Epidemiological, Genetic, and Phenotypic Characteristics of Non-Typhoidal Salmonella in Young Children, as Obtained from a Tertiary Hospital in Guangzhou, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design and Specimen Collection
2.3. Questionnaire
2.4. Salmonella Strains, DNA Extraction, and WGS
2.5. Antimicrobial Susceptibility Testing
2.6. Quality Control
2.7. Statistical Analysis
3. Results
3.1. Prevalence of NTS
3.2. Serotyping, MLST, and Phylogenetic Analysis
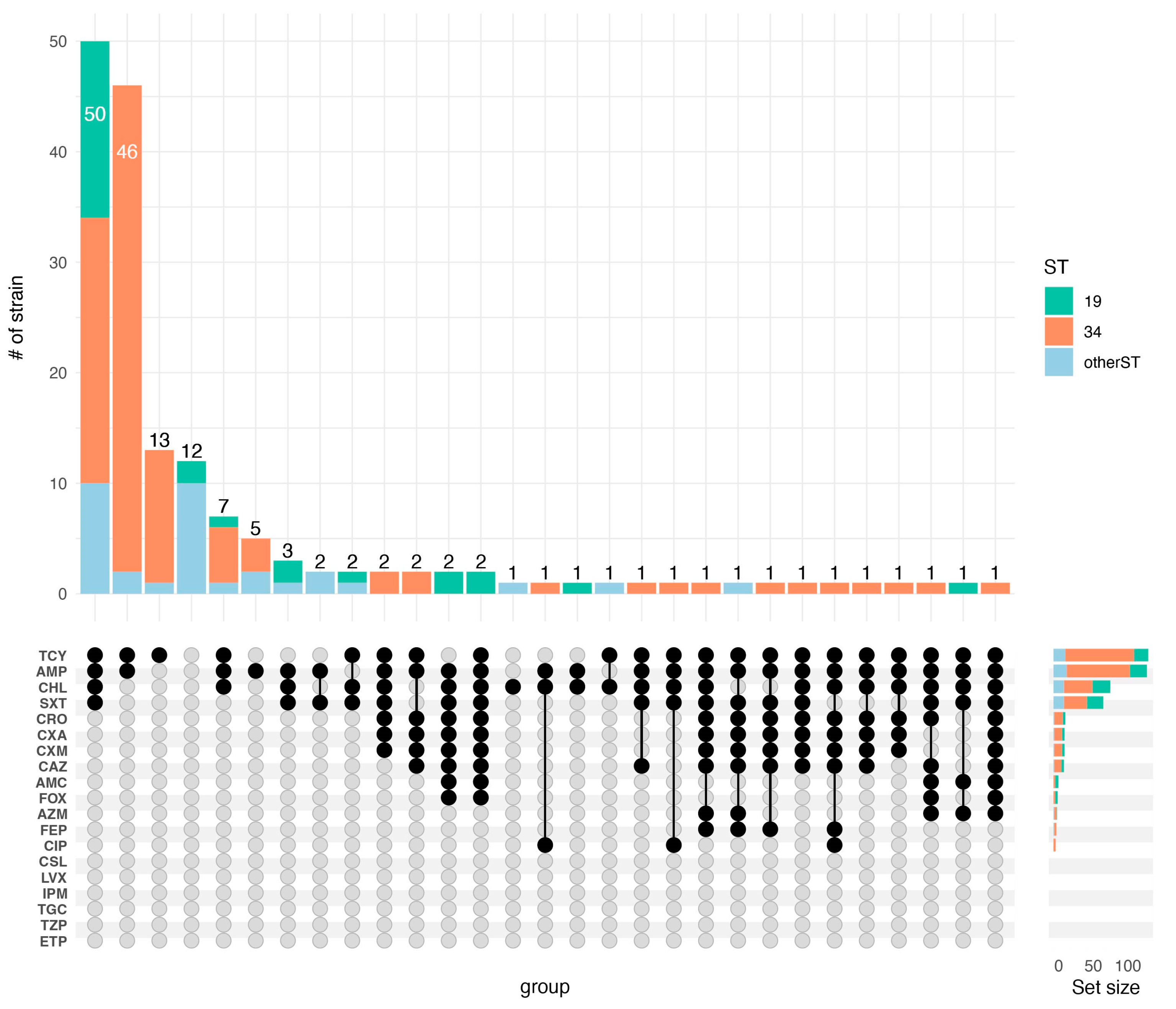
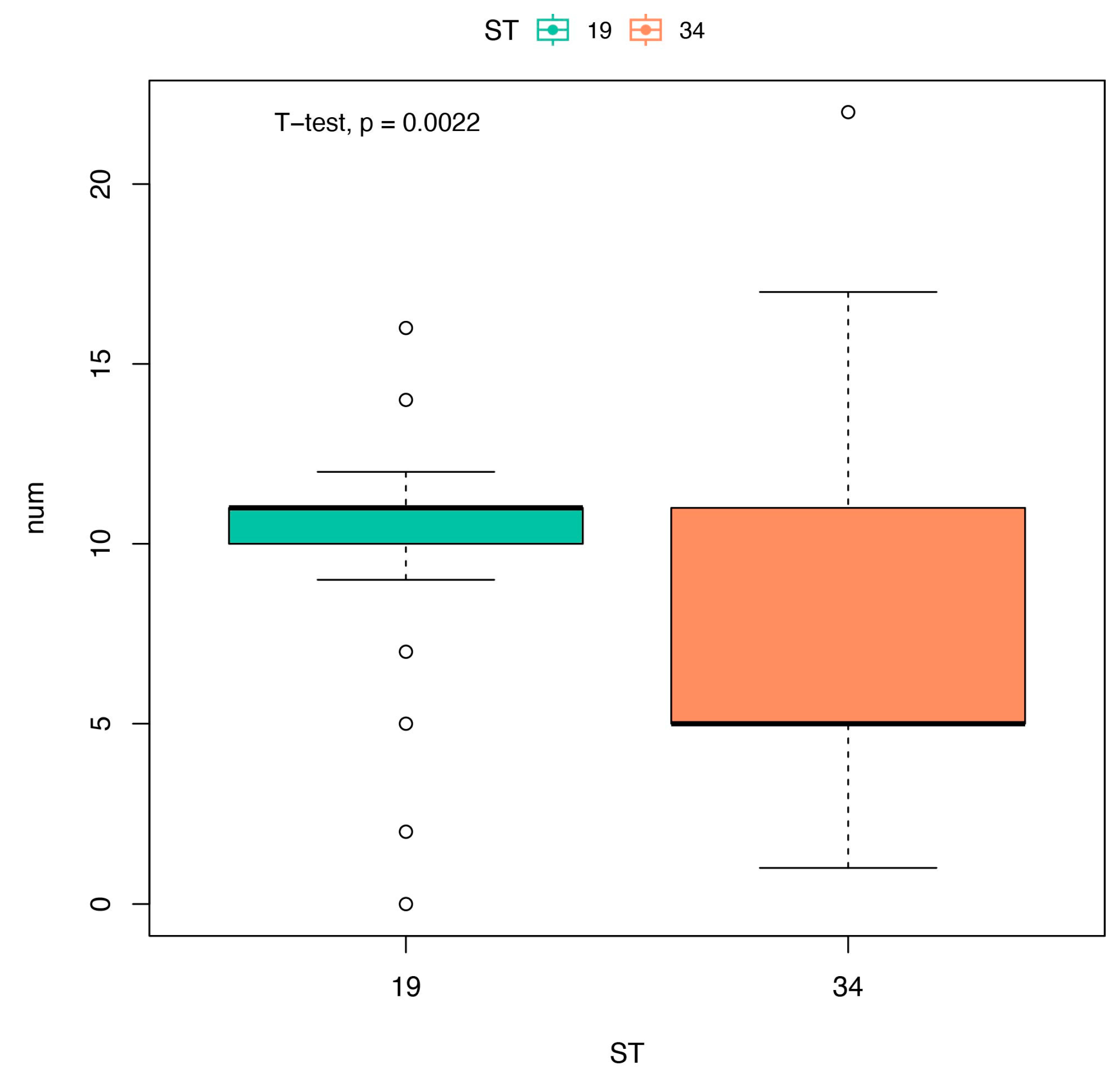
3.3. Antibiotic Susceptibility Pattern and Resistance Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 20 July 2023).
- Wu, L.J.; Luo, Y.; Shi, G.L.; Li, Z.Y. Prevalence, clinical characteristics and changes of antibiotic resistance in children with nontyphoidal Salmonella infections from 2009–2018 in Chongqing, China. Infect. Drug. Resist. 2021, 13, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Nolan, V.G.; Dunn, J.R.; Banerjee, P. Sources of human infection by Salmonella enterica serotype Javiana: A systematic review. PLoS ONE 2019, 14, e0222108. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.R.; Griffin, P.M.; Cole, D.; Walsh, K.A.; Chai, S.J. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1239–1244. [Google Scholar] [CrossRef]
- Müller, L.; Kjelsø, C.; Frank, C.; Jensen, T.; Torpdahl, M.; Søborg, B.; Dorleans, F.; Rabsch, W.; Prager, R.; Gossner, C.M.; et al. Outbreak of Salmonella Strathcona caused by datterino tomatoes, Denmark, 2011. Epidemiol. Infect. 2016, 144, 2802–2811. [Google Scholar] [CrossRef]
- Marshall, K.E.H.; Tewell, M.; Tecle, S.; Leeper, M.; Sinatra, J.; Kissler, B.; Fung, A.; Brown, K.; Wagner, D.; Trees, E.; et al. Protracted outbreak of Salmonella newport infections linked to ground beef: Possible role of dairy cows—21 States, 2016–2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.; Bottichio, L.; Weiss, J.; Higa, J.; McDonald, E.; Sowadsky, R.; Fejes, D.; Saupe, A.; Provo, G.; Seelman, S.; et al. Multistate outbreak of Salmonella Poona infections associated with imported cucumbers, 2015–2016. Epidemiol. Infect. 2019, 147, e270. [Google Scholar] [CrossRef]
- Sloan-Gardner, T.S.; Waters, N.; Marmor, A.; Mude, W. Free range eggs does not mean safe eggs: An outbreak of Salmonella Typhimurium linked to free range eggs. Commun. Dis. Intell. 2019, 15, 43. [Google Scholar] [CrossRef]
- Laidlow, T.A.; Stafford, R.; Jennison, A.V.; Bell, R.; Graham, R.; Graham, T.; Musgrave, N.; Myerson, M.; Kung, N.; Crook, A.; et al. A multi-jurisdictional outbreak of Salmonella Typhimurium infections linked to backyard poultry-Australia, 2020. Zoonoses. Public. Health. 2022, 69, 835–842. [Google Scholar] [CrossRef]
- Butler, A.J.; Thomas, M.K.; Pintar, K.D.M. Expert elicitation as a means to attribute 28 enteric pathogens to foodborne, waterborne, animal contact, and person-to-person transmission routes in Canada. Foodborne. Pathog. Dis. 2015, 12, 335–344. [Google Scholar] [CrossRef]
- Christidis, T.; Hurst, M.; Rudnick, W.; Pintar, K.D.M.; Pollari, F. A comparative exposure assessment of foodborne, animal contact and waterborne transmission routes of Salmonella in Canada. Food. Control. 2020, 109, 106899. [Google Scholar] [CrossRef]
- Xu, L.; He, Q.; Tang, Y.; Wen, W.; Chen, L.; Li, Y.; Yi, C.; Fu, B. Multi-locus sequence and drug resistance analysis of Salmonella infection in children with diarrhea in Guangdong to identify the dominant ST and cause of antibiotic-resistance. Exp. Ther. Med. 2022, 24, 678. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Salmonella Surveillance Annual Report, 2016; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2018. [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- Grattarola, C.; Gallina, S.; Giorda, F.; Pautasso, A.; Ballardini, M.; Iulini, B.; Varello, K.; Goria, M.; Peletto, S.; Masoero, L.; et al. First report of Salmonella 1,4,[5],12:i:- in free-ranging striped dolphins (Stenella coeruleoalba), Italy. Sci. Rep. 2019, 9, 6061. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Y.; Lv, S.L.; Qu, C.; Lan, L.; Tan, D.M.; Li, X.G.; Bai, L. Serotypes, antibiotic resistance, and molecular characteriza-tion of non-typhoidal Salmonella isolated from diarrheic patients in Guangxi Zhuang Autonomous Region, China, 2014–2017. Food Control. 2021, 120, 107478. [Google Scholar] [CrossRef]
- Baker, S.; Thomson, N.; Weill, F.X.; Holt, K.E. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Science 2018, 360, 733–738. [Google Scholar] [CrossRef]
- Zhang, H.; Xiang, Y.; Huang, Y.; Liang, B.; Xu, X.; Xie, J.; Du, X.; Yang, C.; Liu, H.; Liu, H.; et al. Genetic characterization of mcr-1-positive multidrug-resistant Salmonella enterica serotype Typhimurium isolated from intestinal infection in children and pork offal in China. Front. Microbiol. 2022, 12, 774797. [Google Scholar] [CrossRef]
- Wang, X.; Biswas, S.; Paudyal, N.; Pan, H.; Li, X.; Fang, W.; Yue, M. Antibiotic resistance in Salmonella Typhimurium isolates recovered from the food chain through national antimicrobial resistance monitoring system between 1996 and 2016. Front. Microbiol. 2019, 10, 985. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Chen, M.; Hu, J.; Zhang, H.; Xiang, Y.; Yang, H.; Qiu, S.; Song, H. Plasmid-borne colistin resistance gene mcr-1 in a multidrug resistant Salmonella enterica serovar Typhimurium isolate from an infant with acute diarrhea in China. Int. J. Infect. Dis. 2021, 103, 13–18. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Lyu, N.; Li, Z.; Ma, S.; Cao, D.; Pan, Y.; Hu, Y.; Huang, H.; Gao, G.F.; et al. The temporal dynamics of antimicrobial-resistant Salmonella enterica and predominant serovars in China. Natl. Sci. Rev. 2022, 10, nwac269. [Google Scholar] [CrossRef]
- Zeng, S.; Zhuo, Z.; Huang, Y.; Luo, J.; Feng, Y.; Gong, B.; Huang, X.; Wu, A.; Zhuo, C.; Li, X. Prevalence of chromosomally located blaCTX-M-55 in Salmonella Typhimurium ST34 isolates recovered from a tertiary hospital in Guangzhou, China. Microbiol. Spectr. 2022, 10, e0277121. [Google Scholar] [CrossRef] [PubMed]
- Bioinformatics, B. FastQC a Quality Control Tool for High Throughput Sequencedata. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 4 June 2023).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.E.; Kruczkiewicz, P.; Laing, C.R.; Lingohr, E.J.; Gannon, V.P.; Nash, J.H.; Taboada, E.N. The Salmonella in Silico Typing Resource (SISTR): An open web-accessible tool for rapidly typing and subtyping draft Salmonella genome assemblies. PLoS ONE 2016, 11, e0147101. [Google Scholar] [CrossRef]
- Thomsen, M.C.; Ahrenfeldt, J.; Cisneros, J.L.; Jurtz, V.; Larsen, M.V.; Hasman, H.; Aarestrup, F.M.; Lund, O. A bacterial analysis platform: An integrated system for analysing bacterial whole genome sequencing data for clinical diagnostics and surveillance. PLoS ONE 2016, 11, e0157718. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Jones, R.N.; Barry, A.L.; Packer, R.R.; Gregory, W.W.; Thornsberry, C. In vitro antimicrobial spectrum, occurrence of synergy, and recommendations for dilution susceptibility testing concentrations of the cefoperazone-sulbactam combination. J. Clin. Microbiol. 1987, 25, 1725–1729. [Google Scholar] [CrossRef]
- Woh, P.Y.; Yeung, M.P.S.; Goggins, W.B., 3rd; Lo, N.; Wong, K.T.; Chow, V.; Chau, K.Y.; Fung, K.; Chen, Z.; Ip, M. Genomic epidemiology of multidrug-resistant nontyphoidal Salmonella in young children hospitalized for gastroenteritis. Microbiol. Spectr. 2021, 9, e0024821. [Google Scholar] [CrossRef]
- Gao, F.; Huang, Z.; Xiong, Z.; Zheng, H.; Deng, Q.; Zhong, H.; Zhu, S.; Long, Y.; Wang, J. Prevalence, serotype, and antimicrobial resistance profiles of children infected with Salmonella in Guangzhou, southern China, 2016–2021. Front. Pediatr. 2023, 11, 1077158. [Google Scholar] [CrossRef]
- Deng, X.; Ran, L.; Wu, S.; Ke, B.; He, D.; Yang, X.; Zhang, Y.; Ke, C.; Klena, J.D.; Yan, M.; et al. Laboratory-based surveillance of non-typhoidal Salmonella infections in Guangdong Province, China. Foodborne Pathog. Dis. 2012, 9, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, L.P.; Yu, J.X.; Chen, X.; Wang, R.N.; Yang, X.Z.; Zheng, S.F.; Yu, F.; Zhang, Z.K.; Liu, S.J.; et al. Prevalence of enteropathogens in outpatients with acute diarrhea from urban and rural areas, southeast China, 2010–2014. Am. J. Trop. Med. Hyg. 2019, 101, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Chen, H.; Ou, Y.; Huang, T.; Chen, S.; Zhou, L.; Zhang, J.; Hu, Q.; Zhou, Y.; Ma, W. Prevalence, serotypes, and antimicrobial resistance of Salmonella isolates from patients with diarrhea in Shenzhen, China. BMC Microbiol. 2020, 20, 197. [Google Scholar] [CrossRef]
- Wang, P.; Goggins, W.B.; Chan, E.Y.Y. Associations of Salmonella hospitalizations with ambient temperature, humidity and rainfall in Hong Kong. Environ. Int. 2018, 120, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Lu, W.; Liu, W.; Zhu, P.; Chen, Q.; Zhu, Z. Non-typhoidal Salmonella infections among children in a tertiary hospital in Ningbo, Zhejiang, China, 2012–2019. PLoS Negl. Trop. Dis. 2020, 14, e0008732. [Google Scholar] [CrossRef]
- Jiang, C.; Shaw, K.S.; Upperman, C.R.; Blythe, D.; Mitchell, C.; Murtugudde, R.; Sapkota, A.R.; Sapkota, A. Climate change, extreme events and increased risk of salmonellosis in Maryland, USA: Evidence for coastal vulnerability. Environ. Int. 2015, 83, 58–62. [Google Scholar] [CrossRef]
- Rowe, S.Y.; Rocourt, J.R.; Shiferaw, B.; Kassenborg, H.D.; Segler, S.D.; Marcus, R.; Daily, P.J.; Hardnett, F.P.; Slutsker, L.; Emerging Infections Program FoodNet Working Group. Breast-feeding decreases the risk of sporadic salmonellosis among infants in foodnet sites. Clin. Infect. Dis. 2004, 38, S262–S270. [Google Scholar] [CrossRef]
- Jones, T.F.; Ingram, L.A.; Fullerton, K.E.; Marcus, R.; Anderson, B.J.; McCarthy, P.V.; Vugia, D.; Shiferaw, B.; Haubert, N.; Wedel, S.; et al. A case-control study of the epidemiology of sporadic Salmonella infection in infants. Pediatrics 2006, 118, 2380–2387. [Google Scholar] [CrossRef]
- Ehlayel, M.S.; Bener, A.; Abdulrahman, H.M. Protective effect of breastfeeding on diarrhea among children in a rapidly growing newly developed society. Turk. J. Pediatr. 2009, 51, 527–533. [Google Scholar]
- Kovats, R.S.; Edwards, S.J.; Hajat, S.; Armstrong, B.G.; Ebi, K.L.; Menne, B. The effect of temperature on food poisoning: A time-series analysis of salmonellosis in ten European countries. Epidemiol. Infect. 2004, 132, 443–453. [Google Scholar] [CrossRef]
- Qin, X.; Yang, M.; Cai, H.; Liu, Y.; Gorris, L.; Aslam, M.Z.; Jia, K.; Sun, T.; Wang, X.; Dong, Q. Antibiotic resistance of Salmonella Typhimurium monophasic variant 1,4,[5],12:i:-in China: A systematic review and meta-analysis. Antibiotics 2022, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, H.; Zhong, H.; Cheng, F.; Wu, Z.; Shi, T. Non-typhoidal Salmonella infections among children in Fuzhou, Fujian, China: A 10-year retrospective review from 2012 to 2021. Infect. Drug. Resist. 2023, 16, 2737–2749. [Google Scholar] [CrossRef] [PubMed]
- Helmuth, I.G.; Espenhain, L.; Ethelberg, S.; Jensen, T.; Kjeldgaard, J.; Litrup, E.; Schjørring, S.; Müller, L. An outbreak of monophasic Salmonella Typhimurium associated with raw pork sausage and other pork products, Denmark 2018–19. Epidemiol. Infect. 2019, 147, e315. [Google Scholar] [CrossRef]
- Li, C.; Gu, X.; Zhang, L.; Liu, Y.; Li, Y.; Zou, M.; Liu, B. The occurrence and genomic characteristics of mcr-1-harboring Salmonella from retail meats and eggs in Qingdao, China. Foods 2022, 11, 3854. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Sun, J.; He, D.; Li, X.; Liang, Z.; Ke, C.W. Serovar distribution, antimicrobial resistance profiles, and PFGE typing of Salmonella enterica strains isolated from 2007–2012 in Guangdong, China. BMC Infect. Dis. 2014, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ke, B.; Huang, Y.; He, D.; Li, X.; Liang, Z.; Ke, C. The molecular epidemiological characteristics and genetic diversity of Salmonella Typhimurium in Guangdong, China, 2007–2011. PLoS ONE 2014, 9, e113145. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Y.; Xiong, Z.; Ma, Y.; Wei, Y.; Qu, X.; Zhang, H.; Zhang, J.; Liao, M. Highly prevalent multidrug-resistant Salmonella from chicken and pork meat at retail markets in Guangdong, China. Front. Microbiol. 2018, 9, 2104. [Google Scholar] [CrossRef]
- Liang, Z.; Ke, B.; Deng, X.; Liang, J.; Ran, L.; Lu, L.; He, D.; Huang, Q.; Ke, C.; Li, Z.; et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009–2012. BMC Infect. Dis. 2015, 15, 53. [Google Scholar] [CrossRef]
- Gong, B.; Li, H.; Feng, Y.; Zeng, S.; Zhuo, Z.; Luo, J.; Chen, X.; Li, X. Prevalence, Serotype distribution and antimicrobial resistance of non-typhoidal Salmonella in hospitalized patients in Conghua district of Guangzhou, China. Front. Cell. Infect. Microbiol. 2022, 12, 805384. [Google Scholar] [CrossRef]
- Long, L.; You, L.; Wang, D.; Wang, M.; Wang, J.; Bai, G.; Li, J.; Wei, X.; Li, S. Highly prevalent MDR, frequently carrying virulence genes and antimicrobial resistance genes in Salmonella enterica serovar 4,[5],12:i:- isolates from Guizhou Province, China. PLoS ONE 2022, 17, e0266443. [Google Scholar] [CrossRef]
- Egorova, A.; Shelenkov, A.; Kuleshov, K.; Kulikova, N.; Chernyshkov, A.; Manzeniuk, I.; Mikhaylova, Y.; Akimkin, V. Plasmid Composition, Antimicrobial resistance and virulence genes profiles of ciprofloxacin- and third-generation cephalosporin-resistant foodborne Salmonella enterica isolates from Russia. Microorganisms 2023, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Chiou, J.; Zeng, Z.; Liu, L.; Chen, X.; Zeng, L.; Chan, E.W.; Liu, J.H.; Chen, S. Residues distal to the active site contribute to enhanced catalytic activity of variant and hybrid β-Lactamases derived from CTX-M-14 and CTX-M-15. Antimicrob. Agents Chemother. 2015, 59, 5976–5983. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Liu, D.; Li, X.; Jin, S.; Hu, X.; Zhao, X.; Wu, Y. Epidemiology, serotype and resistance of Salmonella isolates from a children’s hospital in Hangzhou, Zhejiang, China, 2006–2021. Infect. Drug. Resist. 2022, 15, 4735–4748. [Google Scholar] [CrossRef]
- Sun, H.; Wan, Y.; Du, P.; Bai, L. The epidemiology of monophasic Salmonella Typhimurium. Foodborne. Pathog. Dis. 2020, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]

| Serogroup (n) | Serotype (n) | ST (n) |
|---|---|---|
| B (149) | S. 1,4,[5],12:i:- (107) | ST34 (103), ST19 (4) |
| S. Typhimurium (25) | ST19 (24), novel ST (1) | |
| S. Stanley (9) | ST29 (9) | |
| S. Derby (8) | ST40 (8) | |
| C1 (6) | S. Rissen (4) | ST469 (4) |
| S. Bareilly (1) | ST203 (1) | |
| S. Infantis (1) | ST32 (1) | |
| C2–C3 (2) | S. Corvallis (1) | ST9826 (1) |
| S. Goldcoast (1) | ST358 (1) | |
| D (5) | S. Enteritidis (5) | ST11 (5) |
| E1 (2) | S. London (2) | ST155 (2) |
| Antimicrobial | Resistant % | Intermediate % | Susceptible % |
|---|---|---|---|
| AMC | 4.3 | 8.5 | 87.2 |
| AZM | 3 | 25 | 72 |
| AMP | 82.3 | 1.8 | 15.9 |
| ETP | 0 | 0 | 100 |
| SXT | 43.9 | 0 | 56.1 |
| CIP | 4.3 | 55.5 | 40.2 |
| CHL | 50 | 0 | 50 |
| TZP | 0 | 0.6 | 99.4 |
| TCY | 83.5 | 1.2 | 15.2 |
| TGC | 0 | 6.7 | 93.3 |
| FEP | 2.4 | 1.8 | 95.7 |
| CXM | 9.8 | 1.8 | 88.4 |
| CXA | 9.8 | 4.3 | 86 |
| CRO | 10.4 | 0 | 89.6 |
| CAZ | 9.1 | 0 | 90.9 |
| FOX | 3.7 | 0 | 96.3 |
| IPM | 0 | 0 | 100 |
| LVX | 0 | 3 | 97 |
| CSL | 0 | 1.2 | 98.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, B.; Feng, Y.; Zhuo, Z.; Song, J.; Chen, X.; Li, X. Epidemiological, Genetic, and Phenotypic Characteristics of Non-Typhoidal Salmonella in Young Children, as Obtained from a Tertiary Hospital in Guangzhou, China. Microorganisms 2023, 11, 2433. https://doi.org/10.3390/microorganisms11102433
Gong B, Feng Y, Zhuo Z, Song J, Chen X, Li X. Epidemiological, Genetic, and Phenotypic Characteristics of Non-Typhoidal Salmonella in Young Children, as Obtained from a Tertiary Hospital in Guangzhou, China. Microorganisms. 2023; 11(10):2433. https://doi.org/10.3390/microorganisms11102433
Chicago/Turabian StyleGong, Baiyan, Yulian Feng, Zhenxu Zhuo, Jingjie Song, Xiankai Chen, and Xiaoyan Li. 2023. "Epidemiological, Genetic, and Phenotypic Characteristics of Non-Typhoidal Salmonella in Young Children, as Obtained from a Tertiary Hospital in Guangzhou, China" Microorganisms 11, no. 10: 2433. https://doi.org/10.3390/microorganisms11102433
APA StyleGong, B., Feng, Y., Zhuo, Z., Song, J., Chen, X., & Li, X. (2023). Epidemiological, Genetic, and Phenotypic Characteristics of Non-Typhoidal Salmonella in Young Children, as Obtained from a Tertiary Hospital in Guangzhou, China. Microorganisms, 11(10), 2433. https://doi.org/10.3390/microorganisms11102433





