Unraveling the Genomic Potential of the Thermophilic Bacterium Anoxybacillus flavithermus from an Antarctic Geothermal Environment
Abstract
1. Introduction
2. Material and Methods
2.1. Knowledge Discovery in Databases: A Systematic Review of the Literature and Molecular Databases
2.2. Sampling Site, Isolation, and Culture Conditions
2.3. DNA Extraction and Genome Sequencing, Assembly, and Annotation
2.4. Phylogenomic Analysis
2.5. Comparative Genomic Analyses
3. Results
3.1. Deep Mining of Anoxybacillus: Knowledge Discovery in Databases
3.2. Genome Features of Anoxybacillus Strains
3.3. Global Similarity and Supermatrix Phylogenomic Analyses
3.4. Potential Functions of Thermophilic A. flavithermus Strains in Cold Environments
4. Discussion
4.1. Adaptations to the Antarctic Geothermal Environments
4.2. A. Flavithermus as Potential Candidate for Biotechnological Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pikuta, E.; Lysenko, A.; Chuvilskaya, N.; Mendrock, U.; Hippe, H.; Suzina, N.; Nikitin, D.; Osipov, G.; Laurinavichius, K. Anoxybacillus pushchinensis gen. nov., sp. nov., a novel anaerobic, alkaliphilic, moderately thermophilic bacterium from manure, and de-scription of Anoxybacillus flavitherms comb. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 2109–2117. [Google Scholar]
- Heinen, W.; Lauwers, A.M.; Mulders, J.W.M. Bacillus flavothermus, a newly isolated facultative thermophile. Antonie van Leeuwenhoek 1982, 48, 265–272. [Google Scholar] [CrossRef]
- Caputo, A.; Fournier, P.-E.; Raoult, D. Genome and pan-genome analysis to classify emerging bacteria. Biol. Direct 2019, 14, 5. [Google Scholar] [CrossRef]
- Lalucat, J.; Mulet, M.; Gomila, M.; García-Valdés, E. Genomics in Bacterial Taxonomy: Impact on the Genus Pseudomonas. Genes 2020, 11, 139. [Google Scholar] [CrossRef]
- Caspers, M.P.M.; Boekhorst, J.; Abee, T.; Siezen, R.J.; Kort, R. Complete Genome Sequence of Anoxybacillus flavithermus TNO-09.006, a Thermophilic Sporeformer Associated with a Dairy-Processing Environment. Genome Announc. 2013, 1, e00010-13. [Google Scholar] [CrossRef]
- Saw, J.H.; Mountain, B.W.; Feng, L.; Omelchenko, M.V.; Hou, S.; Saito, J.A.; Stott, M.B.; Li, D.; Zhao, G.; Wu, J.; et al. Encapsulated in silica: Genome, proteome and physiology of the thermophilic bacterium Anoxybacillus flavithermus WK1. Genome Biol. 2008, 9, R161. [Google Scholar] [CrossRef]
- Tasara, T.; Morach, M.; Klumpp, J.; Stephan, R. Complete Genome Sequence of Anoxybacillus flavithermus Strain 52-1A Isolated from a Heat-Processed Powdered Milk Concentrate. Genome Announc. 2017, 5, e00800-17. [Google Scholar] [CrossRef]
- Goh, K.M.; Gan, H.M.; Chan, K.-G.; Chan, G.F.; Shahar, S.; Chong, C.S.; Kahar, U.M.; Chai, K.P. Analysis of Anoxybacillus Genomes from the Aspects of Lifestyle Adaptations, Prophage Diversity, and Carbohydrate Metabolism. PLoS ONE 2014, 9, e90549. [Google Scholar] [CrossRef]
- Margaryan, A.; Shahinyan, G.; Hovhannisyan, P.; Panosyan, H.; Birkeland, N.K.; Trchounian, A. Geobacillus and Anoxybacillus spp. from terrestrial geothermal springs worldwide: Diversity and biotechnological applications. In Extremophiles in Eurasian ecosystems: Ecology, Diversity, and Applications; Egamberdieva, D., Birkeland, N.K., Panosyan, H., Li, W.J., Eds.; Springer: Singapore, 2021; Volume 8, pp. 119–166. [Google Scholar]
- Poli, A.; Esposito, E.; Lama, L.; Orlando, P.; Nicolaus, G.; de Appolonia, F.; Gambacorta, A.; Nicolaus, B. Anoxybacillus amylolyticus sp. nov., a thermophilic amylase producing bacterium isolated from Mount Rittmann (Antarctica). Syst. Appl. Microbiol. 2006, 29, 300–307. [Google Scholar] [CrossRef]
- Bendia, A.G.; Araujo, G.G.; Pulschen, A.A.; Contro, B.; Duarte, R.T.D.; Rodrigues, F.; Galante, D.; Pellizari, V.H. Surviving in hot and cold: Psychrophiles and thermophiles from Deception Island volcano, Antarctica. Extremophiles 2018, 22, 917–929. [Google Scholar] [CrossRef]
- Schultz, J.; Rosado, A.S. Extreme environments: A source of biosurfactants for biotechnological applications. Extremophiles 2019, 24, 189–206. [Google Scholar] [CrossRef]
- Antranikian, G.; Egorova, K. Extremophiles, a unique resource of biocatalysts for industrial biotechnology. In Physiology and Biochemistry of Extremophiles; Gerday, C., Glansdorff, N., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 361–406. [Google Scholar]
- Schultz, J.; Rosado, A.S. 3. Use of microbes from extreme environments for biotechnological applications. De Gruyter 2019, 33–56. [Google Scholar] [CrossRef]
- Schultz, J.; Argentino, I.C.V.; Kallies, R.; da Rocha, U.N.; Rosado, A.S. Polyphasic Analysis Reveals Potential Petroleum Hydrocarbon Degradation and Biosurfactant Production by Rare Biosphere Thermophilic Bacteria from Deception Island, an Active Antarctic Volcano. Front. Microbiol. 2022, 13, 885557. [Google Scholar] [CrossRef]
- Costa, C.; Fanelli, E.; Marini, S.; Danovaro, R.; Aguzzi, J. Global Deep-Sea Biodiversity Research Trends Highlighted by Science Mapping Approach. Front. Mar. Sci. 2020, 7, 384. [Google Scholar] [CrossRef]
- Martí, J.; Geyer, A.; Aguirrediaz, G.J. Origin and evolution of the Deception Island caldera (South Shetland Islands, Antarctica). Bull. Volcanol. 2013, 75, 732. [Google Scholar] [CrossRef]
- Herbold, C.W.; McDonald, I.R.; Cary, S.C. Mocrobial ecology of geothermal habitats in Antarctica. In Antartic Terrestrial Microbiology; Cowan, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 181–215. [Google Scholar]
- Kusakabe, M.; Nagao, K.; Ohba, T.; Seo, J.H.; Park, S.-H.; Lee, J.I.; Park, B.-K. Noble gas and stable isotope geochemistry of thermal fluids from Deception Island, Antarctica. Antarct. Sci. 2009, 21, 255–267. [Google Scholar] [CrossRef]
- Blanco, Y.; Prieto-Ballesteros Gómez, M.J.; Moreno-Paz, M.; García-Villadangos, M.; Rodríguez-Manfredi, J.A.; Cruz-Gil, P.; Sánchez-Román, M.; Rivas, L.; Parro, V. Prokaryotic communities and operating metabolisms in the surface and the permafrost of Deception Island (Antarctica). Environ. Microbiol. 2012, 14, 2495–2510. [Google Scholar]
- Somoza, L.; Martínez-Frías, J.; Smellie, J.L.; Rey, J.; Maestro, A. Evidence for hydrothermal venting and sediment volcanism dis-charged after recent short-lived volcanic eruptions at Deception Island, Bransfield Strait, Antarctica. Mar. Geol. 2004, 203, 119–140. [Google Scholar]
- Bendia, A.; Signori, C.; Franco, D.; Duarte, R.; Bohannan, B.J.M.; Pellizari, V.H. A Mosaic of Geothermal and Marine Features Shapes Microbial Community Structure on Deception Island Volcano, Antarctica. Front. Microbiol. 2018, 9, 899. [Google Scholar] [CrossRef]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar]
- Méndez, C.; Brana, A.F.; Manzanal, M.B.; Hardisson, C. Role of substrate mycelium in colony development in Streptomyces. Can. J. Microbiol. 1985, 31, 446–450. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Micro-biol. Lett. 1999, 170, 265–270. [Google Scholar]
- Lane, D. Nucleic acid techniques in bacterial systematics. In 16S/23S rRNA Sequencing; Stackebrandt, E., Good Fellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Sanger, F.; Air, G.M.; Barrell, B.G.; Brown, N.L.; Coulson, A.R.; Fiddes, J.C.; Hutchison, I.I.I.C.A.; Slocombe, P.M.; Smith, M. Nucleotide se-quence of bacteriophage φX174 DNA. Nature 1977, 265, 687–695. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinformatics; Babraham Institute: Cambridge, UK, 2010; Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 June 2021).
- Lindgreen, S. AdapterRemoval: Easy cleaning of next-generation sequencing reads. BMC Res. Notes 2012, 5, 337. [Google Scholar] [CrossRef]
- Melsted, P.; Bjarni, V.H. KmerStream: Streaming algorithms for k-mer abundance estimation. Bioinformatics 2014, 30, 3541–3547. [Google Scholar]
- Hernandez, D.; François, P.; Farinelli, L.; Østerås, M.; Schrenzel, J. De novo bacterial genome sequencing: Millions of very short reads assembled on a desktop computer. Genome Res. 2008, 18, 802–809. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [CrossRef]
- Araujo, F.; Barh, D.; Silva, A.; Guimarães, L.C.; Ramos, R.T.J. GO FEAT: A rapid web-based functional annotation tool for genomic and transcriptomic data. Sci. Rep. 2018, 8, 1794. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Blin, K.; Simon, S.; Alexander, M.K.; Zach, C.-P.; Gilles, P.V.W.; Marnix, H.M.; Tilmann, W. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar]
- van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-Time Whole-Genome Sequencing for Routine Typing, Surveillance, and Outbreak Detection of Verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Soares, S.C.; Geyik, H.; Ramos, R.T.; de Sá, P.H.; Barbosa, E.G.; Baumbach, J.; Figueiredo, H.C.; Miyoshi, A.; Tauch, A.; Silva, A.; et al. GIPSy: Genomic island prediction software. J. Biotechnol. 2015, 232, 2–11. [Google Scholar] [CrossRef]
- Padilha, V.A.; Omer, S.A.; Shiraz, A.S.; André, C.; Rolf, B. CRISPRcasIdentifier: Machine learning for accurate identification and classification of CRISPR-Cas systems. GigaScience 2020, 9, giaa062. [Google Scholar]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Glöckner, F.O.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin, Germany, 2016. [Google Scholar]
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef]
- Riley, R.; Nagy, L. Fungal phylogenomics. Methods Mol. Biol. 2018, 1775, 251–266. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome com-parisons. BMC Genom. 2011, 12, 402. [Google Scholar]
- Silas, S.; Lucas-Elio, P.; Jackson, S.A.; Aroca-Crevillen, A.; Hansen, L.L.; Fineran, P.C.; Sanchez-Amat, A. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived var-iants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar]
- Poli, A.; Romano, I.; Cordella, P.; Orlando, P.; Nicolaus, B.; Berrini, C.C. Anoxybacillus thermarum sp. nov., a novel thermophilic bacterium isolated from thermal mud in Euganean hot springs, Abano Terme, Italy. Extremophiles 2009, 13, 867–874. [Google Scholar] [CrossRef]
- Tiwary, B.K. Evolutionary pan-genomics and applications, chapter 3. In Pan-Genomics: Applications, Challenges, and Future Prospects; Barh, D., Soares, S., Tiwari, S., Azevedo, V., Eds.; Academic Press: London, UK, 2020; pp. 65–80. [Google Scholar]
- Wendling, C.C.; Refardt, D.; Hall, A.R. Fitness benefits to bacteria of carrying prophages and prophage-encoded antibi-otic-resistance genes peak in different environments. Evolution 2021, 75, 515–528. [Google Scholar]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The biology of CRISPR-Cas: Backward and forward. Cell 2018, 172, 1239–1259. [Google Scholar]
- Jore, M.M.; Lundgren, M.; Van Duijn, E.; Bultema, J.B.; Westra, E.R.; Waghmare, S.P.; Wiedenheft, B.; Pul, Ü.; Wurm, R.; Wagner, R.; et al. Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nat. Struct. Mol. Biol. 2011, 18, 529–536. [Google Scholar] [CrossRef]
- Wiedenheft, B.; Lander, G.C.; Zhou, K.; Jore, M.M.; Brouns, S.; Van Der Oost, J.; Doudna, J.A.; Nogales, E. Structures of the RNA-guided surveillance complex from a bacterial immune system. Nature 2011, 477, 486–489. [Google Scholar] [CrossRef]
- Stetter, K.O. Extremophiles and their adaptation to hot environments. FEBS Lett. 1999, 452, 22–25. [Google Scholar] [CrossRef]
- Wang, Q.; Cen, Z.; Zhao, J. The Survival Mechanisms of Thermophiles at High Temperatures: An Angle of Omics. Physiology 2015, 30, 97–106. [Google Scholar] [CrossRef]
- Michael, A.J. Biosynthesis of polyamines and polyamine-containing molecules. Biochem. J. 2016, 473, 2315–2329. [Google Scholar] [CrossRef]
- Marsden, C.G.; Dragon, J.A.; Wallace, S.S.; Sweasy, J.B. Base Excision Repair Variants in Cancer. Methods Enzymol. 2017, 591, 119–157. [Google Scholar] [CrossRef]
- Phadtare, S. Recent developments in bacterial cold-shock response. Curr. Issues Mol. Biol. 2004, 6, 125–136. [Google Scholar]
- von König, K.; Kachel, N.; Kalbitzer, H.R.; Kremer, W. RNA and DNA binding epitopes of the cold shock protein tmcsp from the hy-perthermophile Thermotoga maritima. Protein J. 2020, 39, 487–500. [Google Scholar]
- Nelson, K.E.; Clayton, R.A.; Gill, S.R.; Gwinn, M.L.; Dodson, R.J.; Haft, D.H.; Hickey, E.K.; Peterson, J.D.; Nelson, W.C.; Ketchum, K.A.; et al. Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima. Nature 1999, 399, 323–329. [Google Scholar]
- Galperin, M.Y.; Mekhedov, S.L.; Puigbo, P.; Smirnov, S.; Wolf, Y.I.; Rigden, D.J. Genomic determinants of sporulation in Bacilli and Clostridia: Towards the minimal set of sporulation-specific genes. Environ. Microbiol. 2012, 14, 2870–2890. [Google Scholar]
- Zhao, Y.; Caspers, M.P.M.; Metselaar, K.I.; de Boer, P.; Roeselers, G.; Moezelaar, R.; Groot, M.N.; Montijn, R.C.; Abee, T.; Kort, R. Abiotic and Microbiotic Factors Controlling Biofilm Formation by Thermophilic Sporeformers. Appl. Environ. Microbiol. 2013, 79, 5652–5660. [Google Scholar] [CrossRef]
- Burgess, S.A.; Lindsay, D.; Flint, S.H. Thermophilic bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010, 144, 215–225. [Google Scholar] [CrossRef]
- Lamichhane, G.; Ashis, A.; Darbin, K.P.; Babita, A.; Narayan, G.; Purushottam, N.; Sita Ram, P.; Prakriti, B.; Ganesh, B.K.; Niranjan, P. Recent advances in bioethanol production from lignocellulosic biomass. Int. J. Green Energy 2021, 18, 731–744. [Google Scholar]
- Chan, C.S.; Sin, L.L.; Chan, K.-G.; Shamsir, M.S.; Manan, F.A.; Sani, R.K.; Goh, K.M. Characterization of a glucose-tolerant β-glucosidase from Anoxybacillus sp. DT3-1. Biotechnol. Biofuels 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Goh, K.M.; Kahar, U.M.; Chai, Y.Y.; Chong, C.S.; Chai, K.P.; Ranjani, V.; Illias, R.; Chan, K.G. Recent discoveries and applications of Anoxybacillus. Appl. Microbiol. Biotechnol. 2013, 97, 1475–1488. [Google Scholar] [CrossRef]
- Bolton, D.J.; Kelly, C.T.; Fogarty, W.M. Purification and characterization of the α-amylase of Bacillus flavothermus. Enzym. Microb. Technol. 1997, 20, 340–343. [Google Scholar] [CrossRef]
- Ozdemir, S.; Okumus, V.; Ulutas, M.S.; Dundar, A.; Akarsubası, A.T.; Dumonted, S. Isolation of a novel thermophilic Anoxybacillus flavithermus SO-13, production, characterization and industrial applications of its thermostable α-amylase. J. Bioprocess. Biotech. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Ozdemir, S.; Okumus, V.; Ulutas, M.S. Production and characterization of thermostable α-amylase from thermophilic An-oxybacillus flavithermus sp. nov. SO-19. Starch 2016, 68, 1244–1253. [Google Scholar]
- Acer, Ö.; Pırınççiğlu, H.; Bekler, F.M.; Gül-Güven, R. Anoxybacillus sp. AH1, an α-amylase-producing thermophilic bacterium iso-lated from Dargeçit hot spring. Biologia 2015, 70, 853–862. [Google Scholar]
- Al-Kahem Al-Balawi, T.H.; Wood, A.L.; Solis, A.; Cooper, T.; Barabote, R.D. Anoxybacillus sp. strain UARK-01, a new thermophilic soil bacterium with hyperthermostable alkaline laccase activity. Curr. Microbiol. 2017, 74, 762–771. [Google Scholar]
- Atanassova, M.; Derekova, A.; Mandeva, R.; Sjoholm, C.; Kambourova, M. Anoxybacillus bogrovensis sp. nov., a novel thermophilic bacterium isolated from a hot spring in Dolni Bogrov, Bulgaria. Int. J. Syst. Evol. Microbiol. 2008, 58, 2359–2362. [Google Scholar] [CrossRef]
- Belduz, A.O.; Dulger, S.; Demirbag, Z. Anoxybacillus gonensis sp. nov., a moderately thermophilic, xylose-utilizing, endo-spore-forming bacterium. Int. J. Syst. Evol. Microbiol. 2003, 53, 1315–1320. [Google Scholar]
- Chai, Y.Y.; Kahar, U.M.; Md Salleh, M.; Md Illias, R.; Goh, K.M. Isolation and characterization of pullulan-degrading Anoxybacillus species isolated from Malaysian hot springs. Environ. Technol. 2012, 33, 1231–1238. [Google Scholar]
- Chen, J.; Zheng, J.; Li, Y.; Hao, H.-H.; Chen, J.-M. Characteristics of a novel thermophilic heterotrophic bacterium, Anoxybacillus contaminans HA, for nitrification–aerobic denitrification. Appl. Microbiol. Biotechnol. 2015, 99, 10695–10702. [Google Scholar] [CrossRef]
- Chen, X.-G.; Stabnikova, O.; Tay, J.-H.; Wang, J.-Y.; Tay, S.T.-L. Thermoactive extracellular proteases of Geobacillus caldoproteolyticus, sp. nov., from sewage sludge. Extremophiles 2004, 8, 489–498. [Google Scholar] [CrossRef]
- Cheng, J.H.; Wang, Y.; Zhang, X.Y.; Sun, M.L.; Zhang, X.; Song, X.Y.; Zhang, Y.Z.; Zhang, Y.; Chen, X.L. Characterization and Diversity Analysis of the Extracellular Proteases of Thermophilic Anoxybacillus caldiproteolyticus 1A02591 From Deep-Sea Hydro-thermal Vent Sediment. Front. Microbiol. 2021, 12, 643508. [Google Scholar]
- Cihan, A.C.; Ozcan, B.; Cokmus, C. Anoxybacillus salavatliensis sp. nov., an α-glucosidase producing, thermophilic bacterium isolated from Salavatli. Turkey 2010, 51, 136–146. [Google Scholar] [CrossRef]
- Cihan, A.C.; Cokmus, C.; Koc, M.; Ozcan, B. Anoxybacillus calidus sp. nov., a thermophilic bacterium isolated from soil near a thermal power plant. Int. J. Syst. Evol. Microbiol. 2014, 64, 211–219. [Google Scholar] [CrossRef]
- De Clerck, E.; Rodríguez-Díaz, M.; Vanhoutte, T.; Heyrman, J.; Logan, N.A.; De Vos, P. Anoxybacillus contaminans sp. nov. and Bacillus gelatini sp. nov., isolated from contaminated gelatin batches. Int. J. Syst. Evol. Microbiol. 2004, 54, 941–946. [Google Scholar] [CrossRef][Green Version]
- Coorevits, A.; Dinsdale, A.E.; Halket, G.; Lebbe, L.; De Vos, P.; Van Landschoot, A.; Logan, N.A. Taxonomic revision of the genus Geobacillus: Emendation of Geobacillus, G. stearothermophilus, G. jurassicus, G. toebii, G. thermodenitrificans and G. thermogluco-sidans (nom. corrig., formerly ‘thermoglucosidasius’); transfer of Bacillus thermantarcticus to the genus as G. thermantarcticus comb. nov.; proposal of Caldibacillus debilis gen. nov., comb. nov.; transfer of G. tepidamans to Anoxybacillus as A. tepidamans comb. nov.; and proposal of Anoxybacillus caldiproteolyticus sp. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 1470–1485. [Google Scholar]
- Deep, K.; Poddar, A.; Das, S.K. Anoxybacillus suryakundensis sp. nov, a Moderately Thermophilic, Alkalitolerant Bacterium Isolated from Hot Spring at Jharkhand, India. PLoS ONE 2013, 8, e85493. [Google Scholar] [CrossRef]
- Derekova, A.; Sjøholm, C.; Mandeva, R.; Kambourova, M. Anoxybacillus rupiensis sp. Nov., a novel thermophilic bacterium isolated from Rupi basin (Bulgaria). Extremophiles 2007, 11, 577–583. [Google Scholar] [CrossRef]
- Dulger, S.; Demirbag, Z.; Belduz, A.O. Anoxybacillus ayderensis sp. nov. and Anoxybacillus kestanbolensis sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1499–1503. [Google Scholar] [CrossRef]
- Filippidou, S.; Jaussi, M.; Junier, T.; Wunderlin, T.; Jeanneret, N.; Palmieri, F.; Palmieri, I.; Roussel-Delif, L.; Vieth-Hillebrand, A.; Vetter, A.; et al. Anoxybacillus geothermalis sp. nov., a facultatively anaerobic, endospore-forming bacterium isolated from mineral deposits in a geothermal station. Int. J. Syst. Evol. Microbiol. 2016, 66, 2944–2951. [Google Scholar] [CrossRef]
- Gao, Y.; Dai, J.; Peng, H.; Liu, Y.; Xu, T. Isolation and characterization of a novel organic solvent-tolerant Anoxybacillus sp. PGDY12, a thermophilic Gram-positive bacterium. J. Appl. Microbiol. 2010, 110, 472–478. [Google Scholar] [CrossRef]
- Gul-Guven, R.; Guven, K.; Poli, A.; Nicolaus, B. Anoxybacillus kamchatkensis subsp. asaccharedens subsp. nov., a thermophilic bac-terium isolated from a hot spring in Batman. J. Gen. Appl. Microbiol. 2008, 54, 327–334. [Google Scholar]
- Inan, K.; Belduz, A.O.; Canakci, S. Anoxybacillus kaynarcensis sp. nov., a moderately thermophilic, xylanase producing bacterium. J. Basic Microbiol. 2013, 53, 410–419. [Google Scholar]
- Kacagan, M.; Canakci, S.; Sandalli, C.; Inan, K.; Colak, D.N.; Belduz, A.O. Characterization of a xylanase from a thermophilic strain of Anoxybacillus pushchinoensis A8. Biologia 2008, 63, 599–606. [Google Scholar] [CrossRef]
- Kevbrin, V.V.; Zengler, K.; Lysenko, A.M.; Wiegel, J. Anoxybacillus kamchatkensis sp. nov., a novel thermophilic facultative aerobic bacterium with a broad pH optimum from the Geyser valley, Kamchatka. Extremophiles 2005, 9, 391–398. [Google Scholar]
- Khan, I.U.; Habib, N.; Xiao, M.; Devi, A.M.; Habib, M.; Hejazi, M.S.; Salam, N.; Zhi, X.-Y.; Li, W.-J. Anoxybacillus sediminis sp. nov., a novel moderately thermophilic bacterium isolated from a hot spring. Antonie van Leeuwenhoek 2018, 111, 2275–2282. [Google Scholar] [CrossRef]
- Khanna, K.; Mishra, K.P.; Chanda, S.; Ganju, L.; Singh, S.B.; Kumar, B. Effect of Synbiotics on Amelioration of Intestinal Inflammation Under Hypobaric Hypoxia. High Alt. Med. Biol. 2021, 22, 32–44. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, Y.-J.; Ryu, N.; Park, S.; Jeong, H.; Kim, B.-C.; Lee, D.-W.; Lee, H.-S. Draft Genome Sequence of the Thermophilic Bacterium Anoxybacillus kamchatkensis G10. J. Bacteriol. 2012, 194, 6684–6685. [Google Scholar] [CrossRef]
- Matpan-Bekler, F.; Guven, K. Isolation and production of thermostable α-amylase from thermophilic Anoxybacillus sp. KP1 from Diyadin hot spring in Ağri, Turkey. Biologia 2014, 69, 419–427. [Google Scholar]
- Matpan-Bekler, F.; Yalaz, S.; Guven, K. Molecular characterisation and numerical analysis of novel moderately thermophile Anoxybacillus sp. FMB1. Rom. Biotechnol. Lett. 2018, 23, 13964–13975. [Google Scholar]
- Mittal, P.; Saxena, R.; Sharma, V.K. Draft Genome Sequence of Anoxybacillus mongoliensis Strain MB4, a Sulfur-Utilizing Aerobic Thermophile Isolated from a Hot Spring in Tattapani, Central India. Genome Announc. 2017, 5, e01709-16. [Google Scholar] [CrossRef]
- Najar, I.; Sherpa, M.T.; Das, S.; Das, S.; Thakur, N. Microbial ecology of two hot springs of Sikkim: Predominate population and geochemistry. Sci. Total Environ. 2018, 637–638, 730–745. [Google Scholar] [CrossRef]
- Namsaraev, Z.B.; Babasanova, O.B.; Dunaevsky, Y.E.; Akimov, V.N.; Barkhutova, D.D.; Gorlenko, V.M. Anoxybacillus mongoliensis sp. nov., a novel thermophilic proteinase producing bacterium isolated from alkaline hot spring, Central Mongolia. Microbiology 2010, 79, 491–499. [Google Scholar] [CrossRef]
- Ottesen, A.; Ramachandran, P.; Reed, E.; White, J.R.; Hasan, N.; Subramanian, P.; Ryan, G.; Jarvis, K.; Grim, C.; Daquiqan, N.; et al. Enrichment dynamics of Listeria monocytogenes and the associated microbiome from naturally contaminated ice cream linked to a listeriosis outbreak. BMC Microbiol. 2016, 16, 275. [Google Scholar]
- Patel, B.K.C. Draft Genome Sequence of Anoxybacillus Strain BCO1, Isolated from a Thermophilic Microbial Mat Colonizing the Outflow of a Bore Well of the Great Artesian Basin of Australia. Genome Announc. 2015, 3, e01547-14. [Google Scholar] [CrossRef]
- Dos Reis, S.V.; Beys-Da-Silva, W.O.; Tirloni, L.; Santi, L.; Seixas, A.; Termignoni, C.; Da Silva, M.V.; Macedo, A.J. The extremophile Anoxybacillus sp. PC2 isolated from Brazilian semiarid region (Caatinga) produces a thermostable keratinase. J. Basic Microbiol. 2020, 60, 809–815. [Google Scholar] [CrossRef]
- Sahm, K.; John, P.; Nacke, H.; Wemheuer, B.; Grote, R.; Daniel, R.; Antranikian, G. High abundance of heterotrophic prokaryotes in hydrothermal springs of the Azores as revealed by a network of 16S rRNA gene-based methods. Extremophiles 2013, 17, 649–662. [Google Scholar] [CrossRef]
- Salam, L.B.; Obayori, O.S. Remarkable shift in structural and functional properties of an animal charcoal-polluted soil accentuated by inorganic nutrient amendment. J. Genet. Eng. Biotechnol. 2020, 18, 1–20. [Google Scholar] [CrossRef]
- Wu, S.; Wang, G.; Angert, E.R.; Wang, W.; Li, W.; Zou, H. Composition, Diversity, and Origin of the Bacterial Community in Grass Carp Intestine. PLoS ONE 2012, 7, e30440. [Google Scholar] [CrossRef]
- Xia, W.; Dong, H.; Zheng, C.; Cui, Q.; He, P.; Tang, Y. Hydrocarbon degradation by a newly isolated thermophilic Anoxybacillus sp. with bioemulsifier production and new alkB genes. RSC Adv. 2015, 5, 102367–102377. [Google Scholar] [CrossRef]
- Yadav, P.; Maharjan, J.; Korpole, S.; Prasad, G.S.; Sahni, G.; Bhattarai, T.; Sreerama, L. Production, Purification, and Characterization of Thermostable Alkaline Xylanase From Anoxybacillus kamchatkensis NASTPD13. Front. Bioeng. Biotechnol. 2018, 6, 65. [Google Scholar] [CrossRef]
- Yumoto, I.; Hirota, K.; Kawahara, T.; Nodasaka, Y.; Okuyama, H.; Matsuyama, H.; Yokota, Y.; Nakajima, K.; Hoshino, T. Anoxybacillus voinovskiensis sp. nov., a moderately thermophilic bacterium from a hot spring in Kamchatka. Int. J. Syst. Evol. Microbiol. 2004, 54, 1239–1242. [Google Scholar] [CrossRef]
- Zhang, C.-M.; Huang, X.-W.; Pan, W.-Z.; Zhang, J.; Wei, K.-B.; Klenk, H.-P.; Tang, S.-K.; Li, W.-J.; Zhang, K.-Q. Anoxybacillus tengchongensis sp. nov. and Anoxybacillus eryuanensis sp. nov., facultatively anaerobic, alkalitolerant bacteria from hot springs. Int. J. Syst. Evol. Microbiol. 2011, 61, 118–122. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.Q.; Zhang, Z.L.; Wu, N.; Zhu, X.F.; Wu, M. Anoxybacillus vitaminiphilus sp. nov., a strictly aerobic and moderately ther-mophilic bacterium isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2013, 63, 4064–4071. [Google Scholar]
- Zhao, C.; Chu, Y.; Li, Y.; Yang, C.; Chen, Y.; Wang, X.; Liu, B. High-throughput pyrosequencing used for the discovery of a novel cellulase from a thermophilic cellulose-degrading microbial consortium. Biotechnol. Lett. 2016, 39, 123–131. [Google Scholar] [CrossRef]

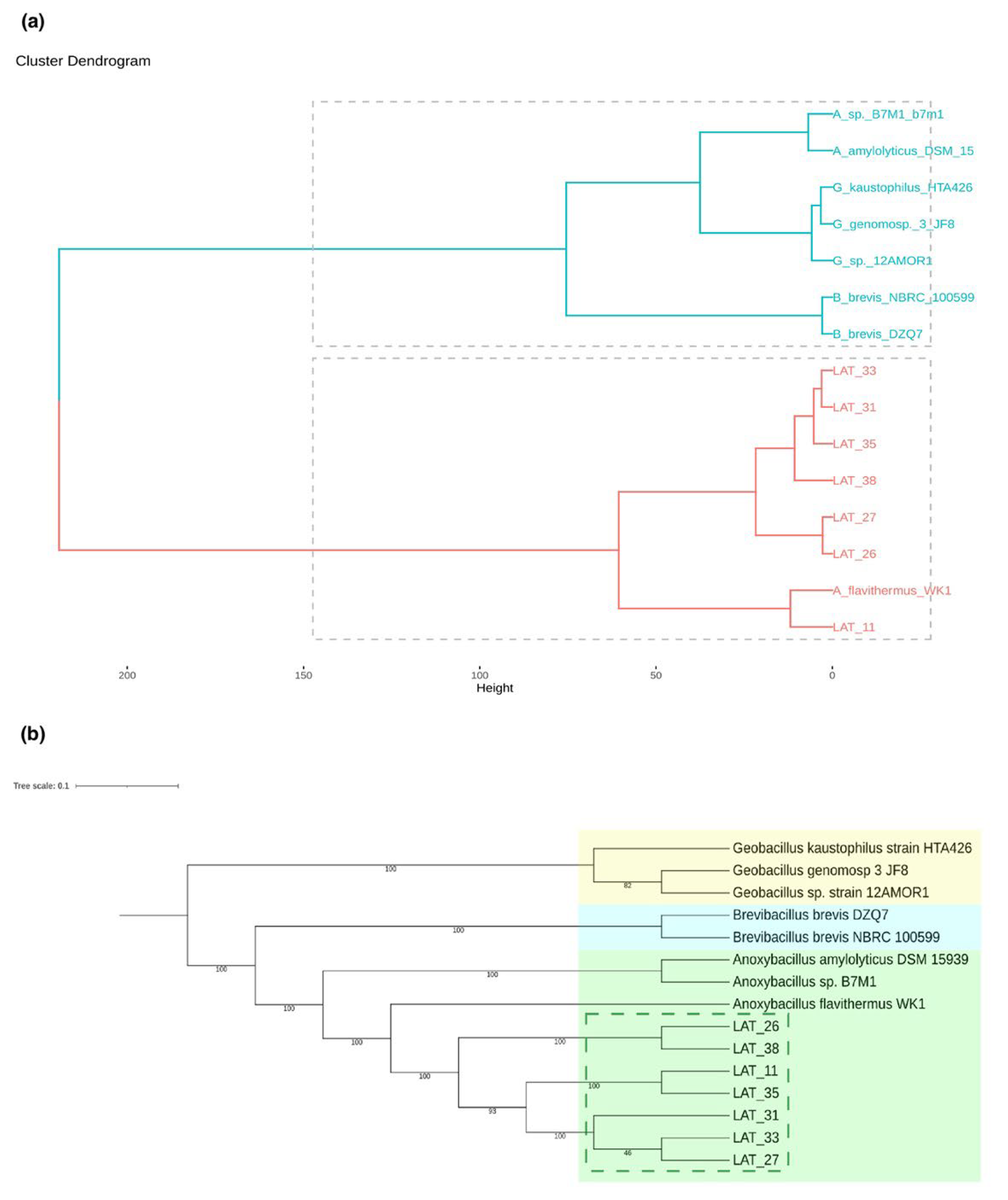
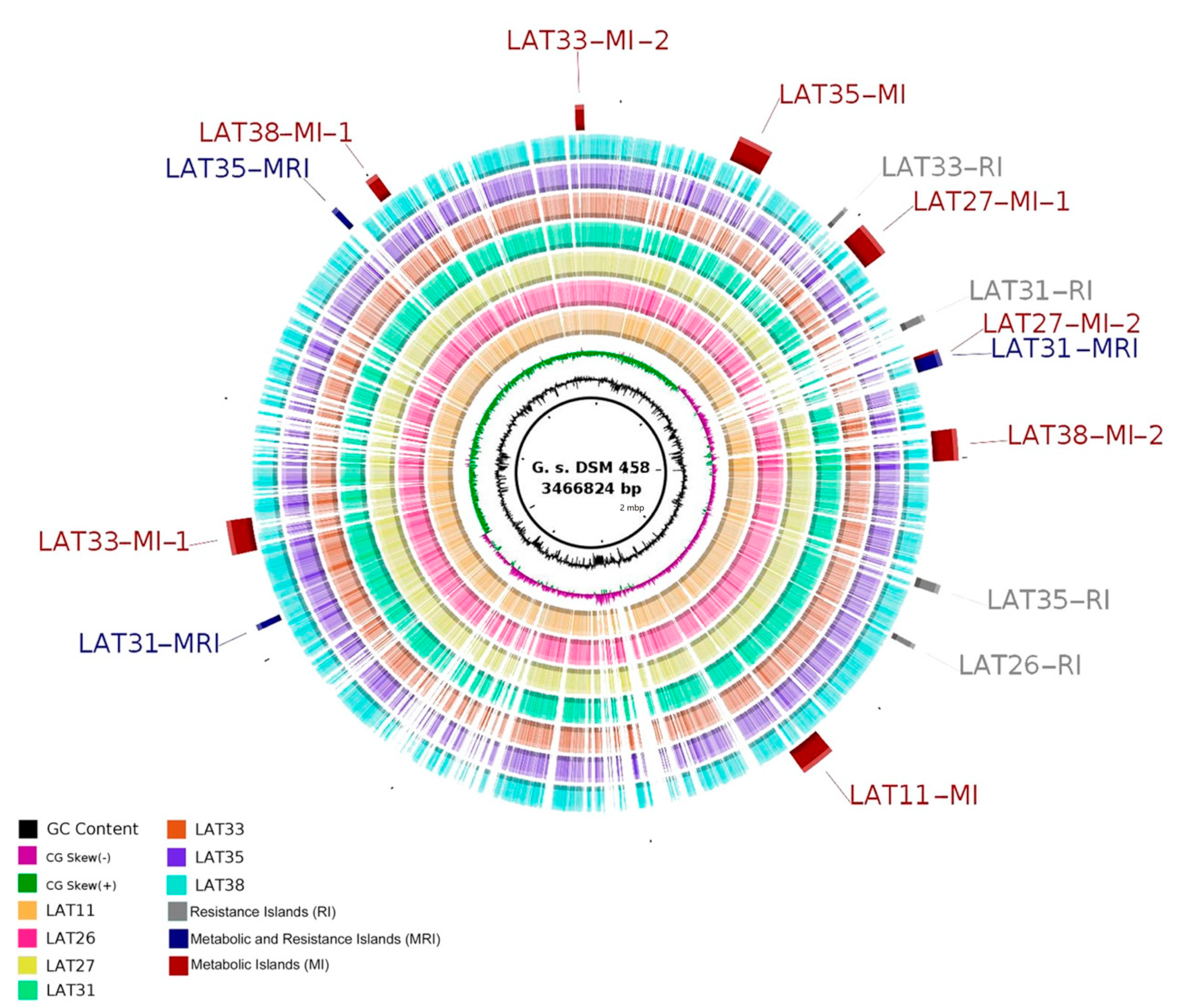
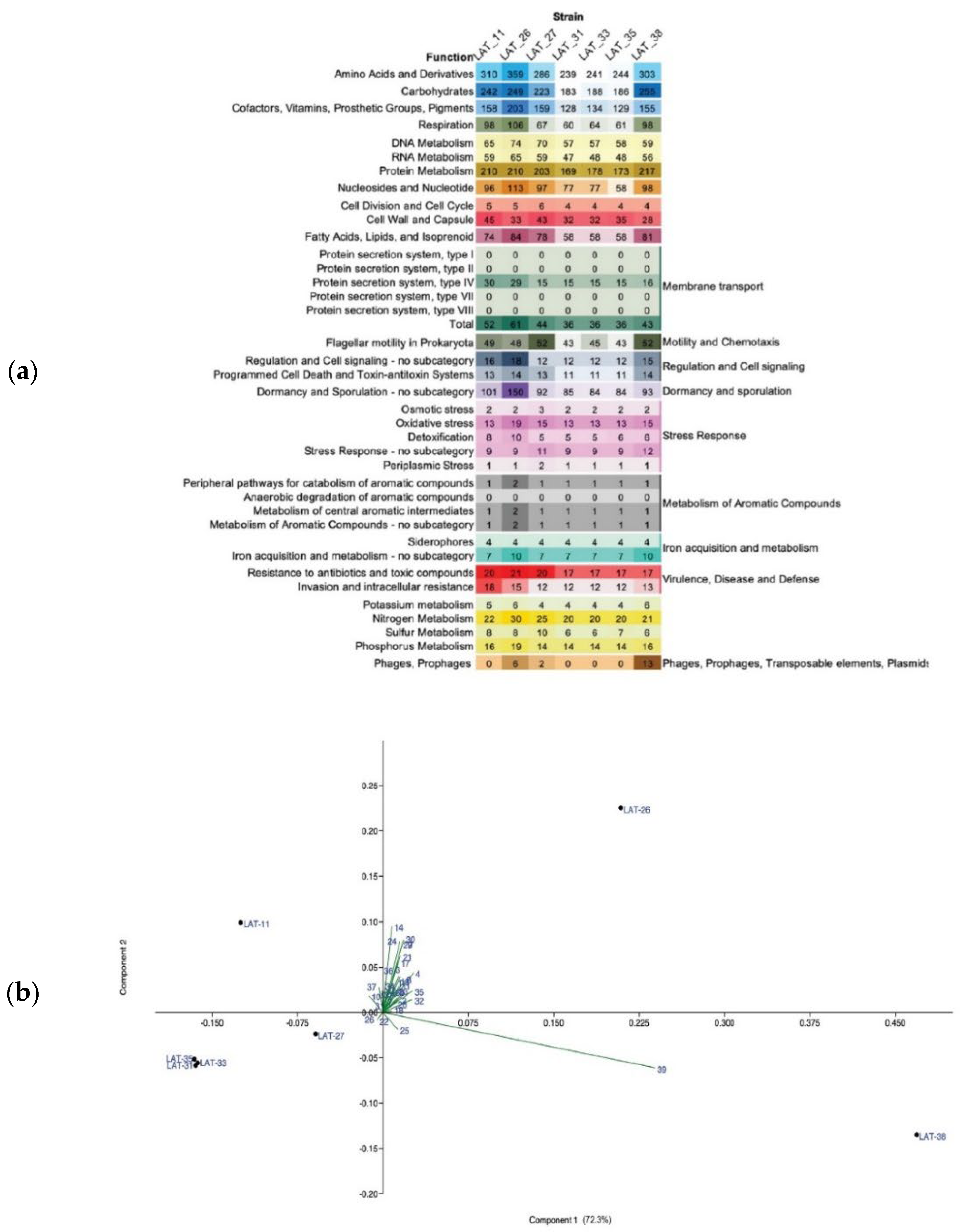
| Anoxybacillus Strain | Location | Environmental Temperature | Conditions of Isolation | Temperature Range of Growth |
|---|---|---|---|---|
| LAT_11 | Fumarole Bay, Deception Island, Antarctica | 100 °C | Lysogeny agar, 55 °C | 30–70 °C |
| LAT_26 | Fumarole Bay, Deception Island, Antarctica | 55 °C | DSMZ 260 medium, 55 °C | 30–70 °C |
| LAT_27 | Fumarole Bay, Deception Island, Antarctica | 70 °C | Lysogeny agar, 55 °C | 30–70 °C |
| LAT_31 | Fumarole Bay, Deception Island, Antarctica | 70 °C | DSMZ 260 medium, 55 °C | 30–70 °C |
| LAT_33 | Fumarole Bay, Deception Island, Antarctica | 70 °C | DSMZ 260 medium, 55 °C | 30–70 °C |
| LAT_35 | Fumarole Bay, Deception Island, Antarctica | 80 °C | Lysogeny agar, 55 °C | 30–70 °C |
| LAT_38 | Whalers Bay, Deception Island, Antarctica | 50 °C | Lysogeny agar, 55 °C | 30–70 °C |
| Strain | Genome Size (bp) | G + C Content (%) | Number of CDS | Count of RNA | Number of Contigs | Accession Number |
|---|---|---|---|---|---|---|
| LAT_11 | 3,296,946 | 41.8 | 3668 | 100 | 402 | JAILSF000000000 |
| LAT_26 | 3,572,987 | 42.0 | 3993 | 120 | 249 | JAILSG000000000 |
| LAT_27 | 3,160,393 | 42.0 | 3575 | 131 | 491 | JAIWIK000000000 |
| LAT_31 | 2,713,308 | 41.6 | 2885 | 100 | 102 | JAILSH000000000 |
| LAT_33 | 2,730,467 | 41.7 | 2932 | 109 | 123 | JAILSI000000000 |
| LAT_35 | 2,720,400 | 41.7 | 2931 | 102 | 129 | JAILSJ000000000 |
| LAT_38 | 3,368,374 | 43.5 | 4297 | 127 | 1122 | JAILSK000000000 |
| Gene | System Subtype | Strain |
|---|---|---|
| CAS12f4_0 | V-F | LAT_11, LAT_26, LAT_27, LAT_35, LAT_33, and LAT_31 |
| CAS7_2 | I-B | LAT_27 |
| CSX1_7 | III | LAT_27 |
| CSB3_0 | I-U | LAT_11 |
| CSE1_0 | I-E | LAT_38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultz, J.; Parise, M.T.D.; Parise, D.; Medeiros, L.G.; Sousa, T.J.; Kato, R.B.; Uetanabaro, A.P.T.; Araújo, F.; Ramos, R.T.J.; de Castro Soares, S.; et al. Unraveling the Genomic Potential of the Thermophilic Bacterium Anoxybacillus flavithermus from an Antarctic Geothermal Environment. Microorganisms 2022, 10, 1673. https://doi.org/10.3390/microorganisms10081673
Schultz J, Parise MTD, Parise D, Medeiros LG, Sousa TJ, Kato RB, Uetanabaro APT, Araújo F, Ramos RTJ, de Castro Soares S, et al. Unraveling the Genomic Potential of the Thermophilic Bacterium Anoxybacillus flavithermus from an Antarctic Geothermal Environment. Microorganisms. 2022; 10(8):1673. https://doi.org/10.3390/microorganisms10081673
Chicago/Turabian StyleSchultz, Júnia, Mariana Teixeira Dornelles Parise, Doglas Parise, Laenne G. Medeiros, Thiago J. Sousa, Rodrigo B. Kato, Ana Paula Trovatti Uetanabaro, Fabrício Araújo, Rommel Thiago Jucá Ramos, Siomar de Castro Soares, and et al. 2022. "Unraveling the Genomic Potential of the Thermophilic Bacterium Anoxybacillus flavithermus from an Antarctic Geothermal Environment" Microorganisms 10, no. 8: 1673. https://doi.org/10.3390/microorganisms10081673
APA StyleSchultz, J., Parise, M. T. D., Parise, D., Medeiros, L. G., Sousa, T. J., Kato, R. B., Uetanabaro, A. P. T., Araújo, F., Ramos, R. T. J., de Castro Soares, S., Brenig, B., de Carvalho Azevedo, V. A., Góes-Neto, A., & Rosado, A. S. (2022). Unraveling the Genomic Potential of the Thermophilic Bacterium Anoxybacillus flavithermus from an Antarctic Geothermal Environment. Microorganisms, 10(8), 1673. https://doi.org/10.3390/microorganisms10081673











