Enhancing Biocrust Development and Plant Growth through Inoculation of Desiccation-Tolerant Cyanobacteria in Different Textured Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Cyanobacterial Species
2.2. Screening of Desiccation Tolerant Cyanobacterial Strain
2.2.1. Revival Potential
2.2.2. EPS Production
2.3. Mass Cultivation and Experimental Design
2.4. Estimation of Chlorophyll-a and EPS in the Biocrusts
2.5. Rehydration Assay and Chlorophyll Fluorescence Measurements of Biocrust
2.6. Agronomic Effect of Revived Cyanobacterial Biocrust on Rice Seedlings
2.6.1. Seed Germination, Root and Shoot Length
2.6.2. Seedlings’ Dry Weight
2.6.3. Photosynthetic Pigments of Rice Seedlings
2.6.4. Flavonoids and Phenol Content of Rice Seedlings
2.7. Statistical Analysis
3. Results and Discussion
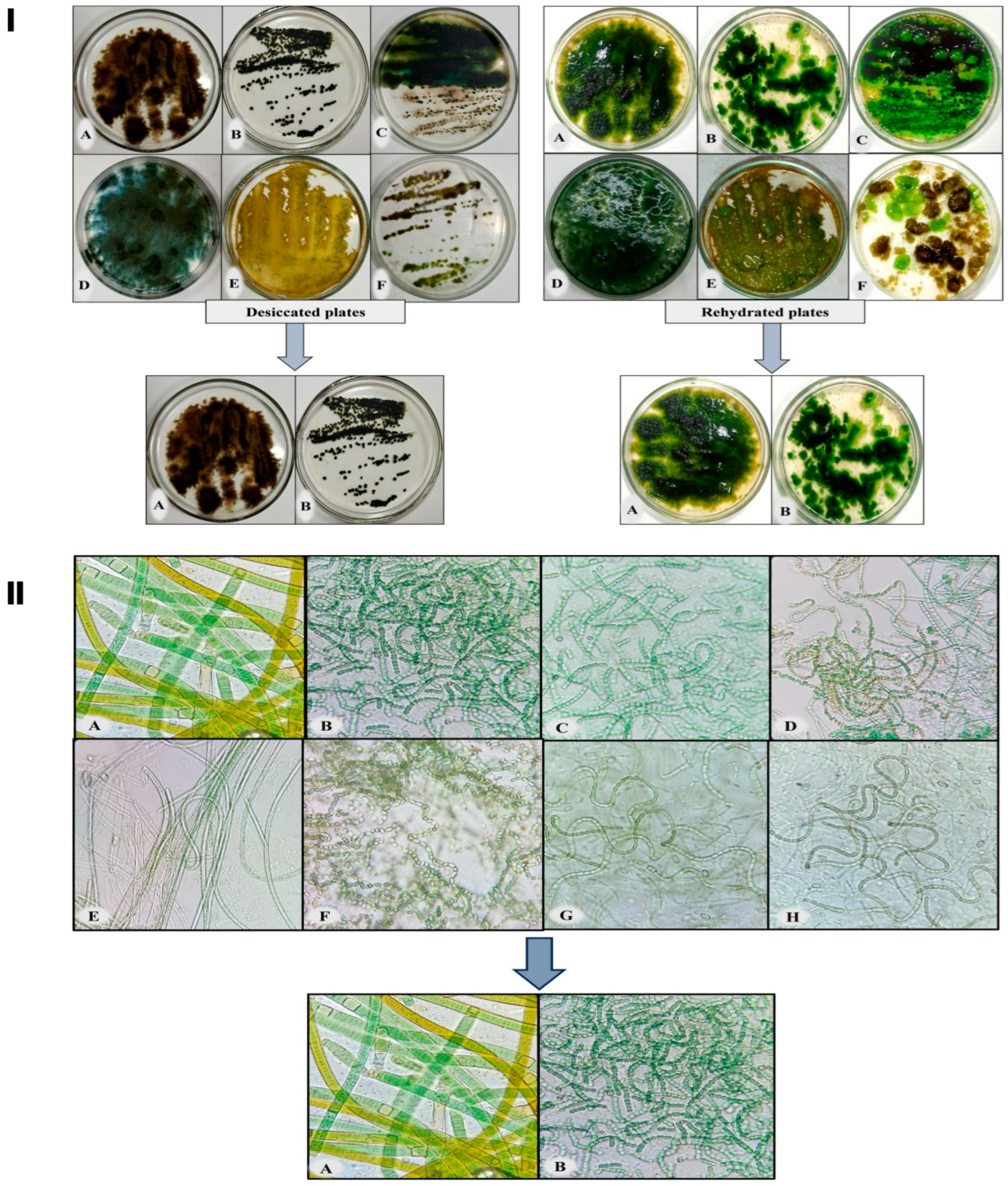
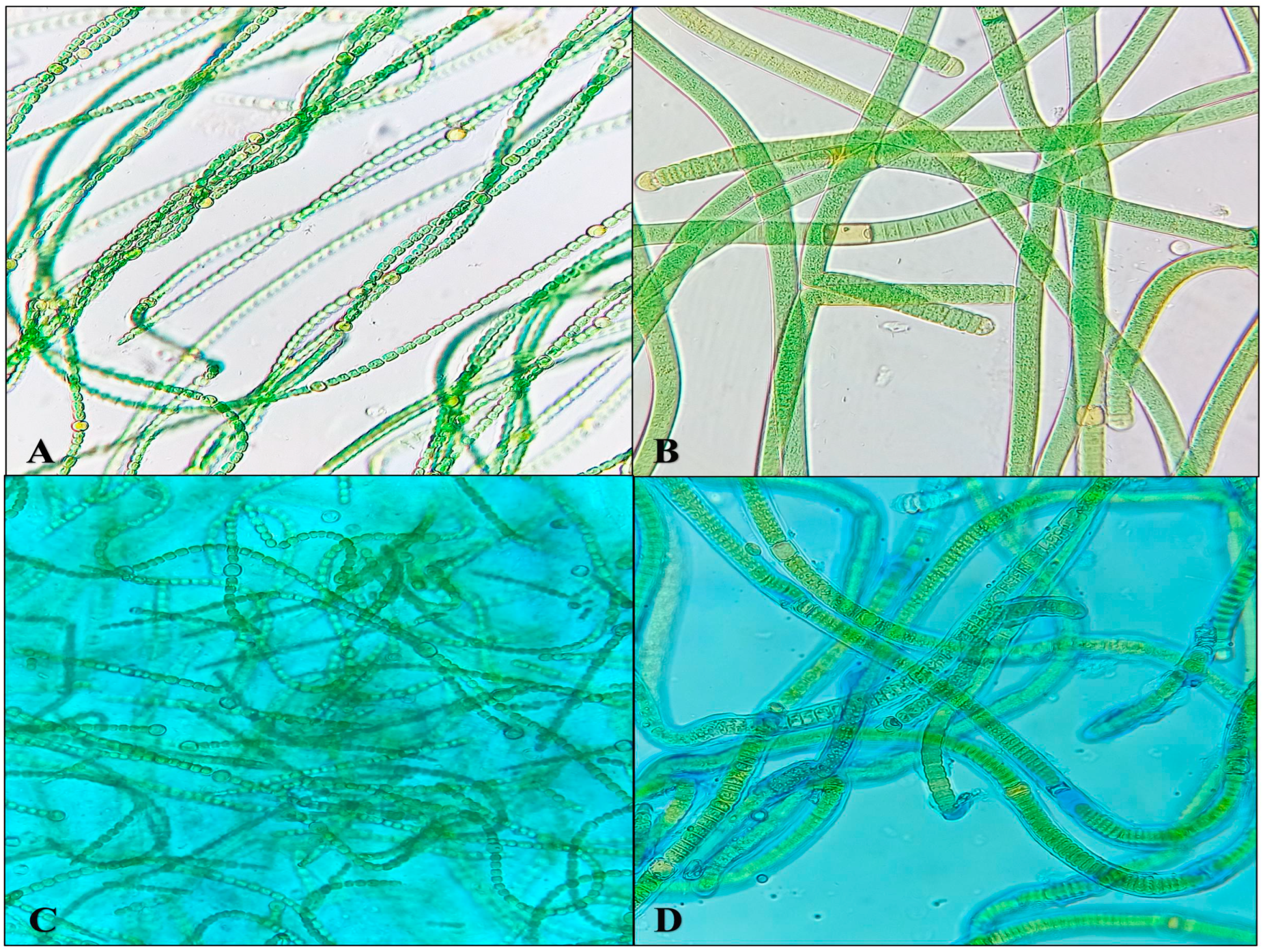
3.1. Screening of Desiccation Tolerant Cyanobacterium
3.2. Characteristics of the Biocrusts
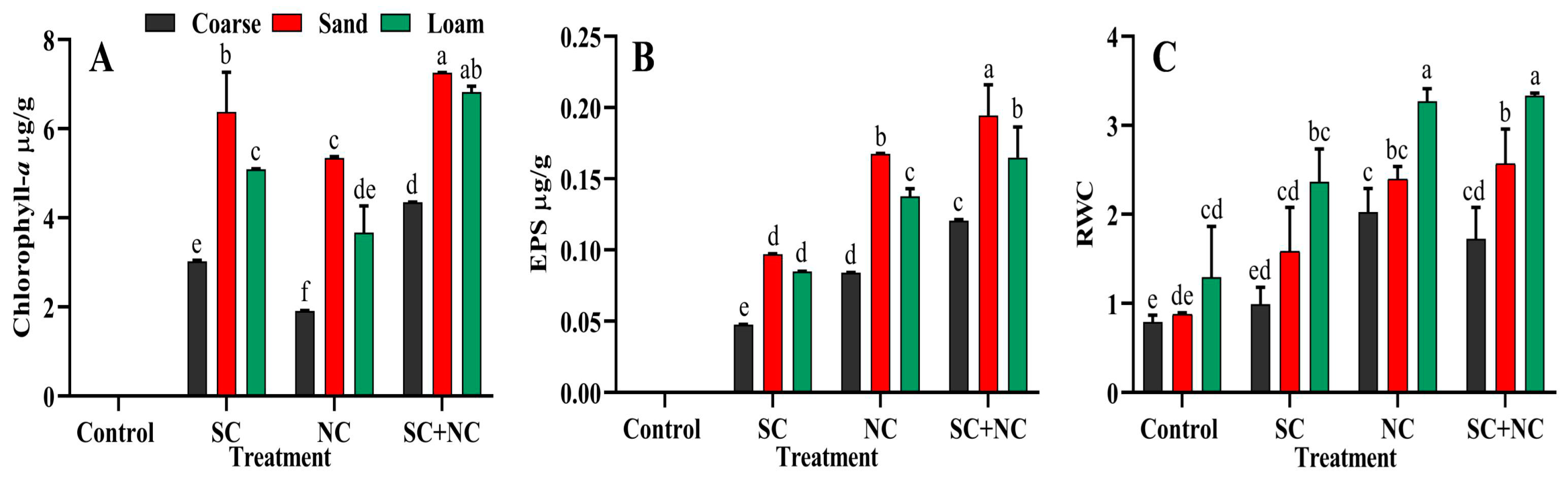
3.3. Estimation of Chlorophyll-a and EPS in the Biocrusts
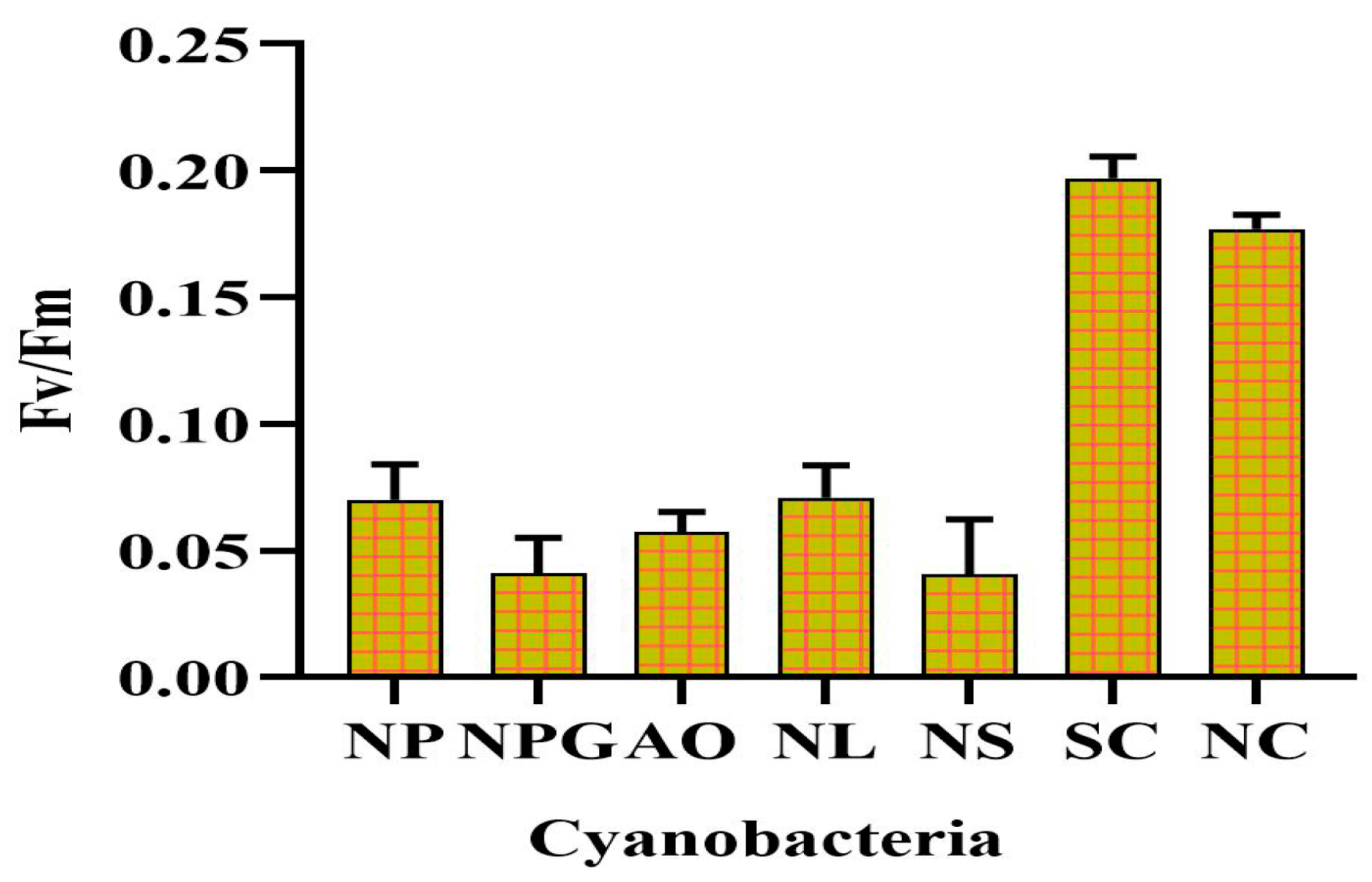
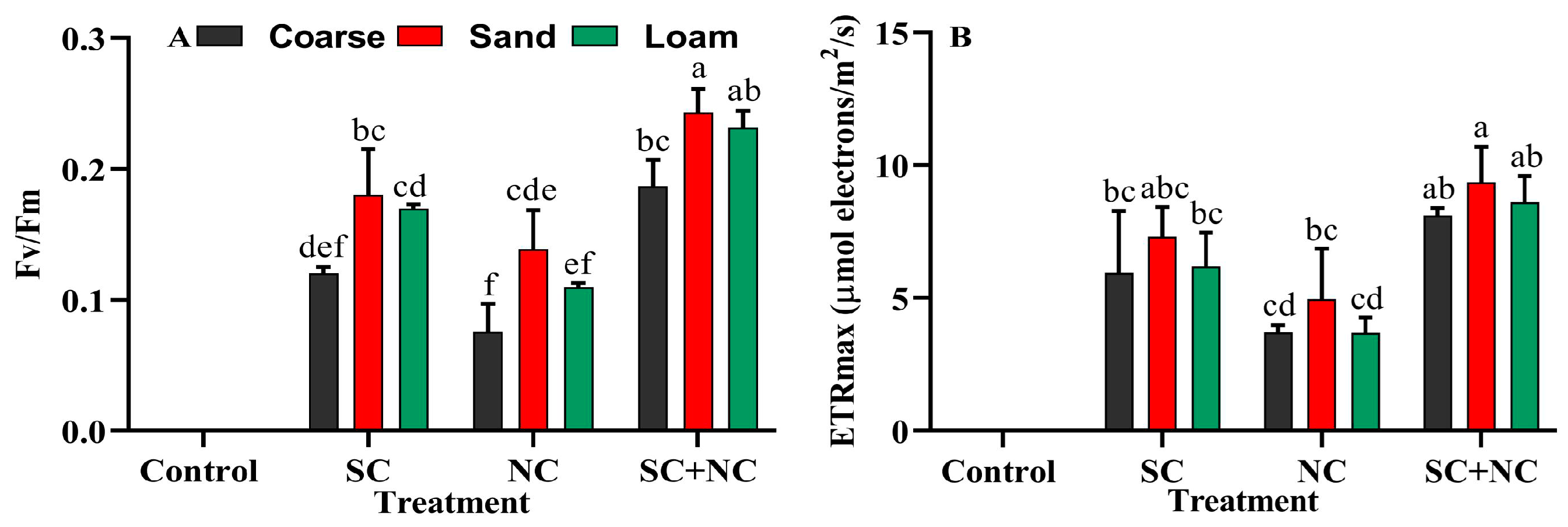
3.4. Revival Potential of Biocrust after Rehydration
3.5. Seed Germination, Seedlings Root–Shoot Length, and Dry Weight
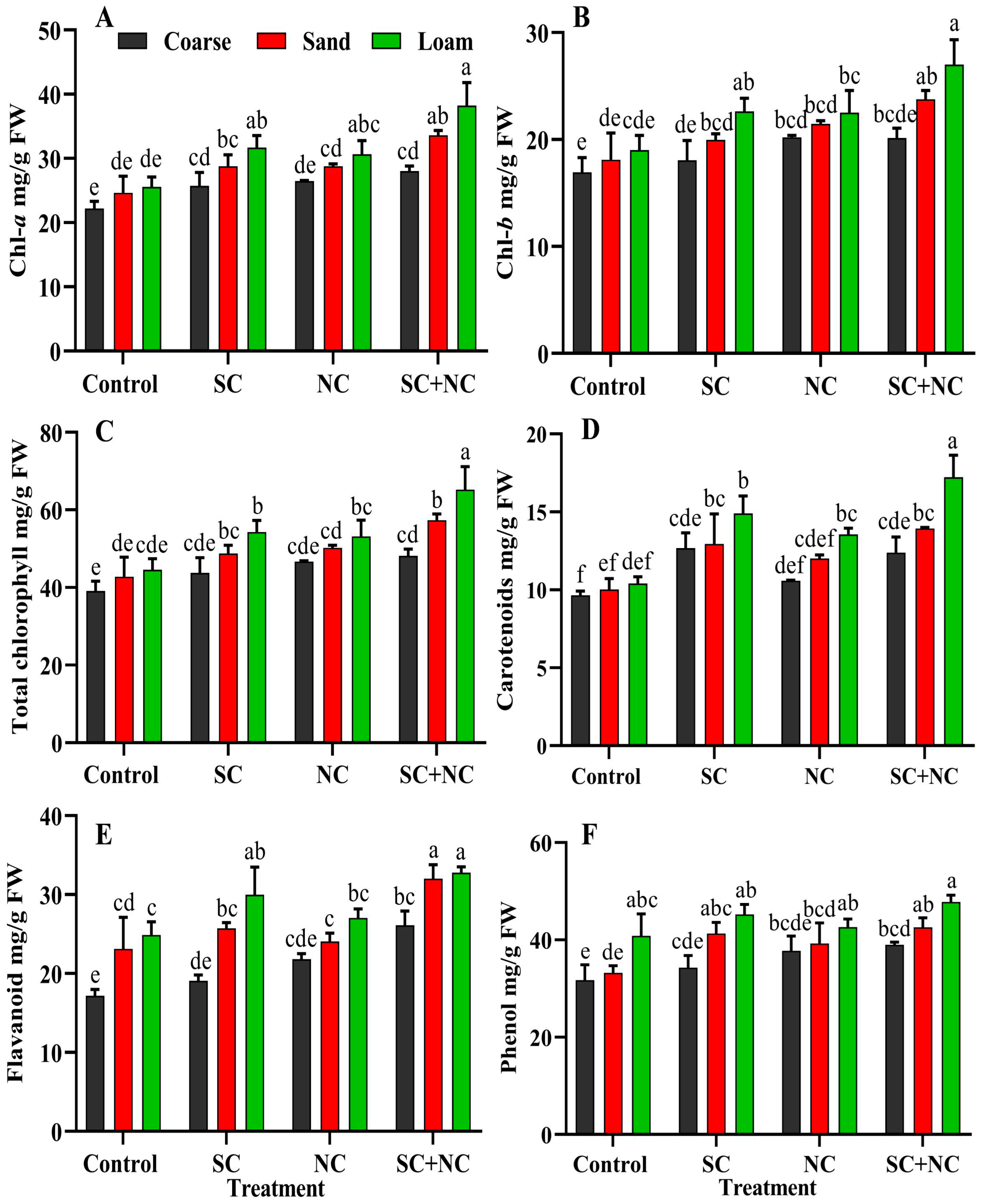
3.6. Effect of Revived Cyanobacterial Biocrust on Growth of Rice Seedling
3.7. Phenol and Flavonoid Content of Rice Seedlings
4. Implications for Soil Restoration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wigley, T.M.L.; Jones, P.D. Influences of precipitation changes and direct CO2 effects on streamflow. Nature 1985, 314, 149–152. [Google Scholar] [CrossRef]
- Adhikari, K.; Hartemink, A.E. Linking soils to ecosystem services—A global review. Geoderma 2016, 262, 101–111. [Google Scholar] [CrossRef]
- Lan, S.; Wu, L.; Zhang, D.; Hu, C. Analysis of environmental factors determining development and succession in biological soil crusts. Sci. Total Environ. 2015, 538, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Singh, R.P.; Rana, S.; Joshi, D.; Kumar, D.; Bhardwaj, N.; Gupta, R.K.; Kumar, A. Mechanisms of stress tolerance in cyanobacteria under extreme conditions. Stresses 2022, 2, 531–549. [Google Scholar] [CrossRef]
- D’Acqui, L.P. Use of indigenous cyanobacteria for sustainable improvement of biogeochemical and physical fertility of marginal soils in semiarid tropics. In Bioformulations: For Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 213–232. [Google Scholar]
- Rossi, F.; Li, H.; Liu, Y.; De Philippis, R. Cyanobacterial inoculation (cyanobacterisation): Perspectives for the development of a standardized multifunctional technology for soil fertilization and desertification reversal. Earth Sci. Rev. 2017, 171, 28–43. [Google Scholar] [CrossRef]
- Park, C.H.; Li, X.R.; Zhao, Y.; Jia, R.L.; Hur, J.S. Rapid development of cyanobacterial crust in the field for combating desertification. PLoS ONE 2017, 12, e0179903. [Google Scholar] [CrossRef]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef]
- Lan, S.; Zhang, Q.; Wu, L.; Liu, Y.; Zhang, D.; Hu, C. Artificially accelerating the reversal of desertification: Cyanobacterial inoculation facilitates the succession of vegetation communities. Environ. Sci. Technol. 2014, 48, 307–315. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Li, D.; Hu, C.; Rao, B. Feasibility of cyanobacterial inoculation for biological soil crusts formation in desert area. Soil Biol. Biochem. 2009, 41, 926–929. [Google Scholar] [CrossRef]
- Velasco Ayuso, S.; Giraldo Silva, A.; Nelson, C.; Barger, N.N.; Garcia-Pichel, F. Microbial nursery production of high-quality biological soil crust biomass for restoration of degraded dryland soils. Appl. Environ. Microbi. 2017, 83, e02179-16. [Google Scholar] [CrossRef]
- Rodriguez-Caballero, E.; Belnap, J.; Büdel, B.; Crutzen, P.J.; Andreae, M.O.; Pöschl, U.; Weber, B. Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosci. 2018, 11, 185–189. [Google Scholar] [CrossRef]
- Mehda, S.; Muñoz-Martín, M.Á.; Oustani, M.; Hamdi-Aïssa, B.; Perona, E.; Mateo, P. Microenvironmental conditions drive the differential cyanobacterial community composition of biocrusts from the Sahara Desert. Microorganisms 2021, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Ramos, B.; Muñoz-Martín, M.Á.; Chamizo, S.; Fernández-Valbuena, L.; Mendoza, D.; Perona, E.; Cantón, Y.; Mateo, P. Polyphasic evaluation of key cyanobacteria in biocrusts from the most arid region in Europe. PeerJ 2019, 7, e6169. [Google Scholar] [CrossRef] [PubMed]
- Antoninka, A.; Faist, A.; Rodriguez-Caballero, E.; Young, K.E.; Chaudhary, V.B.; Condon, L.A.; Pyke, D.A. Biological soil crusts in ecological restoration: Emerging research and perspectives. Restor. Ecol. 2020, 28, S3–S8. [Google Scholar] [CrossRef]
- Xiao, B.; Hu, K.; Veste, M.; Kidron, G.J. Natural recovery rates of moss biocrusts after severe disturbance in a semiarid climate of the Chinese Loess Plateau. Geoderma 2019, 337, 402–412. [Google Scholar] [CrossRef]
- Belnap, J.; Weber, B.; Büdel, B. Biological Soil Crusts as an Organizing Principle in Drylands; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 3–13. [Google Scholar]
- Deng, S.; Zhang, D.; Wang, G.; Zhou, X.; Ye, C.; Fu, T.; Ke, T.; Zhang, Y.; Liu, Y.; Chen, L. Biological soil crust succession in deserts through a 59-year-long case study in China: How induced biological soil crust strategy accelerates desertification reversal from decades to years. Soil Biol. Biochem. 2020, 141, 107665. [Google Scholar] [CrossRef]
- Miralles, I.; de Guevara, M.L.; Chamizo, S.; Rodríguez-Caballero, E.; Ortega, R.; van Wesemael, B.; Cantón, Y. Soil CO2 exchange controlled by the interaction of biocrust successional stage and environmental variables in two semiarid ecosystems. Soil Biol. Biochem. 2018, 124, 11–23. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Certini, G.; De Philippis, R. Cyanobacteria inoculation as a potential tool for stabilization of burned soils. Restor. Ecol. 2020, 28, S106–S114. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Román, J.R.; Roncero-Ramos, B.; Erickson, T.E.; Merritt, D.J.; Aguila-Carricondo, P.; Cantón, Y. Cyanobacteria inoculation enhances carbon sequestration in soil substrates used in dryland restoration. Sci. Total Environ. 2018, 636, 1149–1154. [Google Scholar] [CrossRef]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Pringault, O. Cyanobacteria track water in desert soils. Nature 2001, 413, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Billi, D.; Potts, M. Life and death of dried prokaryotes. Res. Microbiol. 2002, 153, 7–12. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ashraf, M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: Growth, photosynthesis, water relations and oxidative defence mechanism. J. Agron. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Golldack, D.; Lüking, I.; Yang, O. Plant tolerance to drought and salinity: Stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 2011, 30, 1383–1391. [Google Scholar] [CrossRef]
- Hirai, M.; Yamakawa, R.; Nishio, J.; Yamaji, T.; Kashino, Y.; Koike, H.; Satoh, K. Deactivation of photosynthetic activities is triggered by loss of a small amount of water in a desiccation-tolerant cyanobacterium, Nostoc commune. Plant Cell Physiol. 2004, 45, 872–878. [Google Scholar] [CrossRef][Green Version]
- Yadav, P.; Singh, R.P.; Alodaini, H.A.; Hatamleh, A.; Santoyo, G.; Kumar, A.; Gupta, R.K. Impact of dehydration on the physiochemical properties of Nostoc calcicola BOT1 and its untargeted metabolic profiling through UHPLC-HRMS. Front. Plant Sci. 2023, 14, 1954. [Google Scholar] [CrossRef]
- Chennu, A.; Grinham, A.; Polerecky, L.; De Beer, D.; Al-Najjar, M.A. Rapid reactivation of cyanobacterial photosynthesis and migration upon rehydration of desiccated marine microbial mats. Front. Microbiol. 2015, 6, 1472. [Google Scholar] [CrossRef]
- Rozenstein, O.; Zaady, E.; Katra, I.; Karnieli, A.; Adamowski, J.; Yizhaq, H. The effect of sand grain size on the development of cyanobacterial biocrusts. Aeolian Res. 2014, 15, 217–226. [Google Scholar] [CrossRef]
- Lan, S.; Wu, L.; Yang, H.; Zhang, D.; Hu, C. A new biofilm based microalgal cultivation approach on shifting sand surface for desert cyanobacterium Microcoleus vaginatus. Bioresour. Technol. 2017, 238, 602–608. [Google Scholar] [CrossRef]
- Mugnai, G.; Rossi, F.; Felde, V.J.M.N.L.; Colesie, C.; Büdel, B.; Peth, S.; Kaplan, A.; De Philippis, R. Development of the polysaccharidic matrix in biocrusts induced by a cyanobacterium inoculated in sand microcosms. Biol. Fertil. Soils 2018, 54, 27–40. [Google Scholar] [CrossRef]
- Issa, O.M.; Valentin, C.; Rajot, J.L.; Cerdan, O.; Desprats, J.F.; Bouchet, T. Runoff generation fostered by physical and biological crusts in semi-arid sandy soils. Geoderma 2011, 167, 22–29. [Google Scholar] [CrossRef]
- Chamizo, S.; Cantón, Y.; Lázaro, R.; Solé-Benet, A.; Domingo, F. Crust composition and disturbance drive infiltration through biological soil crusts in semiarid ecosystems. Ecosystems 2012, 15, 148–161. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Y.; Hu, C.; Chen, L.; Li, D. Relationships between the biomass of algal crusts in fields and their compressive strength. Soil Biol. Biochem. 2007, 39, 567–572. [Google Scholar] [CrossRef]
- Li, H.; Rao, B.; Wang, G.; Shen, S.; Li, D.; Hu, C.; Liu, Y. Spatial heterogeneity of cyanobacteria-inoculated sand dunes significantly influences artificial biological soil crusts in the Hopq Desert (China). Environ. Earth Sci. 2014, 71, 245–253. [Google Scholar] [CrossRef]
- Hawes, I.; Howard-Williams, C.; Vincent, W.F. Desiccation and recovery of Antarctic cyanobacterial mats. Polar Biol. 1992, 12, 587–594. [Google Scholar] [CrossRef]
- Gray, D.W.; Lewis, L.A.; Cardon, Z.G. Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives. Plant Cell Environ. 2007, 30, 1240–1255. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Tamaru, Y.; Takani, Y.; Yoshida, T.; Sakamoto, T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl. Environ. Microbiol. 2005, 71, 7327–7333. [Google Scholar] [CrossRef]
- Zaady, E.; Bouskila, A. Lizard burrows association with successional stages of biological soil crusts in an arid sandy region. J. Arid Environ. 2002, 50, 235–246. [Google Scholar] [CrossRef]
- Castle, S.C.; Morrison, C.D.; Barger, N.N. Extraction of chlorophyll a from biological soil crusts: A comparison of solvents for spectrophotometric determination. Soil Biol. Biochem. 2011, 43, 853–856. [Google Scholar] [CrossRef]
- Dubois, M.; Guilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Calorimetric method for the determination of sugars and related substances. Analyt. Chem. 1956, 18, 350–356. [Google Scholar] [CrossRef]
- Rossi, F.; Mugnai, G.; De Philippis, R. Complex role of the polymeric matrix in biological soil crusts. Plant Soil 2018, 429, 19–34. [Google Scholar] [CrossRef]
- Ogawa, T.; Misumi, M.; Sonoike, K. Estimation of photosynthesis in cyanobacteria by pulse-amplitude modulation chlorophyll fluorescence: Problems and solutions. Photosynth. Res. 2017, 133, 63–673. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, A.C.; Charles, S.Y. Plankton pigment nomographs. J. Mar. Res. 1956, 15, 92–101. [Google Scholar]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Ordonez, A.A.L.; Gomez, J.D.; Vattuone, M.A. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Aboal, M.; Werner, O.; García-Fernández, M.E.; Palazón, J.A.; Cristóbal, J.C.; Williams, W. Should ecomorphs be conserved? The case of Nostoc flagelliforme, an endangered extremophile cyanobacteria. J. Nat. Conserv. 2016, 30, 52–64. [Google Scholar] [CrossRef][Green Version]
- Dojani, S.; Kauff, F.; Weber, B.; Büdel, B. Genotypic and phenotypic diversity of cyanobacteria in biological soil crusts of the Succulent Karoo and Nama Karoo of southern Africa. Microb. Ecol. 2014, 67, 286–301. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria inoculation improves soil stability and fertility on different textured soils: Gaining insights for applicability in soil restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Pringault, O.; Garcia-Pichel, F. Hydrotaxis of cyanobacteria in desert crusts. Microb. Ecol. 2004, 47, 366–373. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Adessi, A.; de Carvalho, R.C.; De Philippis, R.; Branquinho, C.; da Silva, J.M. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol. Biochem. 2018, 116, 67–69. [Google Scholar] [CrossRef]
- Chamizo, S.; Cantón, Y.; Rodríguez-Caballero, E.; Domingo, F. Biocrusts positively affect the soil water balance in semiarid ecosystems. Ecohydrology 2016, 9, 1208–1221. [Google Scholar] [CrossRef]
- Wei, M.L.; Zhang, Y.M. Microscopic and submicroscopic structure of leaf cells of Syntrichia caninervis Mitt. in biological soil crusts. J. Desert Res. 2009, 5, 493–498. [Google Scholar]
- Yadav, P.; Singh, R.P.; Gupta, R.K. Role of cyanobacteria in germination and growth of paddy seedlings. Int. J. Phytol. Res. 2022, 2, 11–18. [Google Scholar]
- Boopathi, T.; Balamurugan, V.; Gopinath, S.; Sundararaman, M. Characterization of IAA production by the mangrove cyanobacterium Phormidium sp. MI405019 and its influence on tobacco seed germination and organogenesis. J. Plant Growth Regul. 2013, 32, 758–766. [Google Scholar] [CrossRef]
- Péret, B.; De Rybel, B.; Casimiro, I.; Benková, E.; Swarup, R.; Laplaze, L.; Beeckman, T.; Bennett, M.J. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009, 14, 399–408. [Google Scholar] [CrossRef]
- Katoh, H.; Jun, F.; Kaori, T.Y.; Yasuaki, N. Isolation and purification of an axenic diazotrophic drought-tolerant cyanobacterium, Nostoc commune, from natural cyanobacterial crusts and its utilization for field research on soils polluted with radioisotopes. BBA-Bioenerg. 2012, 1817, 1499–1505. [Google Scholar] [CrossRef][Green Version]
- Joshi, H.; Shourie, A.; Singh, A. Cyanobacteria as a source of biofertilizers for sustainable agriculture. In Advances in Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 385–396. [Google Scholar]
- El Sheek, M.M.; Zayed, M.A.; Elmossel, F.K. Effect of cyanobacteria isolates on rice seeds germination in saline soil. Baghdad Sci. J. 2018, 15, 0016. [Google Scholar] [CrossRef]
- Chittapun, S.; Limbipichai, S.; Amnuaysin, N.; Boonkerd, R.; Charoensook, M. Effects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I: A pot experiment. J. Appl. Phycol. 2018, 30, 79–85. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Stella, A.M.; Storni, M.M.; Zulpa, G.; Zaccaro, M.C. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Nisha, R.; Kiran, B.; Kaushik, A.; Kaushik, C.P. Bioremediation of salt affected soils using cyanobacteria in terms of physical structure, nutrient status and microbial activity. Int. J. Environ. Sci. Technol. 2018, 15, 571–580. [Google Scholar] [CrossRef]
- Potts, M. Mechanisms of desiccation tolerance in cyanobacteria. Eur. J. Phycol. 1999, 34, 319–328. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Aqil, F.; Khan, M.S.; Hayat, S. Diversity and potential of nonsymbiotic diazotrophic bacteria in promoting plant growth. In Plant-Bacteria Interactions: Strategies and Techniques to Promote Plant Growth; Wiley-VCH: Weinheim, Germany, 2008; pp. 81–109. [Google Scholar]
- Nelson, L.M. Plant growth promoting rhizobacteria (PGPR): Prospects for new inoculants. Crop Manag. 2004, 3, 1–7. [Google Scholar] [CrossRef]
- Narasimhan, K.; Basheer, C.; Bajic, V.B.; Swarup, S. Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol. 2003, 132, 146–153. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef]
- Fernández Valiente, E.A.; Ucha, A.; Quesada, F.; Leganés, R.C. Contribution of N2 fixing cyanobacteria to rice production: Availability of nitrogen from 15N-labelled cyanobacteria and ammonium sulphate to rice. Plant Soil 2000, 221, 107–112. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Darki, B.Z.; Homaee, M. Controlling rainfall-induced soil loss from small experimental plots through inoculation of bacteria and cyanobacteria. Catena 2017, 152, 40–46. [Google Scholar] [CrossRef]
- Fattahi, S.M.; Soroush, A.; Huang, N.; Zhang, J.; Abbasi, S.J.; Yu, Y. Laboratory study on biophysicochemical improvement of desert sand. Catena 2020, 190, 104531. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. Metabolites Facilitating Adaptation of Desert Cyanobacteria to Extremely Arid Environments. Plants 2022, 11, 3225. [Google Scholar] [CrossRef] [PubMed]
- Román, J.R.; Chilton, A.M.; Cantón, Y.; Muñoz-Rojas, M. Assessing the viability of cyanobacteria pellets for application in arid land restoration. J. Environ. Manage. 2020, 270, 110795. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichel, F.; Wojciechowski, M.F. The evolution of a capacity to build supra-cellular ropes enabled filamentous cyanobacteria to colonize highly erodible substrates. PLoS ONE 2009, 4, e7801. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Pandey, K.D.; Mesapogu, S.; Singh, D.V. Influence of NaCl on photosynthesis and nitrogen metabolism of cyanobacterium Nostoc calcicola. Appl. Biochem. Microbiol 2015, 51, 720–725. [Google Scholar] [CrossRef]
- Thapar, R.; Srivastava, A.K.; Bhargava, P.; Mishra, Y.; Rai, L.C. Impact of different abiotic stresses on growth, photosynthetic electron transport chain, nutrient uptake and enzyme activities of Cu-acclimated Anabaena doliolum. J. Plant Physiol. 2008, 165, 306–316. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, P.; Singh, R.P.; Hashem, A.; Abd_Allah, E.F.; Santoyo, G.; Kumar, A.; Gupta, R.K. Enhancing Biocrust Development and Plant Growth through Inoculation of Desiccation-Tolerant Cyanobacteria in Different Textured Soils. Microorganisms 2023, 11, 2507. https://doi.org/10.3390/microorganisms11102507
Yadav P, Singh RP, Hashem A, Abd_Allah EF, Santoyo G, Kumar A, Gupta RK. Enhancing Biocrust Development and Plant Growth through Inoculation of Desiccation-Tolerant Cyanobacteria in Different Textured Soils. Microorganisms. 2023; 11(10):2507. https://doi.org/10.3390/microorganisms11102507
Chicago/Turabian StyleYadav, Priya, Rahul Prasad Singh, Abeer Hashem, Elsayed Fathi Abd_Allah, Gustavo Santoyo, Ajay Kumar, and Rajan Kumar Gupta. 2023. "Enhancing Biocrust Development and Plant Growth through Inoculation of Desiccation-Tolerant Cyanobacteria in Different Textured Soils" Microorganisms 11, no. 10: 2507. https://doi.org/10.3390/microorganisms11102507
APA StyleYadav, P., Singh, R. P., Hashem, A., Abd_Allah, E. F., Santoyo, G., Kumar, A., & Gupta, R. K. (2023). Enhancing Biocrust Development and Plant Growth through Inoculation of Desiccation-Tolerant Cyanobacteria in Different Textured Soils. Microorganisms, 11(10), 2507. https://doi.org/10.3390/microorganisms11102507









