Ticks and Chlamydia-Related Bacteria in Swiss Zoological Gardens Compared to in Contiguous and Distant Control Areas
Abstract
:1. Introduction
2. Materials and Methods
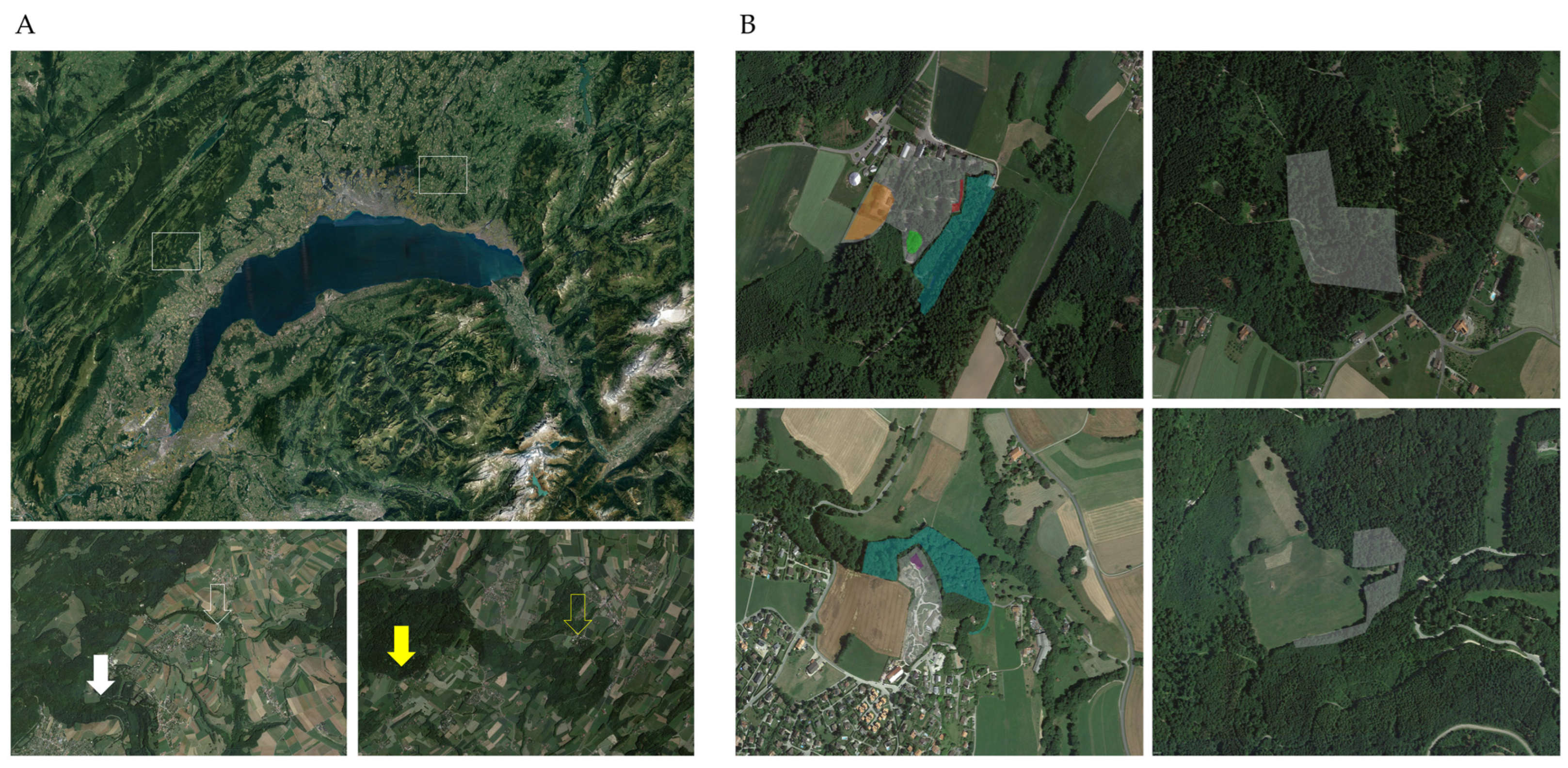
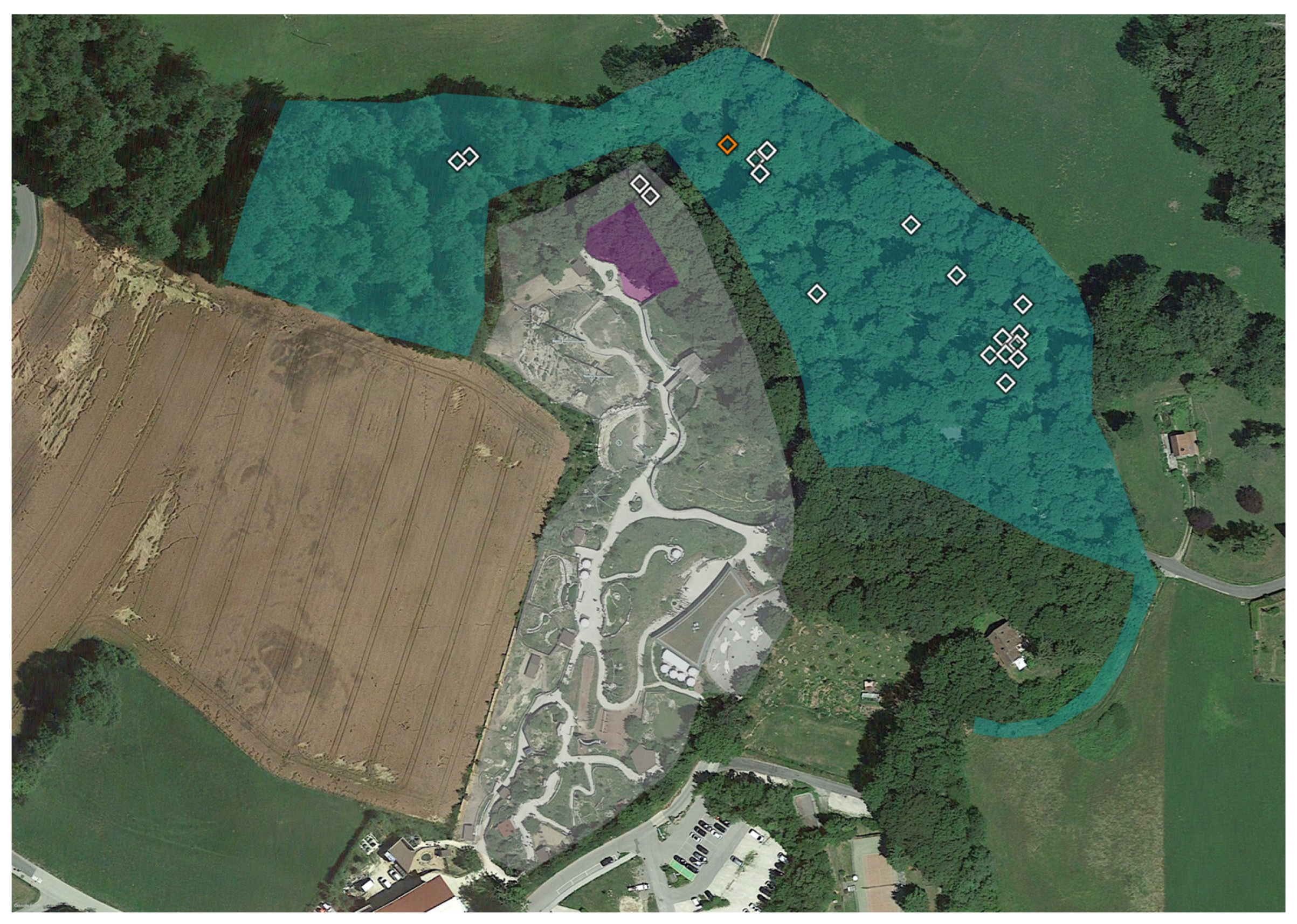
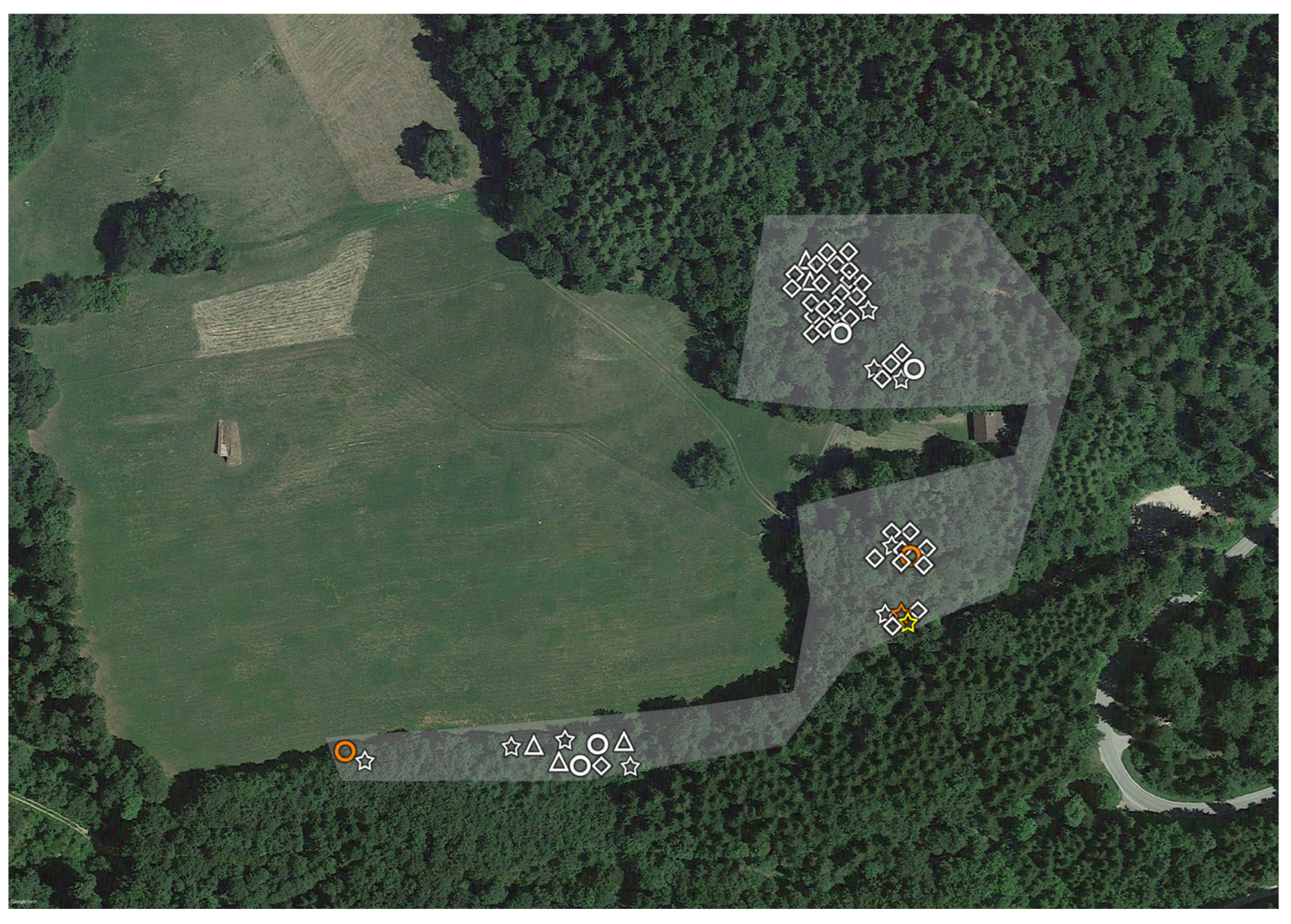
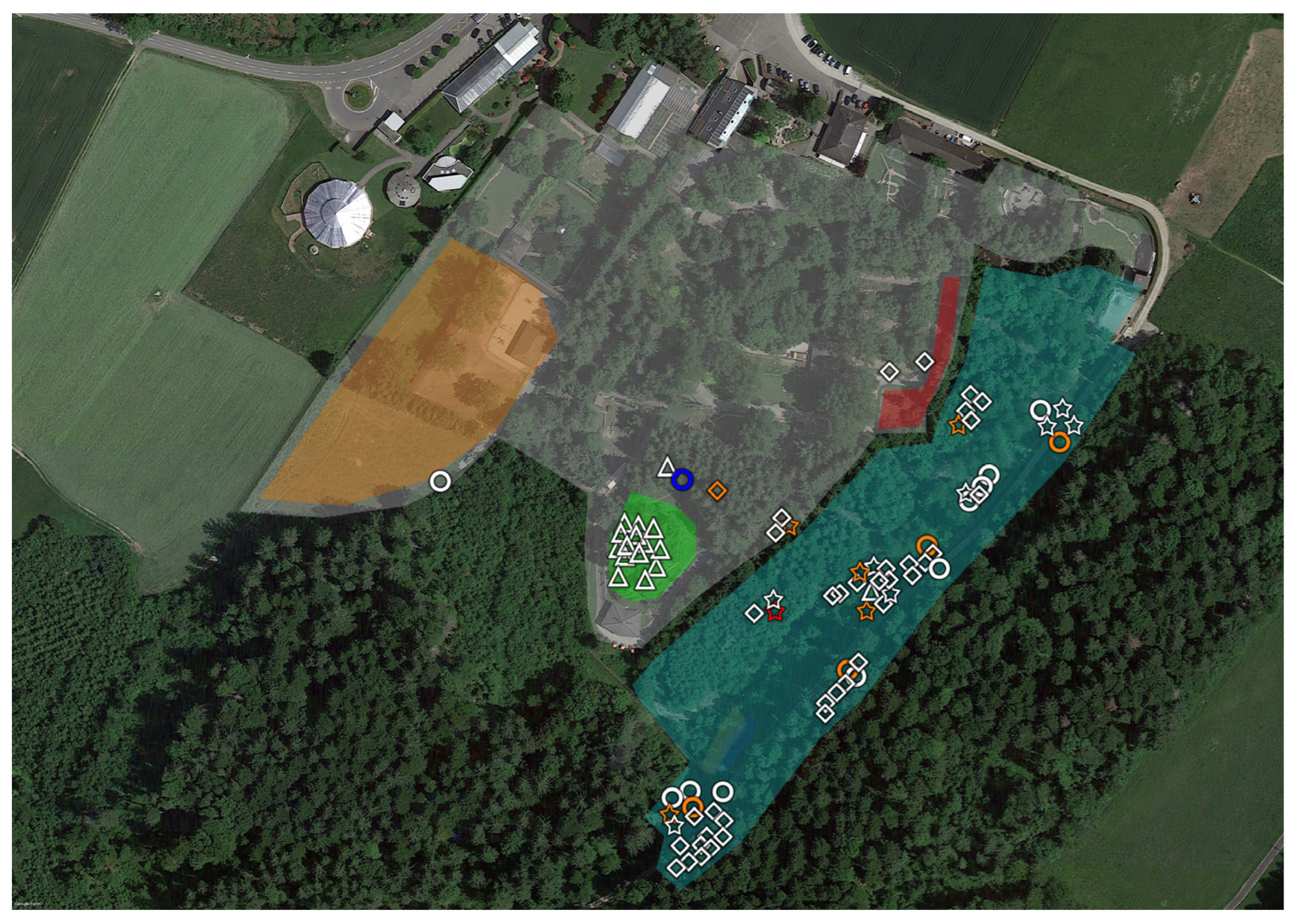
2.1. Tick Sampling
2.2. Ticks Lysis, DNA Extraction and Pan-Chlamydiae TaqMan Quantitative PCR (pC-qPCR)
2.3. Geopositioning and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mermod, C.; Aeschlimann, A.; Graf, J.F. Ecology and ethology of Ixodes ricinus Linné 1758, in Switzerland (Acarina, Ixodoidea). 1st report: Numerical fluctuations. Acarologia 1973, 15, 197–205. [Google Scholar] [PubMed]
- Estrada-Peña, A. Geostatistics as predictive tools to estimate Ixodes ricinus (Acari: Ixodidae) habitat suitability in the Western Palearctic from AVHRR satellite imagery. Exp. Appl. Acarol. 1999, 23, 337–349. [Google Scholar] [CrossRef]
- Porretta, D.; Mastrantonio, V.; Amendolia, S.; Gaiarsa, S.; Epis, S.; Genchi, C.; Bandi, C.; Otranto, D.; Urbanelli, S. Effects of global changes on the climatic niche of the tick Ixodes ricinus inferred by species distribution modelling. Parasit. Vectors 2013, 6, 271. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalská, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef] [PubMed]
- Rochat, E.; Vuilleumier, S.; Aeby, S.; Greub, G.; Joost, S. Nested species distribution models of Chlamydiales in Ixodes ricinus (tick) hosts in Switzerland. Appl. Environ. Microbiol. 2020, 87, e01237-20. [Google Scholar] [CrossRef]
- de la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci.-Landmark 2008, 13, 6938–6946. [Google Scholar] [CrossRef]
- Randolph, S.E. Tick-Borne Disease Systems Emerge from the Shadows: The beauty lies in molecular detail, the message in epidemiology. Parasitology 2009, 136, 1403–1413. [Google Scholar] [CrossRef]
- Hai, V.V.; Almeras, L.; Socolovschi, C.; Raoult, D.; Parola, P.; Pagès, F. Monitoring human tick-borne disease risk and tick bite exposure in Europe: Available tools and promising future methods. Ticks Tick-Borne Dis. 2014, 5, 607–619. [Google Scholar] [CrossRef]
- Pilloux, L.; Aeby, S.; Gaümann, R.; Burri, C.; Beuret, C.; Greub, G. The high prevalence and diversity of Chlamydiales DNA within Ixodes ricinus ticks suggest a role for ticks as reservoirs and vectors of Chlamydia-related bacteria. Appl. Environ. Microbiol. 2015, 81, 8177–8182. [Google Scholar] [CrossRef]
- Fritsche, T.R.; Gautom, R.K.; Seyedirashti, S.; Bergeron, D.L.; Lindquist, T.D. Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lenses. J. Clin. Microbiol. 1993, 31, 1122–1126. [Google Scholar] [CrossRef]
- Thomas, V.; Casson, N.; Greub, G. Criblamydia sequanensis, a new intracellular Chlamydiales isolated from Seine river water using amoebal co-culture. Environ. Microbiol. 2006, 8, 2125–2135. [Google Scholar] [CrossRef]
- Wheelhouse, N.; Hearn, J.; Livingstone, M.; Flockhart, A.; Dagleish, M.; Longbottom, D. Identification of Parachlamydiaceae DNA in nasal and rectal passages of healthy dairy cattle. J. Appl. Microbiol. 2022, 132, 2642–2648. [Google Scholar] [CrossRef]
- Stokes, H.S.; Berg, M.L.; Bennett, A.T.D. A Review of chlamydial infections in wild birds. Pathog. Basel Switz. 2021, 10, 948. [Google Scholar] [CrossRef]
- Thao, M.L.; Baumann, L.; Hess, J.M.; Falk, B.W.; Ng, J.C.K.; Gullan, P.J.; Baumann, P. Phylogenetic evidence for two new insect-associated Chlamydia of the family Simkaniaceae. Curr. Microbiol. 2003, 47, 46–50. [Google Scholar] [CrossRef]
- Moulder, J.W. Interaction of Chlamydiae and host cells in vitro. Microbiol. Rev. 1991, 55, 143–190. [Google Scholar] [CrossRef]
- Everett, K.D.E.; Bush, R.M.; Andersen, A.A. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Evol. Microbiol. 1999, 49, 415–440. [Google Scholar] [CrossRef]
- Greub, G. International committee on systematics of prokaryotes subcommittee on the taxonomy of the Chlamydiae. Int. J. Syst. Evol. Microbiol. 2010, 60, 2691–2693. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Greub, G. Pathogenic potential of novel Chlamydiae and diagnostic approaches to infections due to these obligate intracellular bacteria. Clin. Microbiol. Rev. 2006, 19, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Abe, T.; Nijhof, A.M.; Yamamoto, S.; Jongejan, F.; Ikemura, T.; Sugimoto, C. A novel approach, based on BLSOMs (Batch Learning Self-Organizing Maps), to the microbiome analysis of ticks. ISME J. 2013, 7, 1003–1015. [Google Scholar] [CrossRef]
- Hokynar, K.; Sormunen, J.J.; Vesterinen, E.J.; Partio, E.K.; Lilley, T.; Timonen, V.; Panelius, J.; Ranki, A.; Puolakkainen, M. Chlamydia-like organisms (CLOs) in finnish Ixodes ricinus ticks and human skin. Microorganisms 2016, 4, 28. [Google Scholar] [CrossRef]
- Chisu, V.; Foxi, C.; Tanda, A.; Masala, G. Molecular evidence of Chlamydiales in ticks from wild and domestic hosts in Sardinia, Italy. Parasitol. Res. 2018, 117, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Burnard, D.; Weaver, H.; Gillett, A.; Loader, J.; Flanagan, C.; Polkinghorne, A. Novel Chlamydiales genotypes identified in ticks from australian wildlife. Parasit. Vectors 2017, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Croxatto, A.; Rieille, N.; Kernif, T.; Bitam, I.; Aeby, S.; Péter, O.; Greub, G. Presence of Chlamydiales DNA in ticks and fleas suggests that ticks are carriers of Chlamydiae. Ticks Tick-Borne Dis. 2014, 5, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hrnková, J.; Schneiderová, I.; Golovchenko, M.; Grubhoffer, L.; Rudenko, N.; Černý, J. Role of zoo-housed animals in the ecology of ticks and tick-borne pathogens-A review. Pathog. Basel Switz. 2021, 10, 210. [Google Scholar] [CrossRef]
- Kazimírová, M.; Hamšíková, Z.; Kocianová, E.; Marini, G.; Mojšová, M.; Mahríková, L.; Berthová, L.; Slovák, M.; Rosá, R. Relative density of host-seeking ticks in different habitat types of south-western Slovakia. Exp. Appl. Acarol. 2016, 69, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Froeschke, G.; van der Mescht, L.; McGeoch, M.; Matthee, S. Life history strategy influences parasite responses to habitat fragmentation. Int. J. Parasitol. 2013, 43, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Nelder, M.P.; Reeves, W.K.; Adler, P.H.; Wozniak, A.; Wills, W. Ectoparasites and associated pathogens of free-roaming and captive animals in zoos of South Carolina. Vector Borne Zoonotic Dis. Larchmt. N. 2009, 9, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; VanAcker, M.C.; Fernandez, M.P. Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J. Med. Entomol. 2021, 58, 1546–1564. [Google Scholar] [CrossRef]
- Stoebel, K.; Schoenberg, A.; Streich, W.J. The seroepidemiology of Lyme borreliosis in zoo animals in Germany. Epidemiol. Infect. 2003, 131, 975–983. [Google Scholar] [CrossRef]
- Širmarová, J.; Tichá, L.; Golovchenko, M.; Salát, J.; Grubhoffer, L.; Rudenko, N.; Nowotny, N.; Růžek, D. Seroprevalence of Borrelia burgdorferi sensu lato and tick-borne encephalitis virus in zoo animal species in the Czech Republic. Ticks Tick-Borne Dis. 2014, 5, 523–527. [Google Scholar] [CrossRef]
- Ticha, L.; Golovchenko, M.; Oliver, J.H.; Grubhoffer, L.; Rudenko, N. Sensitivity of Lyme borreliosis spirochetes to serum complement of regular zoo animals: Potential reservoir competence of some exotic vertebrates. Vector Borne Zoonotic Dis. Larchmt. N. 2016, 16, 13–19. [Google Scholar] [CrossRef] [PubMed]
- DiVincenti, L.; Garner, M.; Thomas, B.; Birkenheuer, A. Babesia sp. infection in a zoo-housed polar bear (Ursus maritimus). Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100350. [Google Scholar] [CrossRef] [PubMed]
- Milnes, E.L.; Thornton, G.L.; Delnatte, P.; Léveillé, A.N.; Barta, J.R.; Smith, D.A.; Nemeth, N.M. Molecular detection of Babesia odocoilei in wild, farmed, and zoo cervids in Ontario, Canada. J. Wildl. Dis. 2019, 55, 335–342. [Google Scholar] [CrossRef]
- Fritschi, J.S.; Marti, H.; Seth-Smith, H.; Aeby, S.; Greub, G.; Meli, M.; Hofmann-Lehmann, R.; Mühldorfer, K.; Stokar-Regenscheit, N.; Wiederkehr, D.; et al. Prevalence and phylogeny of Chlamydiae and hemotropic Mycoplasma species in captive and free-living bats. BMC Microbiol. 2020, 20, 182. [Google Scholar] [CrossRef]
- Schautteet, K.; Vanrompay, D. Chlamydiaceae infections in pig. Vet. Res. 2011, 42, 29. [Google Scholar] [CrossRef]
- Wu, S.-M.; Huang, S.-Y.; Xu, M.-J.; Zhou, D.-H.; Song, H.-Q.; Zhu, X.-Q. Chlamydia felis exposure in companion dogs and cats in Lanzhou, China: A Public Health Concern. BMC Vet. Res. 2013, 9, 104. [Google Scholar] [CrossRef]
- Gonzalez, I.; Labruna, M.; Chagas, C.; Salgado, P.; Monticelli, C.; Morais, L.; Moraes, A.; Antunes, T.; Ramos, P.; Martins, T. Ticks infesting captive and free-roaming wild animal species at the São Paulo zoo, São Paulo, Brazil. Rev. Bras. Parasitol. Veterinária 2017, 26, 496–499. [Google Scholar] [CrossRef]
- Gäumann, R.; Mühlemann, K.; Strasser, M.; Beuret, C.M. High-throughput procedure for tick surveys of tick-borne encephalitis virus and its application in a national surveillance study in Switzerland. Appl. Environ. Microbiol. 2010, 76, 4241–4249. [Google Scholar] [CrossRef]
- Ginsberg, H.S.; Ewing, C.P. Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone-star ticks, Amblyomma americanum (Acari: Ixodidae). Exp. Appl. Acarol. 1989, 7, 313–322. [Google Scholar] [CrossRef]
- Gupta, S.K.; Schönberg, A.; Hiepe, T. Prevalence of ticks in relation to their role as vector of Borrelia burgdorferi under autochthone conditions. Appl. Parasitol. 1995, 36, 97–106. [Google Scholar]
- Lienard, J.; Croxatto, A.; Aeby, S.; Jaton, K.; Posfay-Barbe, K.; Gervaix, A.; Greub, G. Development of a new Chlamydiales-specific real-time PCR and its application to respiratory clinical samples. J. Clin. Microbiol. 2011, 49, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Greub, G. Parachlamydia acanthamoebae, an emerging agent of pneumonia. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2009, 15, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Candidatus Rhabdochlamydia Porcellionis Genome Assembly ASM1535681v2. Available online: https://www.ncbi.nlm.nih.gov/data-hub/assembly/GCF_015356815.2/ (accessed on 18 July 2023).
- Williams, C.V.; Steenhouse, J.L.V.; Bradley, J.M.; Hancock, S.I.; Hegarty, B.C.; Breitschwerdt, E.B. Naturally occurring Ehrlichia chaffeensis infection in two prosimian primate species: Ring-tailed lemurs (Lemur catta) and ruffed lemurs (Varecia variegata). Emerg. Infect. Dis. J.—CDC 2002, 8, 12. [Google Scholar] [CrossRef]
- Do Nascimento, K.K.G.; Veríssimo, S.M.M.; Raia, V.d.A.; Guimarães, R.C.S.; Seade, G.C.C.; Azevedo, A.C.P.; Matos, S.P.; de Oliveira, J.M.; Bezerra, I.A.; Martins, T.F. Tick fauna of wild animals received and attended at the Santarém zoological park, Western Pará State, Brazil. Ciênc. Rural. 2017, 47, e20170159. [Google Scholar] [CrossRef]
- Burridge, M.J.; Simmons, L.-A.; Condie, T. Control of an exotic tick (Aponomma komodoense) infestation in a Komodo dragon (Varanus komodoensis) exhibit at a zoo in Florida. J. Zoo. Wildl. Med. Off. Publ. Am. Assoc. Zoo. Vet. 2004, 35, 248–249. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Hung, R.W. Biases associated with several sampling methods used to estimate abundance of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 1997, 34, 615–623. [Google Scholar] [CrossRef]
- Smith, F.D.; Wall, L.E.R. Prevalence of Babesia and Anaplasma in ticks infesting dogs in Great Britain. Vet. Parasitol. 2013, 198, 18–23. [Google Scholar] [CrossRef]
- Wicki, R.; Sauter, P.; Mettler, C.; Natsch, A.; Enzler, T.; Pusterla, N.; Kuhnert, P.; Egli, G.; Bernasconi, M.; Lienhard, R.; et al. Swiss army survey in Switzerland to determine the prevalence of Francisella tularensis, members of the Ehrlichia phagocytophila genogroup, Borrelia burgdorferi Sensu Lato, and tick-borne encephalitis virus in ticks. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2000, 19, 427–432. [Google Scholar] [CrossRef]
- Reye, A.L.; Hübschen, J.M.; Sausy, A.; Muller, C.P. Prevalence and seasonality of tick-borne pathogens in questing Ixodes ricinus ticks from Luxembourg. Appl. Environ. Microbiol. 2010, 76, 2923–2931. [Google Scholar] [CrossRef]
- Corsaro, D.; Thomas, V.; Goy, G.; Venditti, D.; Radek, R.; Greub, G. ‘Candidatus Rhabdochlamydia crassificans’, an intracellular bacterial pathogen of the cockroach Blatta orientalis (Insecta: Blattodea). Syst. Appl. Microbiol. 2007, 30, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lienard, J.; Croxatto, A.; Gervaix, A.; Lévi, Y.; Loret, J.-F.; Posfay-Barbe, K.M.; Greub, G. Prevalence and diversity of Chlamydiales and other amoeba-resisting bacteria in domestic drinking water systems. New Microbes New Infect. 2017, 15, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Greub, G.; Raoult, D. Microorganisms resistant to free-living Amoebae. Clin. Microbiol. Rev. 2004, 17, 413–433. [Google Scholar] [CrossRef]
- Kabeya, H.; Sato, S.; Maruyama, S. Prevalence and characterization of Chlamydia DNA in zoo animals in Japan. Microbiol. Immunol. 2015, 59, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Pilloux, L.; Baumgartner, A.; Jaton, K.; Lienhard, R.; Ackermann-Gäumann, R.; Beuret, C.; Greub, G. Prevalence of Anaplasma phagocytophilum and Coxiella burnetii in Ixodes ricinus ticks in Switzerland: An underestimated epidemiologic risk. New Microbes New Infect. 2019, 27, 22–26. [Google Scholar] [CrossRef]
- Jaton, K.; Peter, O.; Raoult, D.; Tissot, J.-D.; Greub, G. Development of a high throughput PCR to detect Coxiella burnetii and its application in a diagnostic laboratory over a 7-year period. New Microbes New Infect. 2013, 1, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Greub, G.; Sahli, R.; Brouillet, R.; Jaton, K. Ten years of R&D and full automation in molecular diagnosis. Future Microbiol. 2016, 11, 403–425. [Google Scholar] [CrossRef]
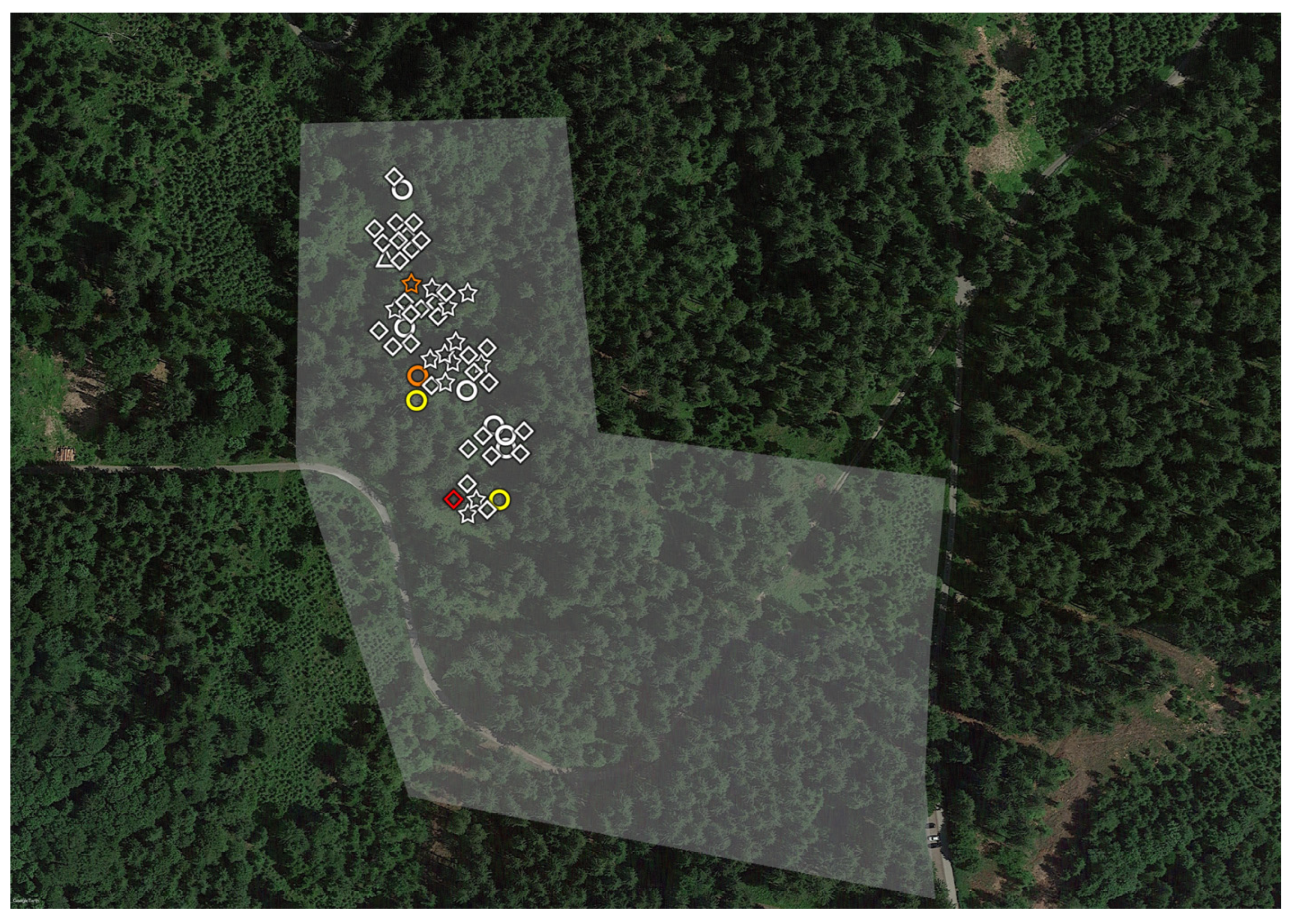
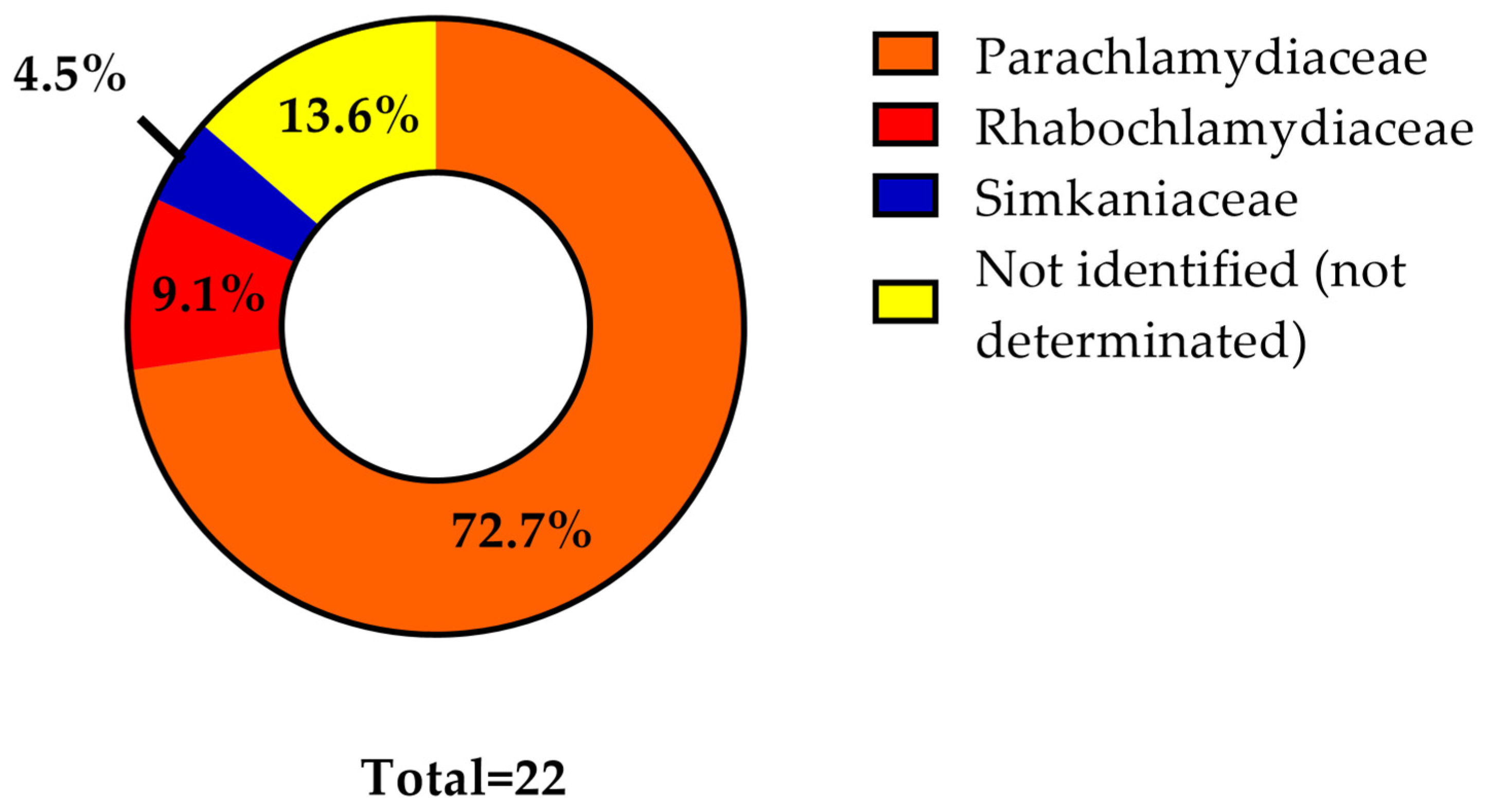
| Place | Ticks Servion | Ticks Garenne | Ticks Garenne and Servion |
|---|---|---|---|
| Zoo | 8 (23) | 2 (2) | 10 (25) |
| -Enclosure | 0 (14) | 0 (0) | 0 (14) |
| -Surrounding | 3 (3) | 2 (2) | 5 (5) |
| -Outside | 5 (6) | 0 (0) | 5 (6) |
| Contiguous area | 59 (60) | 17 (17) | 76 (77) |
| Control area | 54 (55) | 50 (55) | 104 (110) |
| Place | Chlamydia Prevalence in Ticks from Servion (%) 1 | Chlamydia Prevalence in Ticks from Garenne (%) 1 | Chlamydia Prevalence in Ticks from Garenne and Servion (%) 1 |
|---|---|---|---|
| Zoo | 37.5 (3/8) | 0 (0/2) | 30 (3/10) |
| -Enclosure | 0 (0/0) | 0 (0/0) | 0 (0/0) |
| -Surrounding | 0 (0/3) | 0 (0/2) | 0 (0/5) |
| -Outside | 60 (3/5) | 0 (0/0) | 6 (3/5) |
| Contiguous area | 15.25 (9/59) | 5.88 (1/17) | 13.16 (10/76) |
| Control area | 9.26 (5/54) | 8 (4/50) | 8.65 (9/104) |
| Tick Developmental Stage | Number of Ticks | Enclosure | Surrounding and Outside | Contiguous Area | Control Area | Chlamydiae Prevalencein Ticks (%) 1 |
|---|---|---|---|---|---|---|
| Larvae | 22 | 14 | 1 | 1 | 6 | 0 (0/22) |
| Nymphs | 122 | 0 | 7 | 50 | 65 | 2.46 (3/122) |
| Female adults | 30 | 0 | 2 | 13 | 15 | 30 (10/30) |
| Male adults | 38 | 0 | 1 | 13 | 24 | 23.68 (9/38) |
| Total | 212 | 14 | 11 | 77 | 110 | 10.38 (22/212) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanat, V.; Aeby, S.; Greub, G. Ticks and Chlamydia-Related Bacteria in Swiss Zoological Gardens Compared to in Contiguous and Distant Control Areas. Microorganisms 2023, 11, 2468. https://doi.org/10.3390/microorganisms11102468
Vanat V, Aeby S, Greub G. Ticks and Chlamydia-Related Bacteria in Swiss Zoological Gardens Compared to in Contiguous and Distant Control Areas. Microorganisms. 2023; 11(10):2468. https://doi.org/10.3390/microorganisms11102468
Chicago/Turabian StyleVanat, Vincent, Sébastien Aeby, and Gilbert Greub. 2023. "Ticks and Chlamydia-Related Bacteria in Swiss Zoological Gardens Compared to in Contiguous and Distant Control Areas" Microorganisms 11, no. 10: 2468. https://doi.org/10.3390/microorganisms11102468
APA StyleVanat, V., Aeby, S., & Greub, G. (2023). Ticks and Chlamydia-Related Bacteria in Swiss Zoological Gardens Compared to in Contiguous and Distant Control Areas. Microorganisms, 11(10), 2468. https://doi.org/10.3390/microorganisms11102468






