A Quantitative ELISA to Detect Anti-SARS-CoV-2 Spike IgG Antibodies in Infected Patients and Vaccinated Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection
2.3. Dry Blood Spot Elution Procedure
2.4. Indirect ELISA
3. Results
3.1. Development of SARS-CoV-2 Spike Protein-Specific Quantitative IgG ELISA
3.2. Linearity
3.3. Precision and Reproducibility
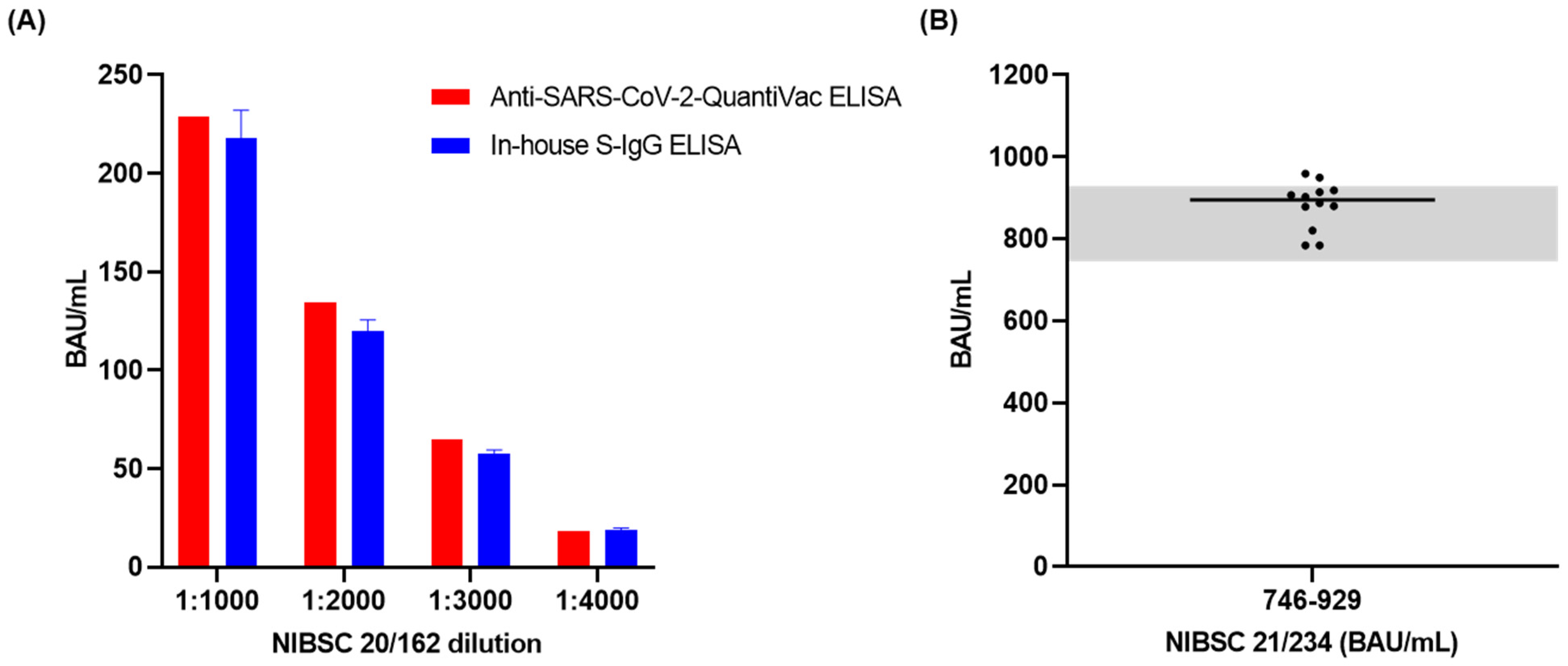
3.4. Accuracy
3.5. Analytical Sensitivity
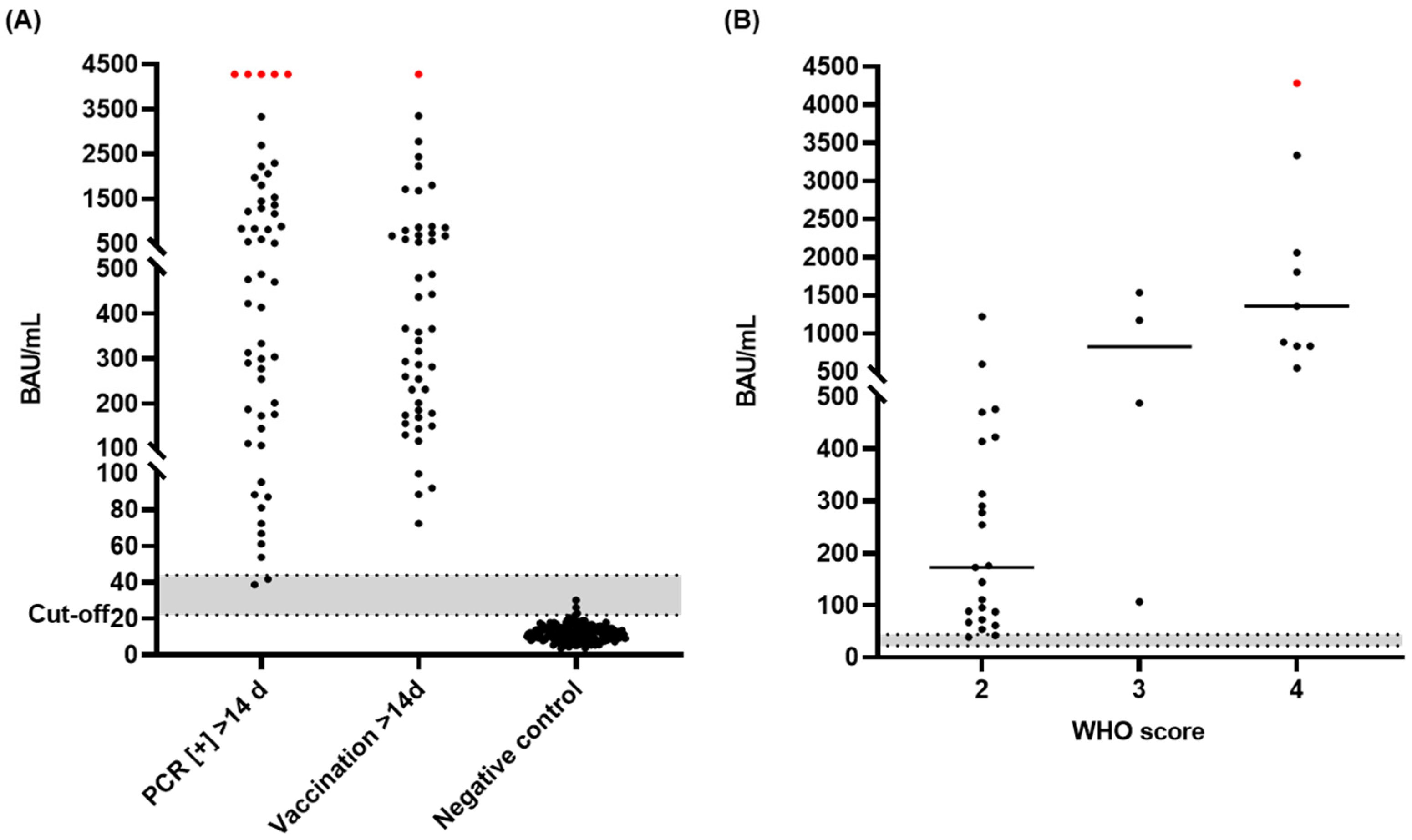
3.6. Diagnostic Sensitivity and Specificity of the S-IgG ELISA
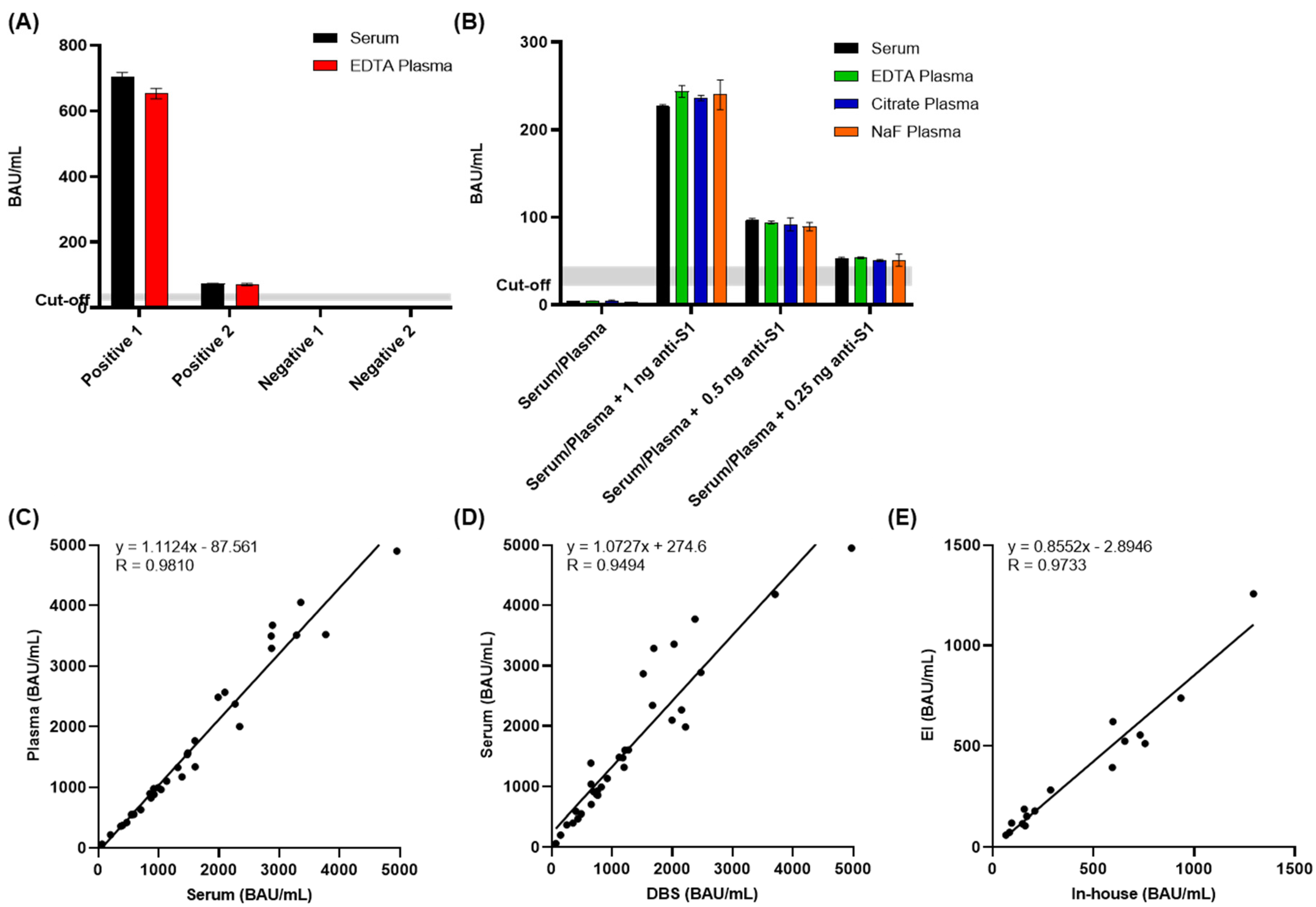
3.7. Interference and Cross-Reactivity
3.8. Detection of IgG Antibodies in Different Sample Matrices
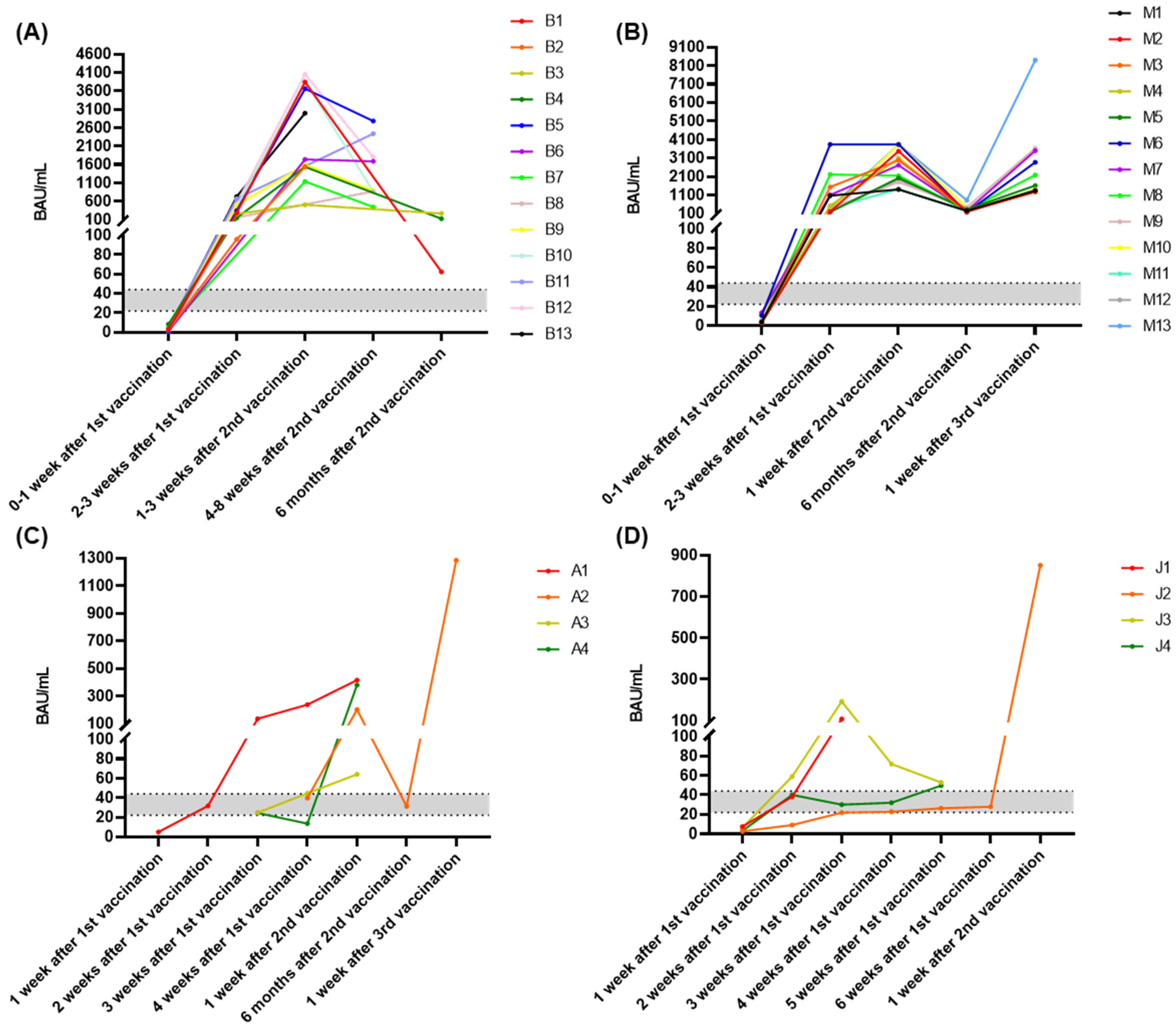
3.9. Time Course of Antibody Response during Vaccination
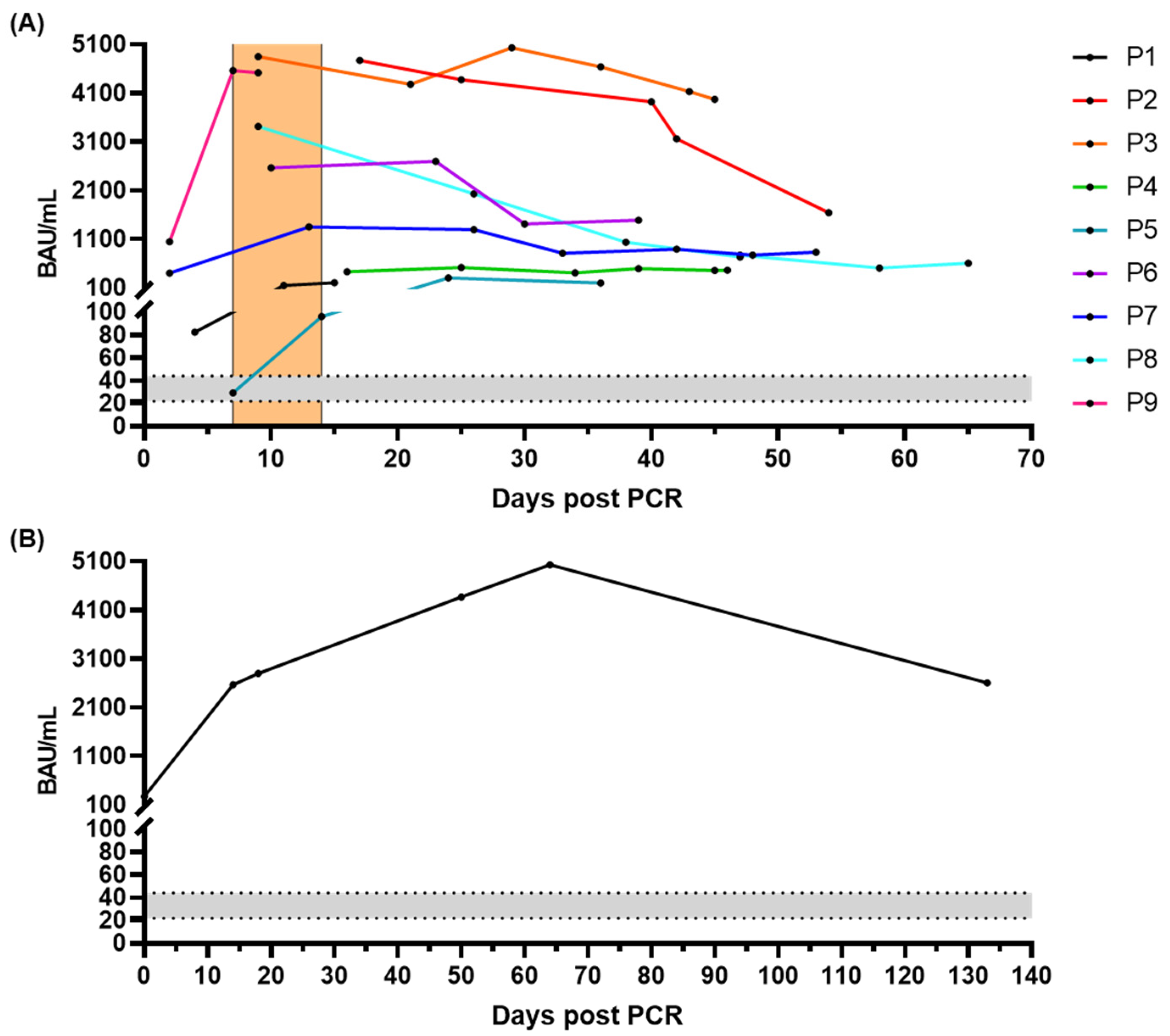
3.10. Time Course of Antibody Response after Infection
3.11. Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weekly Epidemiological Update on COVID-19. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---3-august-2022 (accessed on 3 August 2022).
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Chan, J.F.W.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.W.; Yuan, S.; Yuen, K.Y. Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Ganash, M.; Bakri, M.M.; Qanash, H.; Al-Rajhi, A.M.H.; Elhussieny, N.I. SARS-CoV-2, the Other Face to SARS-CoV and MERS-CoV: Future Predictions. Biomed. J. 2021, 44, 86–93. [Google Scholar] [CrossRef]
- FDA Combating COVID-19 with Therapeutics. Available online: https://www.fda.gov/media/136832/download (accessed on 6 August 2022).
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Biswasroy, P.; Kumar Naik, P.; Ghosh, G.; Rath, G. A Review of Current Interventions for COVID-19 Prevention. Arch. Med. Res. 2020, 51, 363–374. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19: Unravelling the Clinical Progression of Nature’s Virtually Perfect Biological Weapon. Ann. Transl. Med. 2020, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; el Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-COVID-19/treatments-vaccines/COVID-19-vaccines (accessed on 21 August 2022).
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the Novel Coronavirus from the Ongoing Wuhan Outbreak and Modeling of Its Spike Protein for Risk of Human Transmission. Sci. China Life Sci. 2020, 63, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.Y.; Wang, P.; Mok, B.W.Y.; Zhang, A.J.; Chu, H.; Lee, A.C.Y.; Deng, S.; Chen, P.; Chan, K.H.; Song, W.; et al. Attenuated SARS-CoV-2 Variants with Deletions at the S1/S2 Junction. Emerg. Microbes Infect. 2020, 9, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Berger, I.; Schaffitzel, C. The SARS-CoV-2 Spike Protein: Balancing Stability and Infectivity. Cell Res. 2020, 30, 1059–1060. [Google Scholar] [CrossRef]
- Deming, D.; Sheahan, T.; Heise, M.; Yount, B.; Davis, N.; Sims, A.; Suthar, M.; Harkema, J.; Whitmore, A.; Pickles, R.; et al. Vaccine Efficacy in Senescent Mice Challenged with Recombinant SARS-CoV Bearing Epidemic and Zoonotic Spike Variants. PLoS Med. 2006, 3, e525. [Google Scholar] [CrossRef]
- Yasui, F.; Kai, C.; Kitabatake, M.; Inoue, S.; Yoneda, M.; Yokochi, S.; Kase, R.; Sekiguchi, S.; Morita, K.; Hishima, T.; et al. Prior Immunization with Severe Acute Respiratory Syndrome (SARS)-Associated Coronavirus (SARS-CoV) Nucleocapsid Protein Causes Severe Pneumonia in Mice Infected with SARS-CoV. J. Immunol. 2008, 181, 6337–6348. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral Targets for Vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL (accessed on 6 August 2022).
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of Protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Eberhardt, K.A.; Dewald, F.; Heger, E.; Gieselmann, L.; Vanshylla, K.; Wirtz, M.; Kleipass, F.; Johannis, W.; Schommers, P.; Gruell, H.; et al. Evaluation of a New Spike (S)-Protein-Based Commercial Immunoassay for the Detection of Anti-SARS-CoV-2 IgG. Microorganisms 2021, 9, 733. [Google Scholar] [CrossRef]
- Vernet, R.; Charrier, E.; Grogg, J.; Mach, N. A Quantitative Elisa Protocol for Detection of Specific Human Igg against the SARS-CoV-2 Spike Protein. Vaccines 2021, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.; Ward, G. Interferences in Immunoassay. Clin. Biochem. Rev. 2004, 25, 105. [Google Scholar] [PubMed]
- McDade, T.W. Development and Validation of Assay Protocols for Use with Dried Blood Spot Samples. Am. J. Hum. Biol. 2014, 26, 1–9. [Google Scholar] [CrossRef]
- Gaugler, S.; Sottas, P.E.; Blum, K.; Luginbühl, M. Fully Automated Dried Blood Spot Sample Handling and Extraction for Serological Testing of SARS-CoV-2 Antibodies. Drug Test. Anal. 2021, 13, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Toh, Z.Q.; Higgins, R.A.; Anderson, J.; Mazarakis, N.; Do, L.A.H.; Rautenbacher, K.; Ramos, P.; Dohle, K.; Tosif, S.; Crawford, N.; et al. The Use of Dried Blood Spots for the Serological Evaluation of SARS-CoV-2 Antibodies. J. Public Health 2021, 44, e260–e263. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, C.; Croxatto, A.; Coste, A.T.; Pojer, F.; André, C.; Pellaton, C.; Farina, A. Crossm Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population—Based Seroprevalence Studies. J. Virol. 2021, 95, e01828-20. [Google Scholar] [CrossRef]
- Luo, J.; Brakel, A.; Krizsan, A.; Ludwig, T.; Mötzing, M.; Volke, D.; Lakowa, N.; Grünewald, T.; Lehmann, C.; Wolf, J.; et al. Sensitive and Specific Serological ELISA for the Detection of SARS-CoV-2 Infections. Virol. J. 2022, 19, 1–10. [Google Scholar] [CrossRef]
- Padoan, A.; Dall’Olmo, L.; della Rocca, F.; Barbaro, F.; Cosma, C.; Basso, D.; Cattelan, A.; Cianci, V.; Plebani, M. Antibody Response to First and Second Dose of BNT162b2 in a Cohort of Characterized Healthcare Workers. Clin. Chim. Acta 2021, 519, 60–63. [Google Scholar] [CrossRef]
- Samanovic, M.I.; Cornelius, A.R.; Wilson, J.P.; Karmacharya, T.; Gray-Gaillard, S.L.; Allen, J.R.; Hyman, S.W.; Moritz, G.; Ali, M.; Koralov, S.B.; et al. Poor Antigen-Specific Responses to the Second BNT162b2 MRNA Vaccine Dose in SARS-CoV-2-Experienced Individuals. medRxiv 2021. [Google Scholar] [CrossRef]
- Luo, S.; Xu, J.; Cho, C.Y.; Zhu, S.; Whittaker, K.C.; Wang, X.; Feng, J.; Wang, M.; Xie, S.; Fang, J.; et al. Quantitative Detection of Anti-SARS-CoV-2 Antibodies Using Indirect ELISA. Lab Med. 2022, 53, 225–234. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Salvagno, G.L.; Gelati, M.; Peserico, D.; Pighi, L.; de Nitto, S.; Henry, B.M.; Porru, S.; Lippi, G. Comparison of Five Commercial Anti-SARS-CoV-2 Total Antibodies and Igg Immunoassays after Vaccination with Bnt162b2 Mrna. J. Med. Biochem. 2021, 40, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Dashdorj, N.J.; Wirz, O.F.; Röltgen, K.; Haraguchi, E.; Buzzanco, A.S.; Sibai, M.; Wang, H.; Miller, J.A.; Solis, D.; Sahoo, M.K.; et al. Direct Comparison of Antibody Responses to Four SARS-CoV-2 Vaccines in Mongolia. Cell Host Microbe 2021, 29, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination with BNT162b2 and MRNA-1273. JAMA—J. Am. Med. Assoc. 2021, 326, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.P.Y.; Connolly, C.M.; Ruddy, J.A.; Boyarsky, B.J.; Alejo, J.L.; Werbel, W.A.; Massie, A.; Christopher-Stine, L.; Garonzik-Wang, J.; Segev, D.L.; et al. Antibody Response to the Janssen/Johnson & Johnson SARS-CoV-2 Vaccine in Patients with Rheumatic and Musculoskeletal Diseases. Ann. Rheum. Dis. 2021, 80, 1365–1366. [Google Scholar] [PubMed]
- Whitaker, H.J.; Tsang, R.S.M.; Byford, R.; Andrews, N.J.; Sherlock, J.; Sebastian Pillai, P.; Williams, J.; Button, E.; Campbell, H.; Sinnathamby, M.; et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 Vaccine Effectiveness and Immune Response amongst Individuals in Clinical Risk Groups. J. Infect. 2022, 84, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
| Diagnostic Parameter | Value |
|---|---|
| Quantitation range | 428.2 to 0.031 BAU/mL |
| LOD | 0.114 BAU/mL |
| LOQ | 0.345 BAU/mL |
| Precision | Inter-operator: 4.34% CV Inter-day: 7.83% CV |
| Reproducibility | 4.00% CV |
| Cutoff | 22 BAU/mL |
| Sensitivity | 100% [95% CI, 96.8% to 100.0%] |
| Specificity | 98.3% [95% CI, 95.0% to 99.6%] |
| Long-term stability | >29 d (37 °C) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Klett, J.; Gabert, J.; Lipp, T.; Karbach, J.; Jäger, E.; Borte, S.; Hoffmann, R.; Milkovska-Stamenova, S. A Quantitative ELISA to Detect Anti-SARS-CoV-2 Spike IgG Antibodies in Infected Patients and Vaccinated Individuals. Microorganisms 2022, 10, 1812. https://doi.org/10.3390/microorganisms10091812
Luo J, Klett J, Gabert J, Lipp T, Karbach J, Jäger E, Borte S, Hoffmann R, Milkovska-Stamenova S. A Quantitative ELISA to Detect Anti-SARS-CoV-2 Spike IgG Antibodies in Infected Patients and Vaccinated Individuals. Microorganisms. 2022; 10(9):1812. https://doi.org/10.3390/microorganisms10091812
Chicago/Turabian StyleLuo, Ji, Jennifer Klett, Jörg Gabert, Thomas Lipp, Julia Karbach, Elke Jäger, Stephan Borte, Ralf Hoffmann, and Sanja Milkovska-Stamenova. 2022. "A Quantitative ELISA to Detect Anti-SARS-CoV-2 Spike IgG Antibodies in Infected Patients and Vaccinated Individuals" Microorganisms 10, no. 9: 1812. https://doi.org/10.3390/microorganisms10091812
APA StyleLuo, J., Klett, J., Gabert, J., Lipp, T., Karbach, J., Jäger, E., Borte, S., Hoffmann, R., & Milkovska-Stamenova, S. (2022). A Quantitative ELISA to Detect Anti-SARS-CoV-2 Spike IgG Antibodies in Infected Patients and Vaccinated Individuals. Microorganisms, 10(9), 1812. https://doi.org/10.3390/microorganisms10091812





