Novel Probiotic Bacterium Rouxiella badensis subsp. acadiensis (Canan SV-53) Modulates Gut Immunity through Epigenetic Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Probiotic and Prebiotic Solution Preparation
2.3. Study Design
2.3.1. Probiotic-Prebiotic Experiment
2.3.2. Heat-Inactivated Probiotic Experiment
2.4. Histological Sections Preparation
2.5. Identification of IgA, IgG, IL-17A, IL-6, IL-23, and IL-10 Producing Cell Populations by Immunofluorescence
2.6. Determination of IgA, IgG, IL-17A, IL-6, IL-23, and IL-10 Concentrations by ELISA
2.7. Determination of miRNA Expression by Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
2.8. Gut Microbiome Analysis
2.9. Methylome-Wide Profiling and Data Analysis
2.10. Statistical Analysis
3. Results
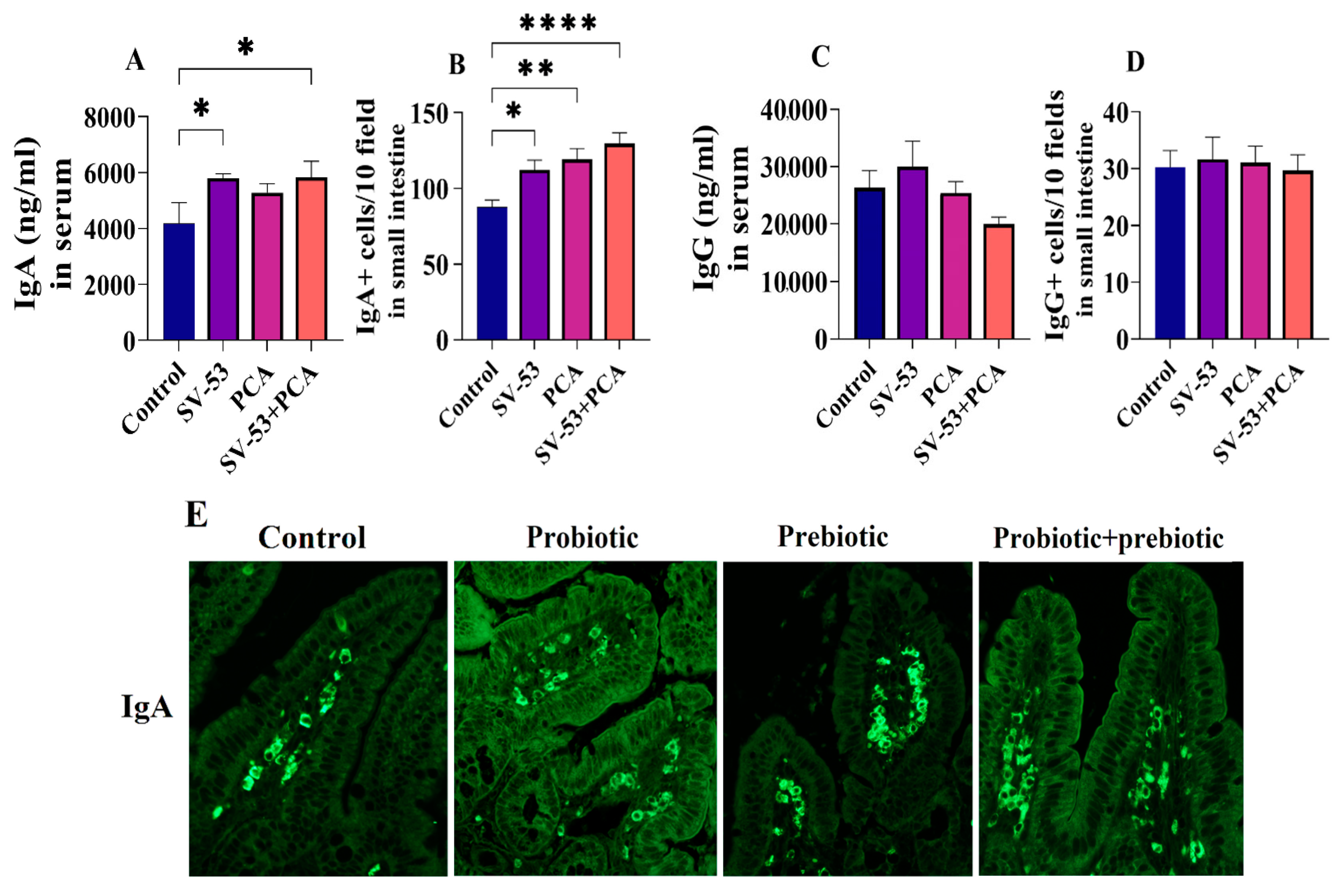
3.1. Effect of Probiotic and Prebiotic Intake on Mucosal Immunity
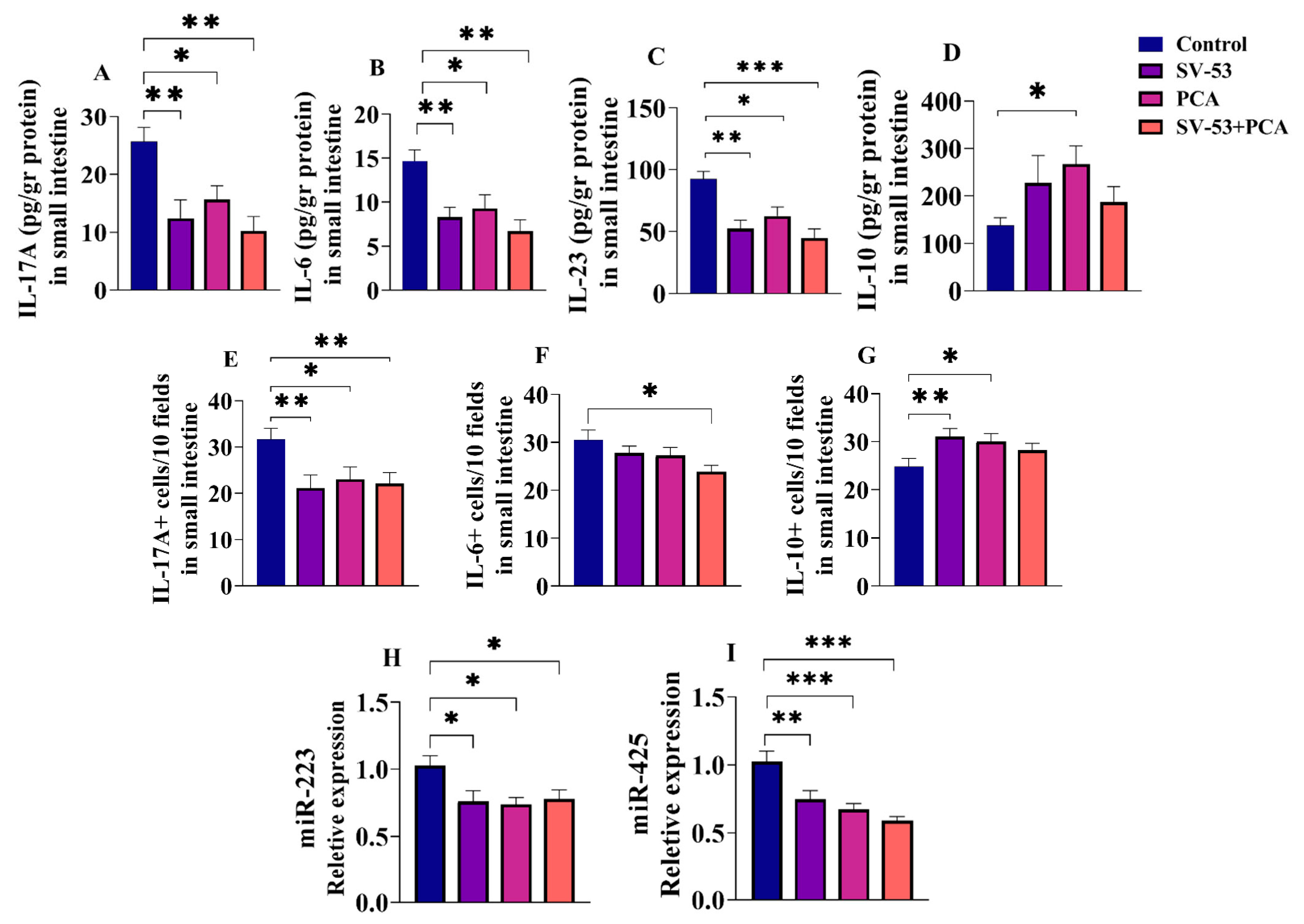
3.2. Effect of Probiotic and Prebiotic Intake on the Expression of Selected Cytokines and miRNAs
3.3. Effect of the Probiotic and Prebiotic Intake on the Gut Microbiome
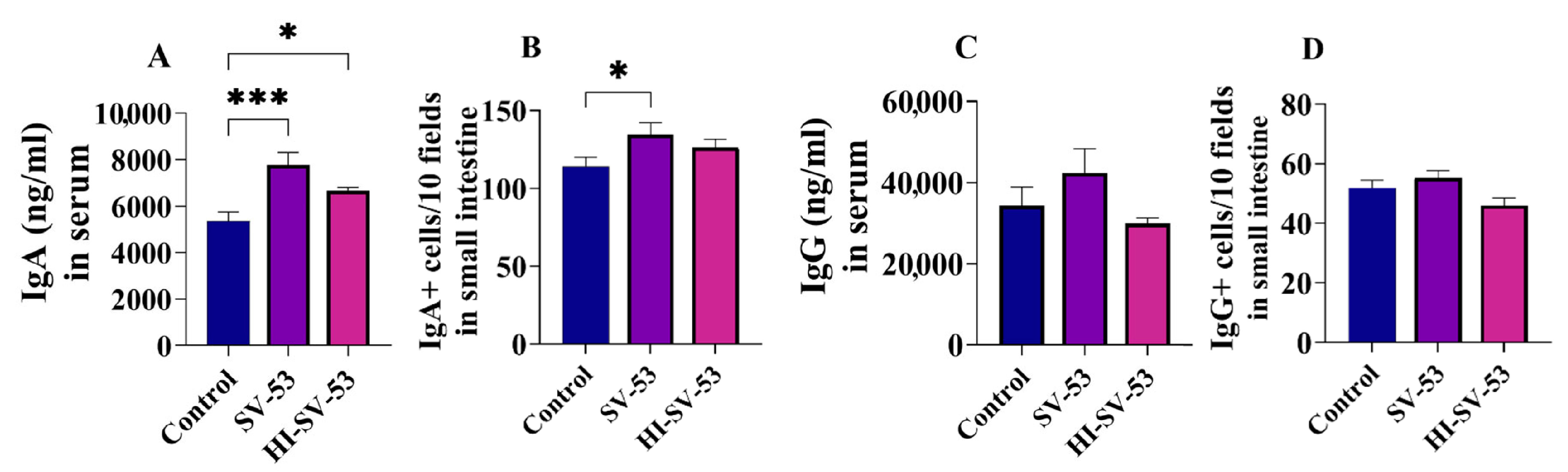
3.4. Effect of Heat-Inactivated SV-53 Intake on Mucosal Immunity
3.5. Effect of Heat-Inactivated SV-53 Intake on the Expression of Selected Cytokines and miRNAs
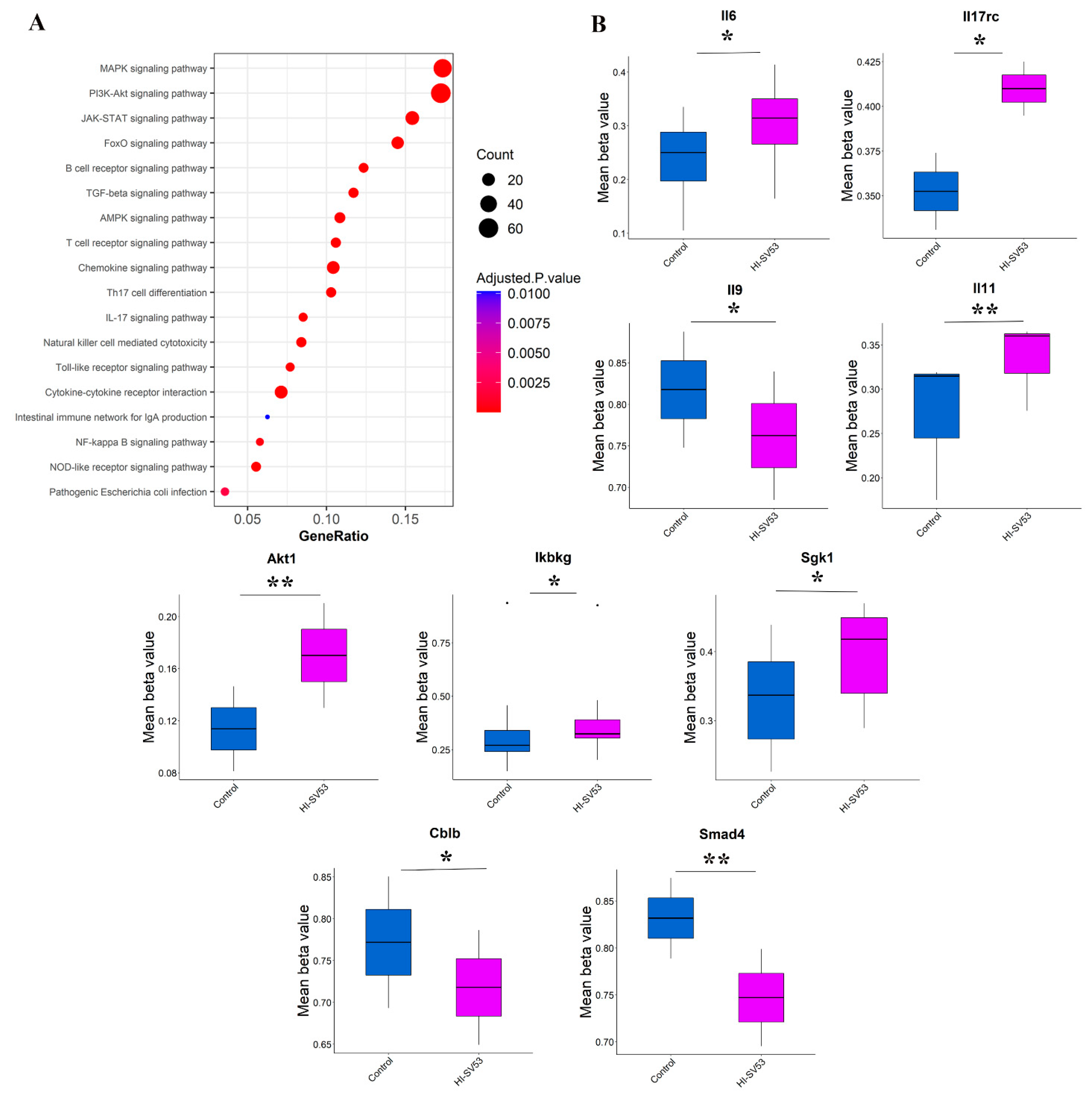
3.6. Effect of Heat-Inactivated SV-53 on DNA Methylation Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahbazi, R.; Yasavoli-Sharahi, H.; Alsadi, N.; Ismail, N.; Matar, C. Probiotics in Treatment of Viral Respiratory Infections and Neuroinflammatory Disorders. Molecules 2020, 25, 4891. [Google Scholar] [CrossRef] [PubMed]
- Santaolalla, R.; Abreu, M.T. Innate immunity in the small intestine. Curr. Opin. Gastroenterol. 2012, 28, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Marsal, J.; Agace, W.W. Targeting T-cell migration in inflammatory bowel disease. J. Intern. Med. 2012, 272, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A.; Heinbockel, L.; Brandenburg, K.; Hornef, M.W. Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa. Gut Microbes 2014, 5, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.B.; Luo, M.M.; Chen, Z.Y.; He, B.H. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020, 2020, 8813558. [Google Scholar] [CrossRef]
- Omenetti, S.; Pizarro, T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front. Immunol. 2015, 6, 639. [Google Scholar] [CrossRef]
- Cabrera-Ortega, A.A.; Feinberg, D.; Liang, Y.; Rossa, C.; Graves, D.T. The Role of Forkhead Box 1 (FOXO1) in the Immune System: Dendritic Cells, T Cells, B Cells, and Hematopoietic Stem Cells. Crit. Rev. Immunol. 2017, 37, 1–13. [Google Scholar] [CrossRef]
- Sharma, M.; Kaveri, S.V.; Bayry, J. Th17 cells, pathogenic or not? TGF-β3 imposes the embargo. Cell. Mol. Immunol. 2013, 10, 101–102. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef]
- Huber, S.; Gagliani, N.; Esplugues, E.; O’Connor, W., Jr.; Huber, F.J.; Chaudhry, A.; Kamanaka, M.; Kobayashi, Y.; Booth, C.J.; Rudensky, A.Y.; et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011, 34, 554–565. [Google Scholar] [CrossRef]
- Littman, D.R.; Rudensky, A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010, 140, 845–858. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+ T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Hedblom, G.A.; Reiland, H.A.; Sylte, M.J.; Johnson, T.J.; Baumler, D.J. Segmented Filamentous Bacteria—Metabolism Meets Immunity. Front. Microbiol. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Luo, A.; Leach, S.T.; Barres, R.; Hesson, L.B.; Grimm, M.C.; Simar, D. The Microbiota and Epigenetic Regulation of T Helper 17/Regulatory T Cells: In Search of a Balanced Immune System. Front. Immunol. 2017, 8, 417. [Google Scholar] [CrossRef]
- Park, E.J.; Shimaoka, M.; Kiyono, H. MicroRNA-mediated dynamic control of mucosal immunity. Int. Immunol. 2017, 29, 157–163. [Google Scholar] [CrossRef]
- Bermúdez-Brito, M.; Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Marzban, H.; Goleij, P.; Sahebkar, A.; Morshedi, K.; Rezaei, S.; Mahjoubin-Tehran, M.; Tarrahimofrad, H.; Hamblin, M.R.; Mirzaei, H. Effects of therapeutic probiotics on modulation of microRNAs. Cell Commun. Signal 2021, 19, 4. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Chan, B.D.; Leung, T.-W.; Chen, M.; Tai, W.C.-S. Beneficial and anti-inflammatory effects of formulated prebiotics, probiotics, and synbiotics in normal and acute colitis mice. J. Funct. Foods 2022, 88, 104871. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological Function of Short-Chain Fatty Acids and Its Regulation on Intestinal Health of Poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.I.; Kumari, A.; Kapila, S.; Kapila, R. Probiotic lactobacilli mediated changes in global epigenetic signatures of human intestinal epithelial cells during Escherichia coli challenge. Ann. Microbiol. 2019, 69, 603–612. [Google Scholar] [CrossRef]
- Kumar, M.; Nagpal, R.; Verma, V.; Kumar, A.; Kaur, N.; Hemalatha, R.; Gautam, S.K.; Singh, B. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr. Rev. 2013, 71, 23–34. [Google Scholar] [CrossRef]
- Mallet, J.F.; Shahbazi, R.; Alsadi, N.; Matar, C. Polyphenol-Enriched Blueberry Preparation Controls Breast Cancer Stem Cells by Targeting FOXO1 and miR-145. Molecules 2021, 26, 4330. [Google Scholar] [CrossRef]
- Novotny-Nuñez, I.; Perdigón, G.; Matar, C.; Martínez Monteros, M.J.; Yahfoufi, N.; Cazorla, S.I.; Maldonado-Galdeano, C. Evaluation of Rouxiella badensis Subsp. Acadiensis (Canan SV-53) as a Potential Probiotic Bacterium. Microorganisms 2023, 11, 1347. [Google Scholar]
- Bošković, M.; Roje, B.; Chung, F.F.; Gelemanović, A.; Cahais, V.; Cuenin, C.; Khoueiry, R.; Vilović, K.; Herceg, Z.; Terzić, J. DNA Methylome Changes of Muscle- and Neuronal-Related Processes Precede Bladder Cancer Invasiveness. Cancers 2022, 14, 487. [Google Scholar] [CrossRef]
- Zhou, W.; Triche, T.J., Jr.; Laird, P.W.; Shen, H. SeSAMe: Reducing artifactual detection of DNA methylation by Infinium BeadChips in genomic deletions. Nucleic Acids Res. 2018, 46, e123. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Peters, T.J.; Buckley, M.J.; Statham, A.L.; Pidsley, R.; Samaras, K.; Lord, R.V.; Clark, S.J.; Molloy, P.L. De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin 2015, 8, 6. [Google Scholar] [CrossRef]
- Yuan, X.-L.; Gao, N.; Xing, Y.; Zhang, H.-B.; Zhang, A.-L.; Liu, J.; He, J.-L.; Xu, Y.; Lin, W.-M.; Chen, Z.-M.; et al. Profiling the genome-wide DNA methylation pattern of porcine ovaries using reduced representation bisulfite sequencing. Sci. Rep. 2016, 6, 22138. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Rhee, J.-K. Genomic Effect of DNA Methylation on Gene Expression in Colorectal Cancer. Biology 2022, 11, 1388. [Google Scholar] [CrossRef]
- Yakoob, J.; Fan, X.G.; Hu, G.L.; Zhang, Z. DNA methylation and carcinogenesis in digestive neoplasms. World J. Gastroenterol. 1998, 4, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Q.; Liu, H. Coronavirus disease 2019 and the gut–lung axis. Int. J. Infect. Dis. 2021, 113, 300–307. [Google Scholar] [CrossRef]
- Seifert, S.; Watzl, B. Inulin and Oligofructose: Review of Experimental Data on Immune Modulation. J. Nutr. 2007, 137, 2563S–2567S. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Ah-Yen, E.G.; Chandrasegaram, R.; Aly, S.; Murack, M.; Kadamani, A.K.; Matar, C.; Ismail, N. Adolescent use of potential novel probiotic Rouxiella badensis subsp. acadiensis (Canan SV-53) mitigates pubertal LPS-Induced behavioral changes in adulthood in a sex-specific manner by modulating 5HT1A receptors expression in specific brain areas. Compr. Psychoneuroendocrinol. 2021, 7, 100063. [Google Scholar] [CrossRef]
- Mallet, J.-F.; Shahbazi, R.; Alsadi, N.; Saleem, A.; Sobiesiak, A.; Arnason, J.T.; Matar, C. Role of a Mixture of Polyphenol Compounds Released after Blueberry Fermentation in Chemoprevention of Mammary Carcinoma: In Vivo Involvement of miR-145. Int. J. Mol. Sci. 2023, 24, 3677. [Google Scholar] [CrossRef]
- Najafi, S.; Sotoodehnejadnematalahi, F.; Amiri, M.M.; Pourshafie, M.R.; Rohani, M. Prophylactic vs. Therapeutic Effect of Probiotics on the Inflammation Mediated by the NF-kappaB Pathway in Inflammatory Bowel Conditions. Biomedicines 2023, 11, 1675. [Google Scholar] [CrossRef]
- D’Hennezel, E.; Abubucker, S.; Murphy, L.O.; Cullen, T.W. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. mSystems 2017, 2, e00046-17. [Google Scholar] [CrossRef]
- Nam, Y.J.; Lee, C.S. Protocatechuic acid inhibits Toll-like receptor-4-dependent activation of NF-κB by suppressing activation of the Akt, mTOR, JNK and p38-MAPK. Int. Immunopharmacol. 2018, 55, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Mallet, J.F.; Kulshreshtha, G.; Hincke, M.; Ismail, N.; Matar, C. Immunomodulation and Intestinal Morpho-Functional Aspects of a Novel Gram-Negative Bacterium Rouxiella badensis subsp. acadiensis. Front. Microbiol. 2021, 12, 569119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the Mucosal Immune System. BioMed Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Aschermann, S.; Lux, A.; Baerenwaldt, A.; Biburger, M.; Nimmerjahn, F. The other side of immunoglobulin G: Suppressor of inflammation. Clin. Exp. Immunol. 2010, 160, 161–167. [Google Scholar] [CrossRef]
- Castro-Dopico, T.; Clatworthy, M.R. IgG and Fcγ Receptors in Intestinal Immunity and Inflammation. Front. Immunol. 2019, 10, 805. [Google Scholar] [CrossRef]
- Vinderola, G.; Perdigón, G.; Duarte, J.; Farnworth, E.; Matar, C. Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 2006, 36, 254–260. [Google Scholar] [CrossRef]
- Castro-Dopico, T.; Dennison, T.W.; Ferdinand, J.R.; Mathews, R.J.; Fleming, A.; Clift, D.; Stewart, B.J.; Jing, C.; Strongili, K.; Labzin, L.I.; et al. Anti-commensal IgG Drives Intestinal Inflammation and Type 17 Immunity in Ulcerative Colitis. Immunity 2019, 50, 1099–1114.e10. [Google Scholar] [CrossRef]
- Cătană, C.-S.; Berindan Neagoe, I.; Cozma, V.; Magdaş, C.; Tăbăran, F.; Dumitraşcu, D.L. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 5823–5830. [Google Scholar] [CrossRef]
- Martin, L.J.; Matar, C. Increase of antioxidant capacity of the lowbush blueberry (Vaccinium angustifolium) during fermentation by a novel bacterium from the fruit microflora. J. Sci. Food Agri. 2005, 85, 1477–1484. [Google Scholar] [CrossRef]
- Vuong, T.; Mallet, J.-F.; Ouzounova, M.; Rahbar, S.; Hernandez-Vargas, H.; Herceg, Z.; Matar, C. Role of a polyphenol-enriched preparation on chemoprevention of mammary carcinoma through cancer stem cells and inflammatory pathways modulation. J. Transl. Med. 2016, 14, 13. [Google Scholar] [CrossRef]
- Chang, H.; Zhao, F.; Xie, X.; Liao, Y.; Song, Y.; Liu, C.; Wu, Y.; Wang, Y.; Liu, D.; Wang, Y.; et al. PPARα suppresses Th17 cell differentiation through IL-6/STAT3/RORγt pathway in experimental autoimmune myocarditis. Exp. Cell Res. 2019, 375, 22–30. [Google Scholar] [CrossRef]
- Schmitt, H.; Neurath, M.F.; Atreya, R. Role of the IL23/IL17 Pathway in Crohn’s Disease. Front. Immunol. 2021, 12, 622934. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Y.; Cui, T.; Zhang, J. IL-6/STAT3 signaling pathway regulates the proliferation and damage of intestinal epithelial cells in patients with ulcerative colitis via H3K27ac. Exp. Ther. Med. 2021, 22, 890. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Boivin, M.; Guo, S.; Hashimi, M.; Ereifej, L.; Ma, T.Y. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE 2014, 9, e85345. [Google Scholar] [CrossRef]
- Wei, H.-X.; Wang, B.; Li, B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front. Immunol. 2020, 11, 1315. [Google Scholar] [CrossRef]
- De Moreno de Leblanc, A.; Del Carmen, S.; Zurita-Turk, M.; Santos Rocha, C.; van de Guchte, M.; Azevedo, V.; Miyoshi, A.; Leblanc, J.G. Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol. 2011, 2011, 892971. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, S.; Sun, S.; Li, Z.; Guo, B. Inflammasome activation has an important role in the development of spontaneous colitis. Mucosal Immunol. 2014, 7, 1139–1150. [Google Scholar] [CrossRef]
- Di Giacinto, C.; Marinaro, M.; Sanchez, M.; Strober, W.; Boirivant, M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-β-bearing regulatory cells. J. Immunol. 2005, 174, 3237–3246. [Google Scholar] [CrossRef]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Fåk, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Weström, B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 2010, 5, e9009. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Mallet, J.F.; Graham, E.; Matar, C. Role of probiotics and prebiotics in immunomodulation. Curr. Opin. Food Sci. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Looijer-van Langen, M.A.C.; Dieleman, L.A. Prebiotics in chronic intestinal inflammation. Inflamm. Bowel Dis. 2009, 15, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Capitán-Cañadas, F.; Ortega-González, M.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; de Medina, F.S.; Martínez-Augustin, O. Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Mol. Nutr. Food Res. 2014, 58, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Zhang, X.; Chen, W.; Diao, H. MicroRNAs Regulate Intestinal Immunity and Gut Microbiota for Gastrointestinal Health: A Comprehensive Review. Genes 2020, 11, 1075. [Google Scholar] [CrossRef]
- Yang, X.; He, Q.; Guo, Z.; Xiong, F.; Li, Y.; Pan, Y.; Gao, C.; Li, L.; He, C. MicroRNA-425 facilitates pathogenic Th17 cell differentiation by targeting forkhead box O1 (Foxo1) and is associated with inflammatory bowel disease. Biochem. Biophys. Res. Commun. 2018, 496, 352–358. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Fernández-Caballero, J.A.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. The Administration of Escherichia coli Nissle 1917 Ameliorates Development of DSS-Induced Colitis in Mice. Front. Pharmacol. 2018, 9, 468. [Google Scholar] [CrossRef]
- Wang, H.; Chao, K.; Ng, S.C.; Bai, A.H.; Yu, Q.; Yu, J.; Li, M.; Cui, Y.; Chen, M.; Hu, J.-F.; et al. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol. 2016, 17, 58. [Google Scholar] [CrossRef]
- Jiao, P.; Wang, X.-P.; Luoreng, Z.-M.; Yang, J.; Jia, L.; Ma, Y.; Wei, D.-W. miR-223: An Effective Regulator of Immune Cell Differentiation and Inflammation. Int. J. Biol. Sci. 2021, 17, 2308–2322. [Google Scholar] [CrossRef]
- Balzano, F.; Deiana, M.; Dei Giudici, S.; Oggiano, A.; Pasella, S.; Pinna, S.; Mannu, A.; Deiana, N.; Porcu, B.; Masala, A.G.E.; et al. MicroRNA Expression Analysis of Centenarians and Rheumatoid Arthritis Patients Reveals a Common Expression Pattern. Int. J. Med. Sci. 2017, 14, 622–628. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Garcia, F.; Olivares, M.; Rodríguez-Cabezas, M.E.; Gálvez, J. Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: Impact on microRNAs expression and microbiota composition. Mol. Nutr. Food Res. 2017, 61, 1700144. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on microRNAs expression and gut microbiota composition. J. Nutr. Biochem. 2018, 61, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; Tremblay, J.; Arbour, M.; Mallet, J.F.; Masson, L.; Matar, C. Complete PacBio Single-Molecule Real-Time Sequence of a Novel Probiotic-Like Bacterium, Rouxiella badensis subsp. acadiensis, Isolated from the Biota of Wild Blueberries in the Acadian Forest. Microbiol. Resour. Announc. 2023, 12, e0134022. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Garcia-Gil, L.J. Escherichia coli in chronic inflammatory bowel diseases: An update on adherent invasive Escherichia coli pathogenicity. World J. Gastrointest. Pathophysiol. 2014, 5, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Looft, T.; Allen, H.K. Collateral effects of antibiotics on mammalian gut microbiomes. Gut Microbes 2012, 3, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116. [Google Scholar] [CrossRef]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Xiao, H.; Chen, S.; Liu, G.; Tan, B.; Li, N.; Peng, Y.; Li, T.; Zeng, B.; et al. Intestinal Microbiota-Derived GABA Mediates Interleukin-17 Expression during Enterotoxigenic Escherichia coli Infection. Front. Immunol. 2017, 7, 685. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections with Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef]
- Castro-Herrera, V.M.; Rasmussen, C.; Wellejus, A.; Miles, E.A.; Calder, P.C. In Vitro Effects of Live and Heat-Inactivated Bifidobacterium animalis Subsp. Lactis, BB-12 and Lactobacillus rhamnosus GG on Caco-2 Cells. Nutrients 2020, 12, 1719. [Google Scholar] [CrossRef]
- Sang, L.-X.; Chang, B.; Wang, B.-Y.; Liu, W.-X.; Jiang, M. Live and heat-killed probiotic: Effects on chronic experimental colitis induced by dextran sulfate sodium (DSS) in rats. Int. J. Clin. Exp. Med. 2015, 8, 20072–20078. [Google Scholar]
- Wehkamp, J.; Harder, J.; Wehkamp, K.; Wehkamp-von Meissner, B.; Schlee, M.; Enders, C.; Sonnenborn, U.; Nuding, S.; Bengmark, S.; Fellermann, K.; et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: A novel effect of a probiotic bacterium. Infect. Immun. 2004, 72, 5750–5758. [Google Scholar] [CrossRef]
- Vinderola, G.; Matar, C.; Perdigon, G. Role of Intestinal Epithelial Cells in Immune Effects Mediated by Gram-Positive Probiotic Bacteria: Involvement of Toll-Like Receptors. Clin. Vaccine Immunol. 2005, 12, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Pyclik, M.J.; Srutkova, D.; Razim, A.; Hermanova, P.; Svabova, T.; Pacyga, K.; Schwarzer, M.; Górska, S. Viability Status-Dependent Effect of Bifidobacterium longum ssp. longum CCM 7952 on Prevention of Allergic Inflammation in Mouse Model. Front. Immunol. 2021, 12, 707728. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Rilling, K.; Baumgart, D.C.; Gargas, K.; Abou-Ghazalé, T.; Raupach, B.; Eckert, J.; Schumann, R.R.; Enders, C.; Sonnenborn, U.; et al. Escherichia coli Nissle 1917 Distinctively Modulates T-Cell Cycling and Expansion via Toll-Like Receptor 2 Signaling. Infect. Immun. 2005, 73, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ota, N.; Peng, I.; Refino, C.J.; Danilenko, D.M.; Caplazi, P.; Ouyang, W. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 2010, 184, 4307–4316. [Google Scholar] [CrossRef]
- Nowak, E.C.; Weaver, C.T.; Turner, H.; Begum-Haque, S.; Becher, B.; Schreiner, B.; Coyle, A.J.; Kasper, L.H.; Noelle, R.J. IL-9 as a mediator of Th17-driven inflammatory disease. J. Exp. Med. 2009, 206, 1653–1660. [Google Scholar] [CrossRef]
- Zhang, X.; Kiapour, N.; Kapoor, S.; Khan, T.; Thamilarasan, M.; Tao, Y.; Cohen, S.; Miller, R.; Sobel, R.A.; Markovic-Plese, S. IL-11 Induces Encephalitogenic Th17 Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. J. Immunol. 2019, 203, 1142–1150. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Nagai, S.; Ikejiri, A.; Ohtani, M.; Ichiyama, K.; Baba, Y.; Yamada, T.; Egami, S.; Hoshii, T.; Hirao, A.; et al. PI3K-Akt-mTORC1-S6K1/2 Axis Controls Th17 Differentiation by Regulating Gfi1 Expression and Nuclear Translocation of RORγ. Cell Rep. 2012, 1, 360–373. [Google Scholar] [CrossRef]
- Johnston, A.M.; Niemela, J.; Rosenzweig, S.D.; Fried, A.J.; Delmonte, O.M.; Fleisher, T.A.; Kuehn, H. A Novel Mutation in IKBKG/NEMO Leads to Ectodermal Dysplasia with Severe Immunodeficiency (EDA-ID). J. Clin. Immunol. 2016, 36, 541–543. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V.K. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013, 496, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Tang, N.; Ma, Y.; Guo, H.; Zhao, Y.; Tang, R.; Yan, C.; Ouyang, S.; Langdon, W.Y.; Yang, H.; et al. Cbl-b restrains priming of pathogenic Th17 cells via the inhibition of IL-6 production by macrophages. iScience 2022, 25, 105151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, G.; Wan, Y.Y. SKI and SMAD4 are essential for IL-21-induced Th17 differentiation. Mol. Immunol. 2019, 114, 260–268. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahbazi, R.; Yasavoli-Sharahi, H.; Mallet, J.-F.; Sharifzad, F.; Alsadi, N.; Cuenin, C.; Cahais, V.; Chung, F.F.-L.; Herceg, Z.; Matar, C. Novel Probiotic Bacterium Rouxiella badensis subsp. acadiensis (Canan SV-53) Modulates Gut Immunity through Epigenetic Mechanisms. Microorganisms 2023, 11, 2456. https://doi.org/10.3390/microorganisms11102456
Shahbazi R, Yasavoli-Sharahi H, Mallet J-F, Sharifzad F, Alsadi N, Cuenin C, Cahais V, Chung FF-L, Herceg Z, Matar C. Novel Probiotic Bacterium Rouxiella badensis subsp. acadiensis (Canan SV-53) Modulates Gut Immunity through Epigenetic Mechanisms. Microorganisms. 2023; 11(10):2456. https://doi.org/10.3390/microorganisms11102456
Chicago/Turabian StyleShahbazi, Roghayeh, Hamed Yasavoli-Sharahi, Jean-François Mallet, Farzaneh Sharifzad, Nawal Alsadi, Cyrille Cuenin, Vincent Cahais, Felicia Fei-Lei Chung, Zdenko Herceg, and Chantal Matar. 2023. "Novel Probiotic Bacterium Rouxiella badensis subsp. acadiensis (Canan SV-53) Modulates Gut Immunity through Epigenetic Mechanisms" Microorganisms 11, no. 10: 2456. https://doi.org/10.3390/microorganisms11102456
APA StyleShahbazi, R., Yasavoli-Sharahi, H., Mallet, J.-F., Sharifzad, F., Alsadi, N., Cuenin, C., Cahais, V., Chung, F. F.-L., Herceg, Z., & Matar, C. (2023). Novel Probiotic Bacterium Rouxiella badensis subsp. acadiensis (Canan SV-53) Modulates Gut Immunity through Epigenetic Mechanisms. Microorganisms, 11(10), 2456. https://doi.org/10.3390/microorganisms11102456





