The Multifunctional Role of Poloxamer P338 as a Biofilm Disrupter and Antibiotic Enhancer: A Small Step forward against the Big Trouble of Catheter-Associated Escherichia coli Urinary Tract Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial Strains
2.2. Cefoxitin Disk Diffusion and Minimum Inhibitory Concentration (MIC) Assays
2.3. Phenotypic AmpC Confirmation Testing
2.4. Biofilm Dispersion Assay of E. coli with P338 under Static Conditions
2.5. Biofilm Dispersion Assay of E. coli with P338 under Dynamic Conditions
2.6. Minimum Biofilm Eradication Concentration (MBEC) Assay
2.7. Anti-Biofilm Efficacy Test under Dynamic Conditions against E. coli with A Combination of P338 and Cefoxitin
2.8. Confocal Laser Scanning Electron Microscopy (CLSM)
2.9. Statistical Analysis
2.10. Ethical Statement
3. Results
3.1. Cefoxitin Resistance Profile
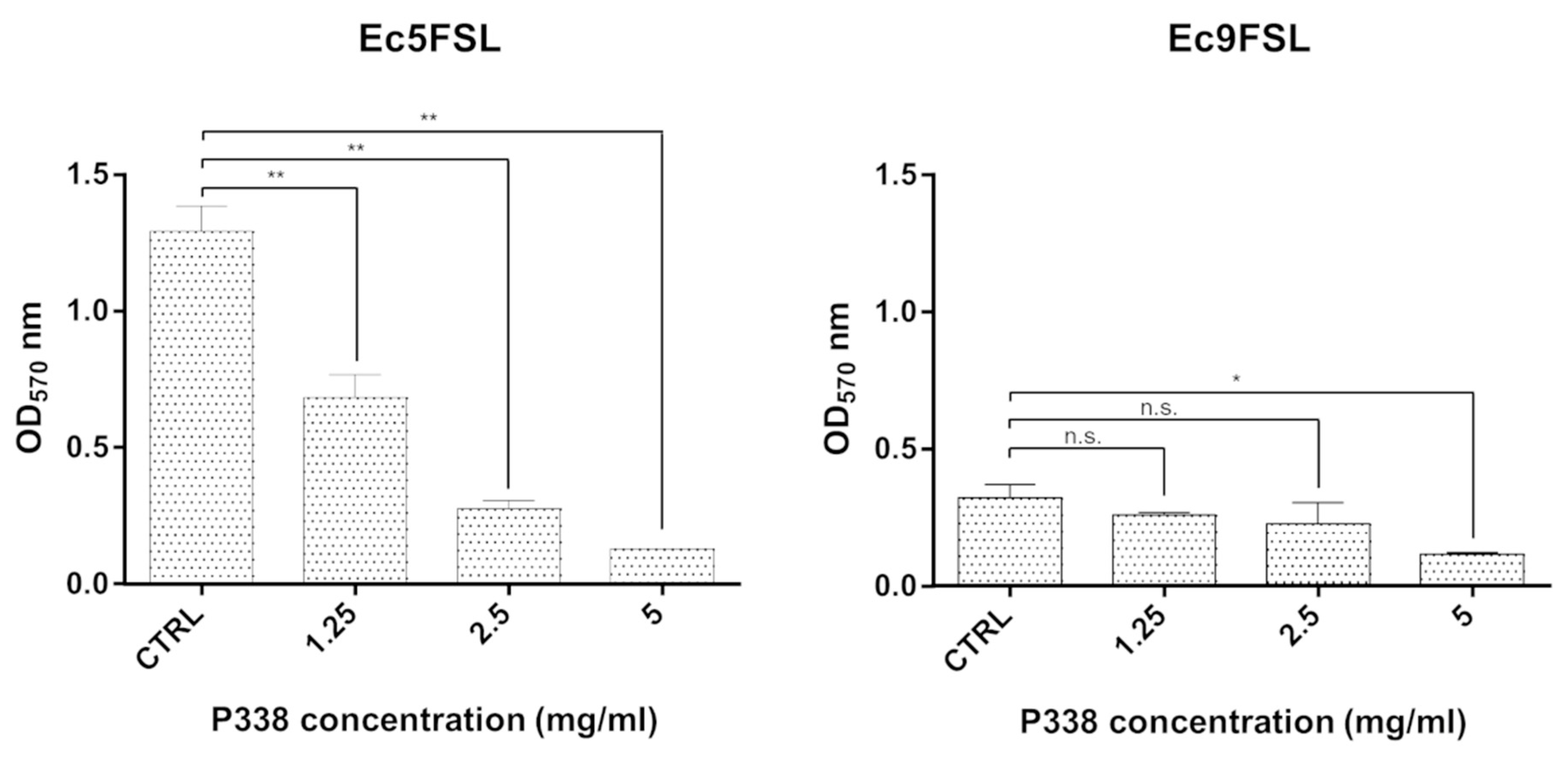
3.2. E. coli Biofilm Dispersion after P338 Treatment under Static Conditions
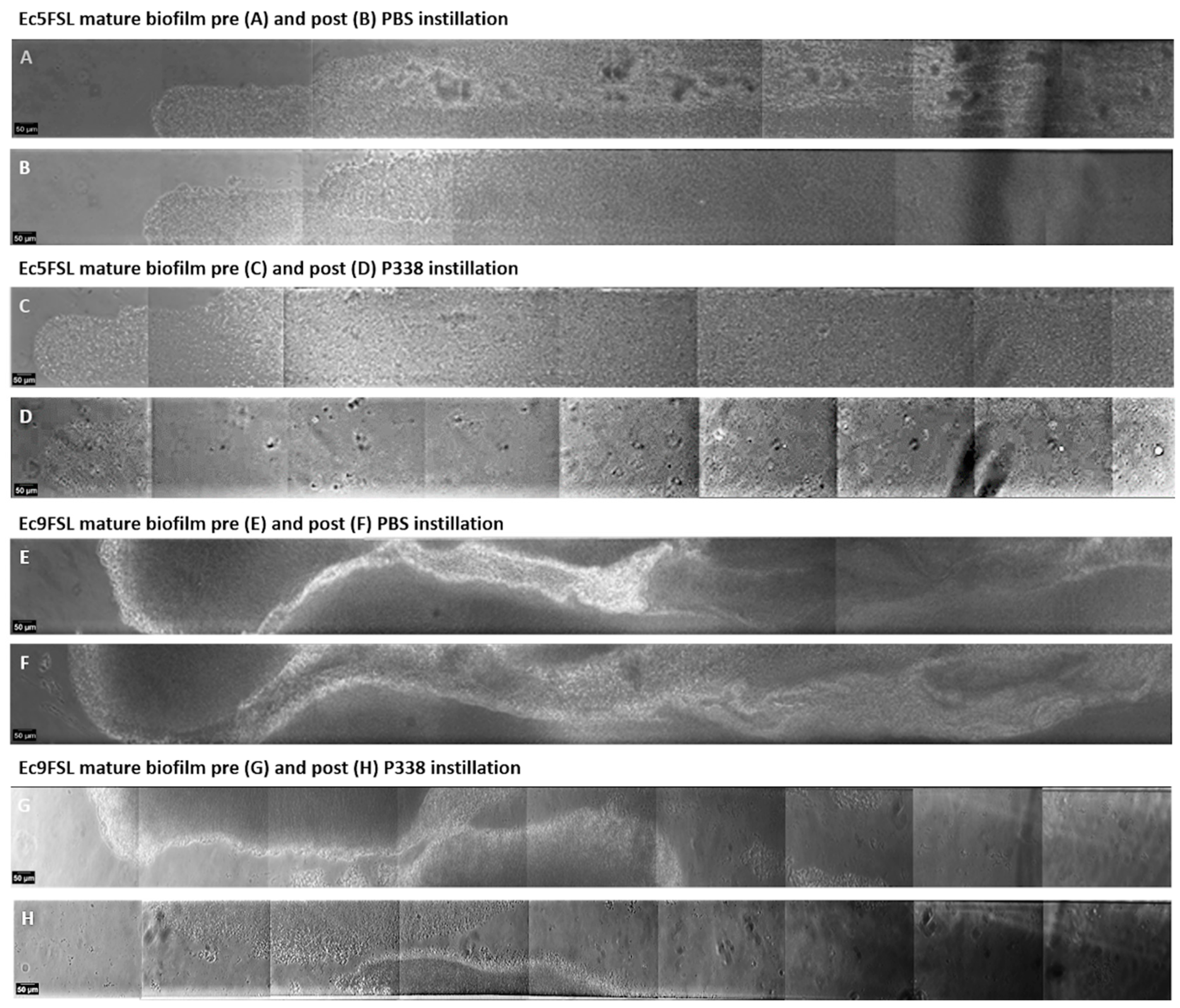
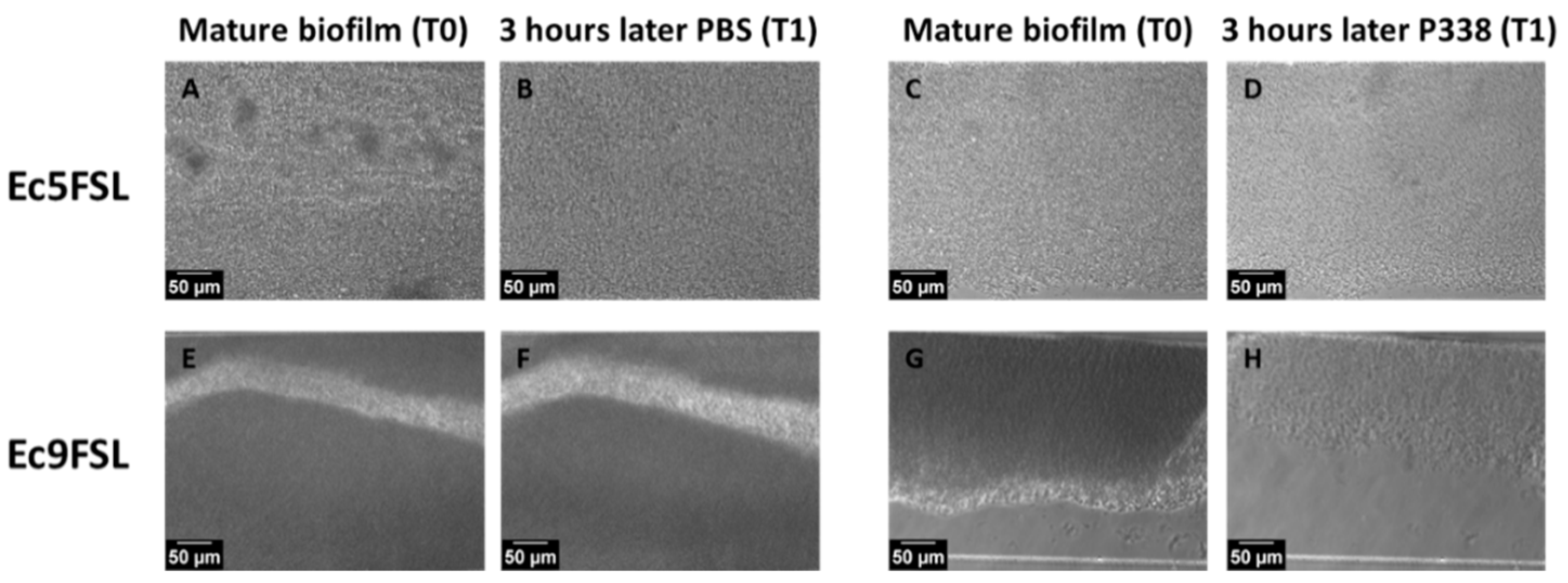
3.3. E. coli Biofilm Dispersion after P338 Treatment under Dynamic Conditions
3.4. Cefoxitin Minimum Biofilm Eradication Concentration (MBEC) against E. coli
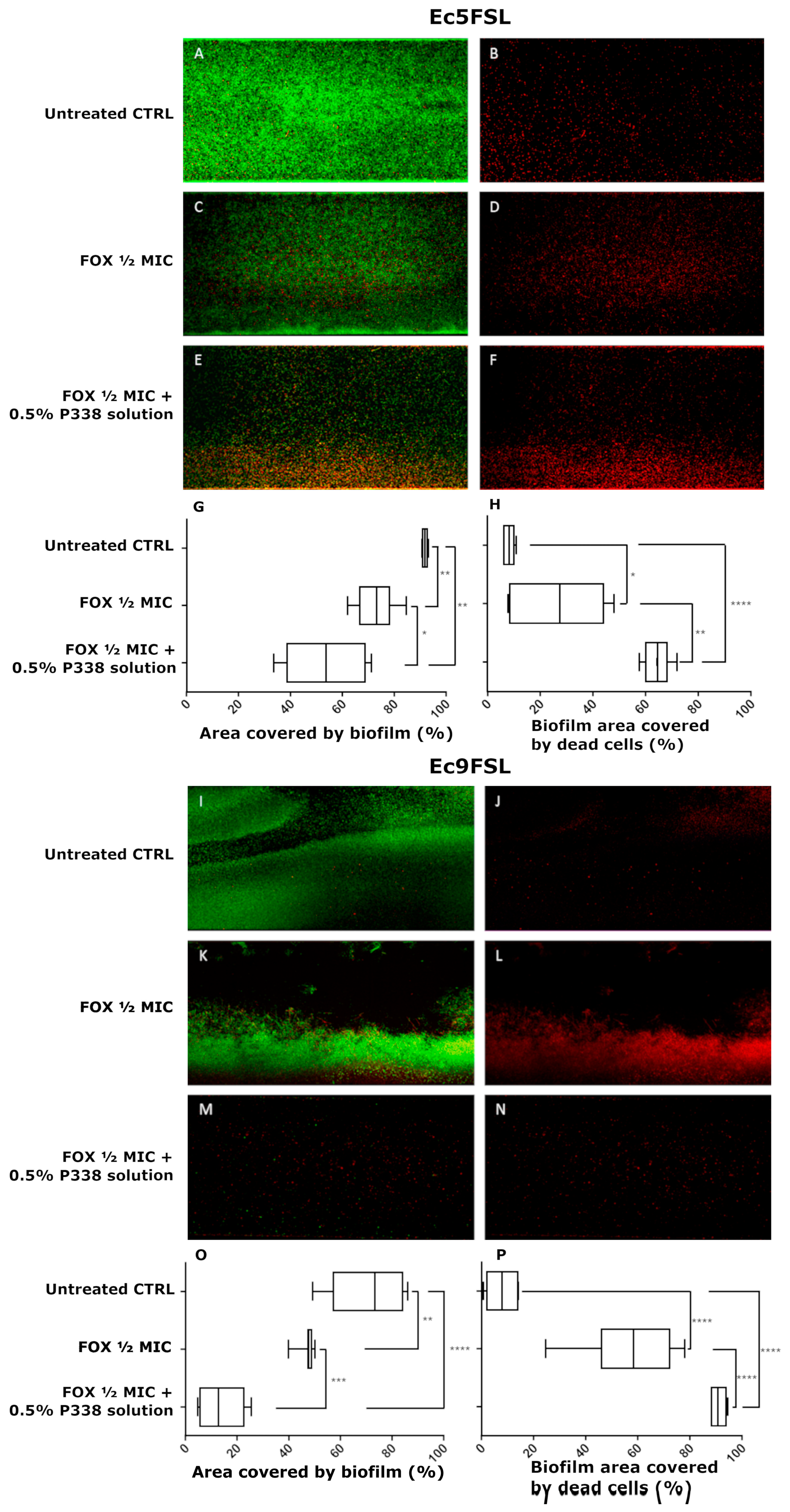
3.5. Anti-Biofilm Efficacy of a Combination of P338 and Cefoxitin against E. coli under Dynamic Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vuotto, C.; Donelli, G. Novel Treatment Strategies for Biofilm-Based Infections. Drugs 2019, 79, 1635–1655. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E.; Yoshikawa, T.T. Urinary tract infection in long-term-care facility residents. Clin. Infect. Dis. 2000, 31, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Pellowe, C.; Pratt, R. Catheter-associated urinary tract infections: Primary care guidelines. Nurs. Times 2004, 100, 53–55. [Google Scholar]
- Pury, J.; Mishra, B.; Mal, A.; Murthy, N.S.; Thakur, A.; Dogra, V.; Singh, D. Catheter associated urinary tract infections in neurology and neurosurgical units. J. Infect. 2002, 44, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Stickler, D.J. Bacterial biofilms in patients with indwelling urinary catheters. Nat. Clin. Pract. Urol. 2008, 5, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Donelli, G.; Vuotto, C. Biofilm-based infections in long-term care facilities. Future Microbiol. 2014, 9, 175–188. [Google Scholar] [CrossRef]
- Maharjan, G.; Khadka, P.; Siddhi Shilpakar, G.; Chapagain, G.; Dhungana, G.R. Catheter-Associated Urinary Tract Infection and Obstinate Biofilm Producers. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 7624857. [Google Scholar] [CrossRef]
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambyah, P.A.; Tenke, P.; et al. Diagnosis, prevention and treatment of catheter-associated urinary tract infection in adults; 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 625–663. [Google Scholar] [CrossRef]
- Bahramian, A.; Khoshnood, S.; Hashemi, N.; Moradi, M.; Karimi-Yazdi, M.; Jalallou, N.; Saki, M. Identification of metallo-β-lactamases and AmpC production among Escherichia coli strains isolated from hemodialysis patients with urinary tract infection. Mol. Biol. 2021, 48, 7883–7892. [Google Scholar] [CrossRef]
- Neupane, S.; Pant, N.D.; Khatiwada, S.; Chaudhary, R.; Banjara, M.R. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob. Resist. Infect. Control 2016, 5, 5. [Google Scholar] [CrossRef]
- Lee, S.; An, J.U.; Guk, J.H.; Song, H.; Yi, S.; Kim, W.H.; Cho, S. Prevalence, Characteristics and Clonal Distribution of Extended-Spectrum β-Lactamase- and AmpC β-Lactamase-Producing Escherichia coli Following the Swine Production Stages, and Potential Risks to Humans. Front. Microbiol. 2021, 12, 710747. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Mayer, D.; Malone, M.; Swanson, T.; Gibson, D.; Schultz, G. Surfactants and their role in wound cleansing and biofilm management. J. Wound Care 2017, 26, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Chen, R.; Mayer, D.; Salisbury, A.M. Mode of action of poloxamer-based surfactants in wound care and efficacy on biofilms. Int. Wound J. 2018, 15, 749–755. [Google Scholar] [CrossRef]
- Alvarado-Gomez, E.; Martínez-Castañon, G.; Sanchez-Sanchez, R.; Ganem-Rondero, A.; Yacaman, M.J.; Martinez-Gutierrez, F. Evaluation of anti-biofilm and cytotoxic effect of a gel formulation with Pluronic F-127 and silver nanoparticles as a potential treatment for skin wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 621–630. [Google Scholar] [CrossRef]
- Stirpe, M.; Brugnoli, B.; Donelli, G.; Francolini, I.; Vuotto, C. Poloxamer 338 affects cell adhesion and biofilm formation in Escherichia coli: Potential applications in the management of catheter-associated urinary tract infections. Pathogens 2020, 9, 88. [Google Scholar] [CrossRef]
- Kozlov, M.Y.; Melik-Nubarov, N.S.; Batrakova, E.V.; Kabanov, A.V. Relationship between Pluronic Block Copolymer Structure, Critical Micellization Concentration and Partitioning Coefficients of Low Molecular Mass Solutes. Macromolecules 2000, 33, 3305–3313. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters. EUCAST Clinical Breakpoint Tables v. 12.0, valid from 2022-01-01. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 26 August 2022).
- Marialouis, X.A.; Santhanam, A. Antibiotic Resistance, RAPD- PCR Typing of Multiple Drug Resistant Strains of Escherichia coli From Urinary Tract Infection (UTI). J. Clin. Diagn. Res. 2016, 10, DC05–DC09. [Google Scholar] [CrossRef]
- Benmouna, Z.; Dalache, F.; Zadi-Karam, H.; Karam, N.E.; Vuotto, C. Ability of three lactic acid bacteria to grow in sessile mode and to inhibit biofilm formation of pathogenic bacteria. Adv. Exp. Med. Biol. 2020, 1282, 105–114. [Google Scholar] [CrossRef]
- Díaz De Rienzo, M.A.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Pseudomonas aeruginosa biofilm disruption using microbial surfactants. J. Appl. Microbiol. 2016, 120, 868–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, M.; Rong, L.; Raad, I.; Versalovic, J. Novel synergistic antibiofilm combinations for salvage of infected catheters. J. Med. Microbiol. 2009, 58, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Samarian, D.; Jakubovics, N.; Luo, T.; Rickard, A. Use of a High-throughput In Vitro Microfluidic System to Develop Oral Multi-Species Biofilms. J. Vis. Exp. 2014, 94, e52467. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Version 2.01 July 2017. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 26 August 2022).
- Vuotto, C.; Moura, I.; Barbanti, F.; Donelli, G.; Spigaglia, P. Subinhibitory concentrations of metronidazole increase biofilm formation in Clostridium difficile strains. Pathog. Dis. 2016, 74, 114. [Google Scholar] [CrossRef] [PubMed]
- Schierholz, J.M.; Beuth, J. Implant infections: A haven for opportunistic bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Singh-Joy, S.D.; McLain, V.C. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, Poloxamer 105 benzoate and Poloxamer 182 dibenzoate as used in cosmetics. Int. J. Toxicol. 2008, 27, 93–128. [Google Scholar] [CrossRef]
- Yang, Q.; Larose, C.; Della Porta, A.C.; Schultz, G.S.; Gibson, D.J. A surfactant-based wound dressing can reduce bacterial biofilms in a porcine skin explant model. Int. Wound J. 2017, 14, 408–413. [Google Scholar] [CrossRef]
- McCoy, C.P.; Irwin, N.J.; Donnelly, L.; Jones, D.S.; Hardy, J.G.; Carson, L. Anti-Adherent Biomaterials for Prevention of Catheter Biofouling. Int. J. Pharm. 2018, 535, 420–427. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. New Adapted In Vitro Technology to Evaluate Biofilm Formation and Antibiotic Activity Using Live Imaging under Flow Conditions. Diagnostics 2021, 11, 1746. [Google Scholar] [CrossRef]
- Dong, L.; Tong, Z.; Linghu, D.; Lin, Y.; Tao, R.; Liu, J.; Tian, Y.; Ni, L. Effects of sub-minimum inhibitory concentrations of antimicrobial agents on Streptococcus mutans biofilm formation. Int. J. Antimicrob. Agents 2012, 39, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Krzyżek, P.; Dworniczek, E.; Adamski, R.; Sroka, Z. In Silico Screening and In Vitro Assessment of Natural Products with Anti-Virulence Activity against Helicobacter pylori. Molecules 2021, 21, 20. [Google Scholar] [CrossRef]
- Díaz De Rienzo, M.A.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Effect of biosurfactants on Pseudomonas aeruginosa and Staphylococcus aureus biofilms in a BioFlux channel. Appl. Microbiol. Biotechnol. 2016, 100, 5773–5779. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and Antimicrobial-Enhancing Activity of Chelidonium majus and Corydalis cheilanthifolia Extracts against Multidrug-Resistant Helicobacter pylori. Pathogens 2021, 16, 1033. [Google Scholar] [CrossRef] [PubMed]
- Díez-Aguilar, M.; Morosini, M.I.; Köksal, E.; Oliver, A.; Ekkelenkamp, M.; Cantón, R. Use of Calgary and Microfluidic BioFlux Systems To Test the Activity of Fosfomycin and Tobramycin Alone and in Combination against Cystic Fibrosis Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2017, 62, e01650-17. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, T.; Ghebeh, H.; Wuerz, T.; Butler, M. Pluronic enhances the robustness and reduces the cell attachment of mammalian cells. Mol. Biotechnol. 2008, 39, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Das Ghatak, P.; Mathew-Steiner, S.S.; Pandey, P.; Roy, S.; Sen, C.K. A surfactant polymer dressing potentiates antimicrobial efficacy in biofilm disruption. Sci. Rep. 2018, 8, 873. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Carreras, I. Activities of beta-lactam antibiotics against Escherichia coli strains producing extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 1990, 34, 858–862. [Google Scholar] [CrossRef]
- Lepeule, R.; Ruppé, E.; Le, P.; Massias, L.; Chau, F.; Nucci, A.; Lefort, A.; Fantin, B. Cefoxitin as an alternative to carbapenems in a murine model of urinary tract infection due to Escherichia coli harboring CTX-M-15-type extended-spectrum β-lactamase. Antimicrob. Agents Chemother. 2012, 56, 1376–1381. [Google Scholar] [CrossRef]
- Kernéis, S.; Valade, S.; Geri, G.; Compain, F.; Lavollay, M.; Rostane, H.; Carbonnelle, E.; Mainardi, J.L. Cefoxitin as a carbapenem-sparing antibiotic for infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Dis. 2015, 47, 789–795. [Google Scholar] [CrossRef]
- El Nekidy, W.S.; Abdelsalam, M.M.; Nusair, A.R.; El Lababidi, R.; Dajani, R.Z.; St. John, T.J.L.; Ghazi, I.M. Is Cefoxitin a Carbapenem Sparing Agent in the Management of Urinary Tract Infections Caused by ESBL Producing Enterobacterales? Hosp. Pharm. 2021, 57, 568–574. [Google Scholar] [CrossRef]
- Senard, O.; Bouchand, F.; Deconinck, L.; Matt, M.; Fellous, L.; Rottman, M.; Perronne, C.; Dinh, A.; Davido, B. Efficacy of cefoxitin for the treatment of urinary tract infection due to extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates. Ther. Adv. Infect. Dis. 2019, 6, 204993611881105. [Google Scholar] [CrossRef] [PubMed]
- Senard, O.; Lafaurie, M.; Lesprit, P.; Nguyen, Y.; Lescure, X.; Therby, A.; Fihman, V.; Oubaya, N.; Lepeule, R. Efficacy of cefoxitin versus carbapenem in febrile male urinary tract infections caused by extended spectrum beta-lactamase-producing Escherichia coli: A multicenter retrospective cohort study with propensity score analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]


| Strains | FOX Zone Diameter a (mm) | FOX MIC c (mg/L) | Interpretation |
|---|---|---|---|
| Ec5FSL | 23 b | 2 | S |
| Ec9FSL | 16 b | 8 | R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henrici De Angelis, L.; Stirpe, M.; Tomolillo, D.; Donelli, G.; Francolini, I.; Vuotto, C. The Multifunctional Role of Poloxamer P338 as a Biofilm Disrupter and Antibiotic Enhancer: A Small Step forward against the Big Trouble of Catheter-Associated Escherichia coli Urinary Tract Infections. Microorganisms 2022, 10, 1757. https://doi.org/10.3390/microorganisms10091757
Henrici De Angelis L, Stirpe M, Tomolillo D, Donelli G, Francolini I, Vuotto C. The Multifunctional Role of Poloxamer P338 as a Biofilm Disrupter and Antibiotic Enhancer: A Small Step forward against the Big Trouble of Catheter-Associated Escherichia coli Urinary Tract Infections. Microorganisms. 2022; 10(9):1757. https://doi.org/10.3390/microorganisms10091757
Chicago/Turabian StyleHenrici De Angelis, Lucia, Mariarita Stirpe, Dario Tomolillo, Gianfranco Donelli, Iolanda Francolini, and Claudia Vuotto. 2022. "The Multifunctional Role of Poloxamer P338 as a Biofilm Disrupter and Antibiotic Enhancer: A Small Step forward against the Big Trouble of Catheter-Associated Escherichia coli Urinary Tract Infections" Microorganisms 10, no. 9: 1757. https://doi.org/10.3390/microorganisms10091757
APA StyleHenrici De Angelis, L., Stirpe, M., Tomolillo, D., Donelli, G., Francolini, I., & Vuotto, C. (2022). The Multifunctional Role of Poloxamer P338 as a Biofilm Disrupter and Antibiotic Enhancer: A Small Step forward against the Big Trouble of Catheter-Associated Escherichia coli Urinary Tract Infections. Microorganisms, 10(9), 1757. https://doi.org/10.3390/microorganisms10091757









