactory Bulb | [108] |
| BASIGIN | Pituitary Gland, Frontal Cortex | [109,110,111,112] |
| Cathepsin L | Pituitary Gland, Spinal Cord | [113,114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, W.; Feng, J.; Zhang, Y.; Chen, L. SARS-CoV-2 and Brain Health: New Challenges in the Era of the Pandemic. Microorganisms 2023, 11, 2511. https://doi.org/10.3390/microorganisms11102511
Ahmed W, Feng J, Zhang Y, Chen L. SARS-CoV-2 and Brain Health: New Challenges in the Era of the Pandemic. Microorganisms. 2023; 11(10):2511. https://doi.org/10.3390/microorganisms11102511
Chicago/Turabian StyleAhmed, Waqas, Jia Feng, Yifan Zhang, and Lukui Chen. 2023. "SARS-CoV-2 and Brain Health: New Challenges in the Era of the Pandemic" Microorganisms 11, no. 10: 2511. https://doi.org/10.3390/microorganisms11102511
APA StyleAhmed, W., Feng, J., Zhang, Y., & Chen, L. (2023). SARS-CoV-2 and Brain Health: New Challenges in the Era of the Pandemic. Microorganisms, 11(10), 2511. https://doi.org/10.3390/microorganisms11102511






