SARS-CoV-2 Vaccination Coverage and Factors Associated with Low Uptake in a Cohort of People Living with HIV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Characteristics of the Study Population
3.2. SARS-CoV-2 Vaccination
3.3. Factors Associated with Low Uptake of SARS-CoV-2 Vaccines
3.4. SARS-CoV-2 Outcomes among Vaccinated and Unvaccinated People Living with HIV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Guidance for COVID-19 and People with HIV; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021; pp. 1–9. [Google Scholar]
- WHO Clinical Platform for COVID-19. Clinical Features and Prognostic Factors of COVID-19 in People Living with HIV Hospitalized with Suspected or Confirmed SARS-CoV-2 Infection; WHO: Geneva, Switzerland, 2021; pp. 1–20. [Google Scholar]
- Nomah, D.K.; Reyes-Urueña, J.; Díaz, Y.; Moreno, S.; Aceiton, J.; Bruguera, A.; Vivanco-Hidalgo, R.M.; Llibre, J.M.; Domingo, P.; Falcó, V.; et al. Sociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: A retrospective cohort study. Lancet HIV 2021, 8, e701–e710. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca Vaccines on Covid-19 Related Symptoms, Hospital Admissions, and Mortality in Older Adults in England: Test Negative Case-Control Study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Coronavirus Disease (COVID-19): COVID-19 Vaccines and People Living with HIV. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-covid-19-vaccines-and-people-living-with-hiv (accessed on 1 August 2022).
- European Medicines Agency. COVID-19 Vaccines. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines (accessed on 20 September 2021).
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A. Safety and Immunogenicity of the ChAdOx1 NCoV-19 (AZD1222) Vaccine against SARS-CoV-2 in HIV Infection: A Single-Arm Substudy of a Phase 2/3 Clinical Trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef]
- Woldemeskel, A.B.; Karaba, A.H.; Garliss, C.C.; Beck, E.J.; Wang, K.H.; Laeyendecker, O.; Cox, A.L.; Blankson, J.N. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living With Human Immunodeficiency Virus (HIV). Clin. Infect. Dis. 2021, 74, 1268–1270. [Google Scholar] [CrossRef]
- Snyman, J.; Hwa, S.-H.; Krause, R.; Muema, D.; Reddy, T.; Ganga, Y.; Karim, F.; Leslie, A.; Sigal, A.; Ndung’U, T.; et al. Similar Antibody Responses Against Severe Acute Respiratory Syndrome Coronavirus 2 in Individuals Living Without and With Human Immunodeficiency Virus on Antiretroviral Therapy During the First South African Infection Wave. Clin. Infect. Dis. 2021, ciab758. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Cicalini, S.; Meschi, S.; Bordoni, V.; Lorenzini, P.; Vergori, A.; Lanini, S.; De Pascale, L.; Matusali, G.; Mariotti, D. Immunogenicity of MRNA Vaccination against SARS-CoV-2 in Persons Living with HIV (PLWHs) with Low CD4 Count or Previous AIDS. Hiv Med. 2021, 22, 21–23. [Google Scholar]
- Consejo International; Sistema Nacional de Salud. Estrategia de Vacunación Frente a COVID-19 en España; Consejo International, Sistema Nacional de Salud: Madrid, Spain, 2021. [Google Scholar]
- Ministerio de Sanidad; Gobierno de España. Gestión Integral de La Vacunación COVID-19 (GIV COVID-19); Ministerio de Sanidad, Gobierno de España: Madrid, Spain, 2021. [Google Scholar]
- Sticchi, L.; Bruzzone, B.; Caligiuri, P.; Rappazzo, E.; Casto, M.L.; De Hoffer, L.; Gustinetti, G.; Viscoli, C.; Di Biagio, A. Seroprevalence and vaccination coverage of vaccine-preventable diseases in perinatally HIV-1-infected patients. Hum. Vaccines Immunother. 2014, 11, 263–269. [Google Scholar] [CrossRef]
- Harrison, N.; Poeppl, W.; Herkner, H.; Tillhof, K.; Grabmeier-Pfistershammer, K.; Rieger, A.; Forstner, C.; Burgmann, H.; Lagler, H. Predictors for and coverage of influenza vaccination among HIV-positive patients: A cross-sectional survey. HIV Med. 2016, 18, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Moyroud, L.; Hustache, S.; Goirand, L.; Hauzanneau, M.; Epaulard, O. Negative perceptions of hepatitis B vaccination among attendees of an urban free testing center for sexually transmitted infections in France. Hum. Vaccines Immunother. 2017, 13, 998–1004. [Google Scholar] [CrossRef]
- Vallée, A.; Fourn, E.; Majerholc, C.; Touche, P.; Zucman, D. COVID-19 Vaccine Hesitancy among French People Living with HIV. Vaccines 2021, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Generalitat de Catalunya; Agència de Qualitat i Avaluació Sanitàries de Catalunya. Programa Públic d’Analítica de Dades per a La Recerca i La Innovació En Salut a Catalunya—PADRIS; Agència de Qualitat i Avaluació Sanitàries de Catalunya: Barcelona, Spain, 2017. [Google Scholar]
- Agència de Qualitat i Avaluació Sanitàries de Catalunya. Nou Indicador Socioeconòmic Del Model d’Assignació de Recursos de l’Atenció Primària; Agència de Qualitat i Avaluació Sanitàries de Catalunya: Barcelona, Spain, 2017. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bull. World Health Organ. 2007, 85, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Global Vaccine Action Plan. Vaccine 2013, 31, B5–B31. [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Moline, H.L.; Whitaker, M.; Deng, L.; Rhodes, J.C.; Milucky, J.; Pham, H.; Patel, K.; Anglin, O.; Reingold, A.; Chai, S.J. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization among Adults Aged ≥ 65 Years—COVID-NET, 13 States, February–April 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1088. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2021, 114, 252–260. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.-K.; Moore, Z.; Zeng, D. Effectiveness of Covid-19 Vaccines over a 9-Month Period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef]
- Gobierno de España; Government of Spain Estrategia de Vacunación COVID-19. Available online: https://www.vacunacovid.gob.es/ (accessed on 19 October 2021).
- Government of Spain-Ministry of Health GIV COVID-19. Government of Spain-Ministry of Health GIV COVID-19. Informe Vacunación COVID-19. Periodo de Los Datos: 27/12/2020–14/07/2021. 2021. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Informe_GIV_comunicacion_20210726.pdf (accessed on 1 September 2021).
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Perez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; de Larrea, N.F.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Robinson, E.; Jones, A.; Lesser, I.; Daly, M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine 2021, 39, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
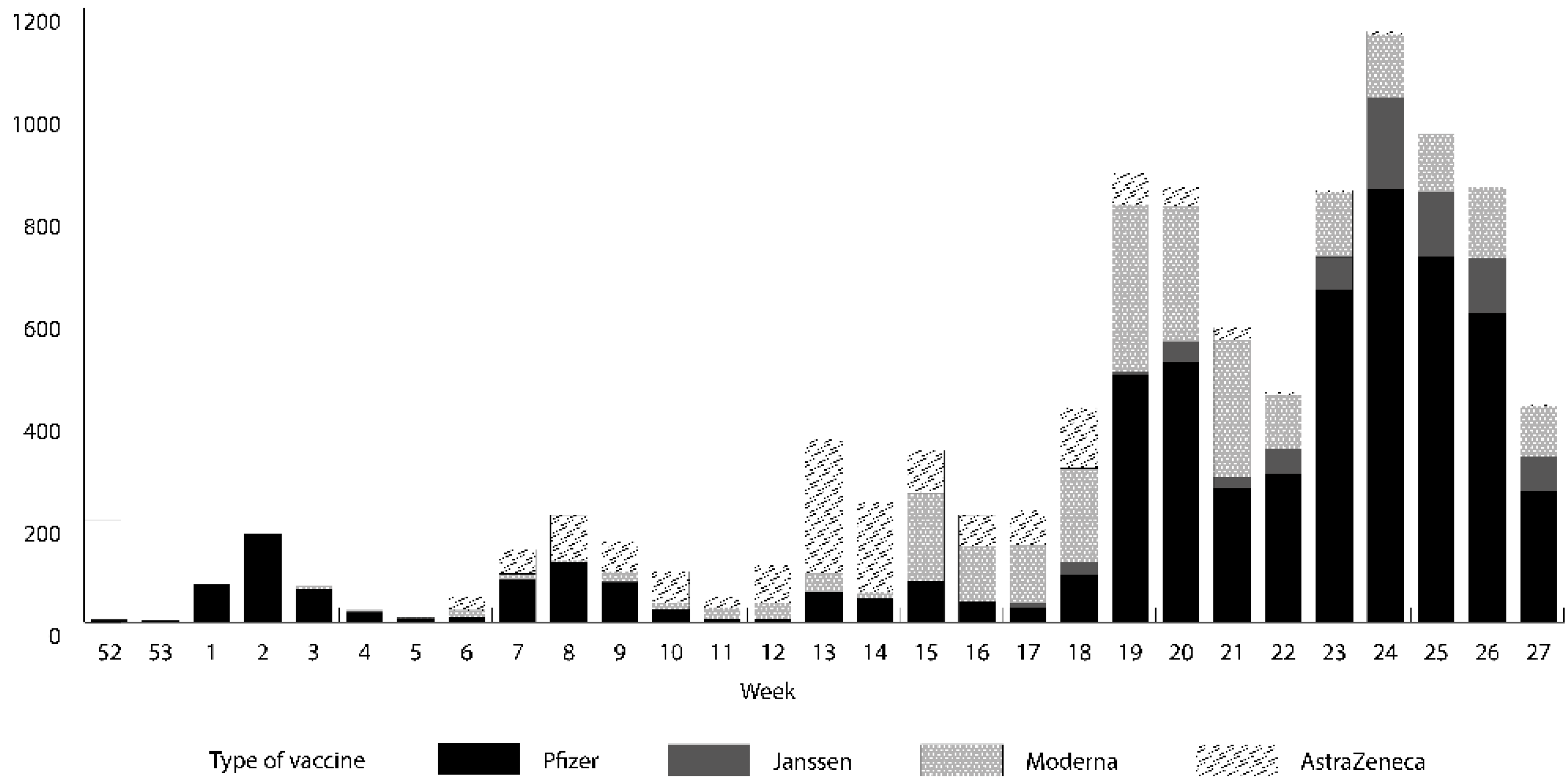
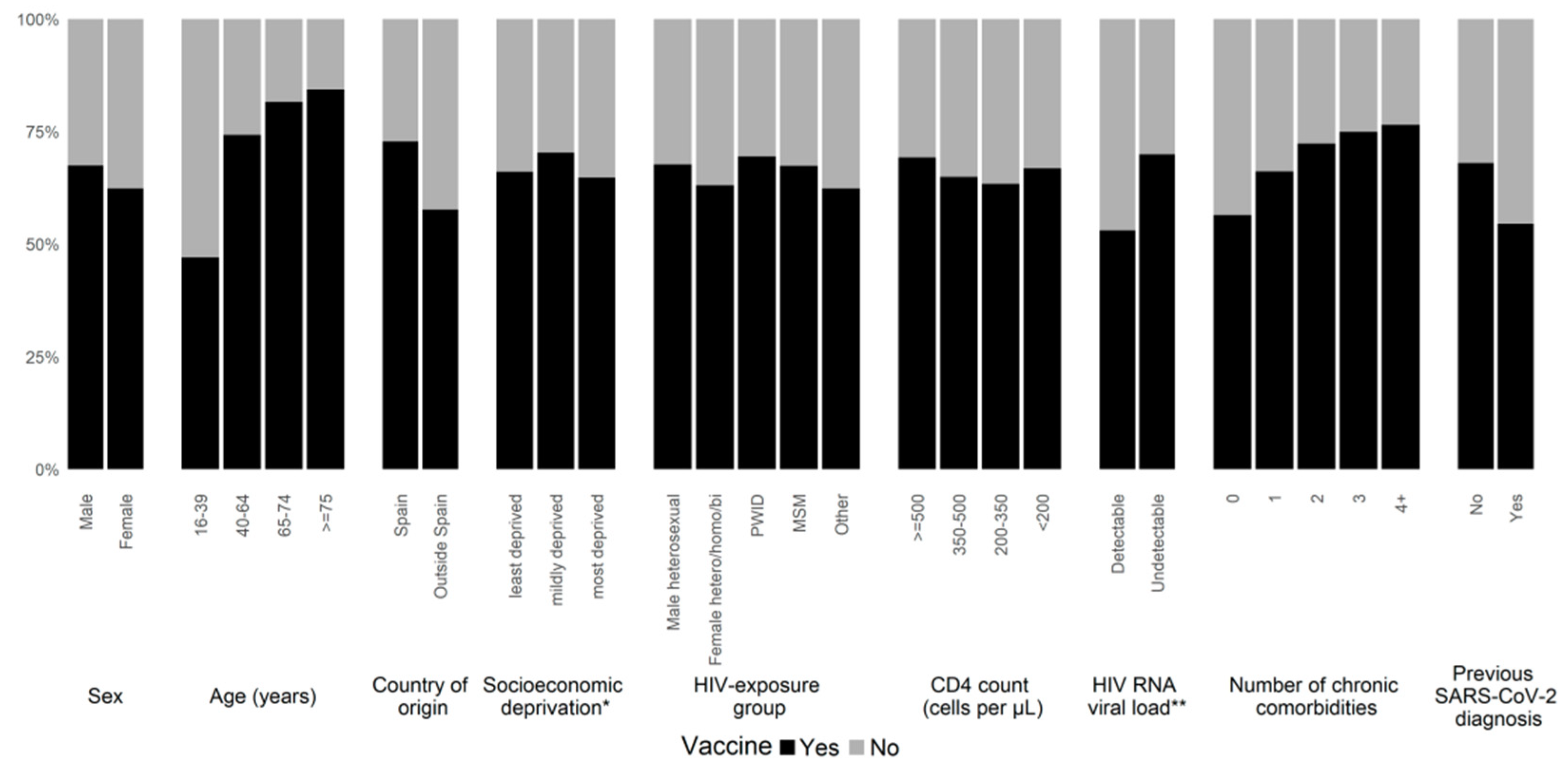
| Overall Cohort (n = 14,942) | Vaccinated x (n = 9945) | Unvaccinated (n = 4997) | p Value | |
|---|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) | |
| Sex a | <0.001 | |||
| Male | 12257 (82.0) | 8271 (83.2) | 3986 (79.8) | |
| Female | 2684 (18.0) | 1673 (16.8) | 1011 (20.2) | |
| Missing | 1 (0.01) | 1 (0.01) | 0 (0) | |
| Age, median (IQR), y b | 46.4 (38.3–54.2) | 49.0 (41.4–55.8) | 40.8 (32.8–49.3) | <0.001 |
| Age category, y b | <0.001 | |||
| 16–39 | 4479 (30.0) | 2106 (21.2) | 2373 (47.5) | |
| 40–64 | 9593 (64.2) | 7124 (71.6) | 2469 (49.4) | |
| 65–74 | 678 (4.5) | 553 (5.6) | 125 (2.5) | |
| ≥75 | 192 (1.3) | 162 (1.6) | 30 (0.6) | |
| Country of origin c | <0.001 | |||
| Spain | 8808 (59.0) | 6409 (64.4) | 2399 (48.0) | |
| Outside Spain | 6132 (41.0) | 3535 (35.6) | 2597 (52.0) | |
| Unknown | 2 (0.01) | 1 (0.01) | 1 (0.02) | |
| Socioeconomic deprivation | ||||
| Least deprived | 7188 (48.1) | 4749 (47.8) | 2439 (48.8) | 0.52 |
| Mildly deprived | 2839 (19.0) | 1997 (20.1) | 842 (16.9) | |
| Most deprived | 4574 (30.6) | 2962 (29.8) | 1612 (32.3) | |
| Missing | 341 (2.3) | 237 (2.4) | 104 (2.1) | |
| HIV transmission route | <0.001 | |||
| PWID | 1727 (11.6) | 1200 (12.1) | 527 (10.6) | |
| MSM | 7835 (52.4) | 5281 (53.1) | 2554 (51.1) | |
| Male heterosexual | 2055 (13.8) | 1391 (14.0) | 664 (13.3) | |
| Female hetero/homo/bisexual | 2008 (13.4) | 1266 (12.7) | 742 (14.9) | |
| Other | 856 (5.7) | 534 (5.4) | 322 (6.4) | |
| Missing | 461 (3.1) | 273 (2.8) | 188 (3.8) | |
| CD4 count (cells/μL) category | <0.001 | |||
| <200 | 3069 (20.5) | 2051 (20.6) | 1018 (20.4) | |
| 200–349 | 1266 (8.5) | 802 (8.1) | 464 (9.3) | |
| 350–499 | 2066 (13.8) | 1341 (13.5) | 725 (14.5) | |
| ≥500 | 7833 (52.4) | 5425 (54.6) | 2408 (48.2) | |
| Missing | 708 (4.7) | 326 (3.3) | 382 (7.6) | |
| HIV-RNA | <0.001 | |||
| Detectable | 1476 (9.9) | 783 (7.9) | 693 (13.9) | |
| Undetectable | 11891 (79.6) | 8317 (83.6) | 3574 (71.5) | |
| Missing | 1575 (10.5) | 845 (8.5) | 730 (14.6) | |
| Number of comorbidities | <0.001 | |||
| 0 | 4849 (32.5) | 2735 (27.5) | 2114 (42.3) | |
| 1 | 3661 (24.5) | 2422 (24.4) | 1239 (24.8) | |
| 2 | 2596 (17.4) | 1878 (18.9) | 718 (14.4) | |
| 3 | 1602 (10.7) | 1201 (12.1) | 401 (8.0) | |
| ≥4 | 2234 (15.0) | 1709 (17.2) | 525 (10.5) | |
| Previous SARS-CoV-2 diagnosis | <0.001 | |||
| Yes | 1610 (10.8) | 878 (8.8) | 732 (14.7) | |
| No | 13332 (89.2) | 9067 (91.2) | 4265 (85.4) |
| Characteristics | OR | aOR a | |
|---|---|---|---|
| Sex | Female | 1.00 (ref) | 1.00 (ref) |
| Male | 1.25 (1.15–1.37) | 1.39 (1.12–1.72) | |
| Age category, y | 16–39 | 1.00 (ref) | 1.00 (ref) |
| 40–64 | 3.25 (3.02–3.50) | 3.01 (2.75–3.30) | |
| 65–74 | 4.98 (4.08–6.13) | 3.77 (3.01–4.77) | |
| ≥75 | 6.08 (4.17–9.19) | 5.08 (3.27–8.24) | |
| Country of origin | Spain | 1.00 (ref) | 1.00 (ref) |
| Outside Spain | 0.51 (0.48–0.55) | 0.64 (0.59–0.70) | |
| Socioeconomic deprivation | Least deprived | 1.00 (ref) | 1.00 (ref) |
| Mildly deprived | 1.22 (1.11–1.34) | 1.21 (1.08–1.35) | |
| Most deprived | 0.94 (0.87–1.02) | 0.97 (0.88–1.07) | |
| HIV transmission route | Male heterosexual | 1.00 (ref) | 1.00 (ref) |
| Female hetero/homo/bisexual | 0.81 (0.72–0.93) | 1.14 (0.88–1.48) | |
| PWID | 1.09 (0.95–1.25) | 0.87 (0.74–1.03) | |
| MSM | 0.99 (0.89–1.09) | 1.43 (1.26–1.62) | |
| Other | 0.79 (0.67–0.94) | 1.06 (0.87–1.30) | |
| CD4 count (cells/μL) category | ≥500 | 1.00 (ref) | 1.00 (ref) |
| 350–499 | 0.82 (0.74–0.91) | 0.79 (0.70–0.88) | |
| 200–349 | 0.77 (0.68–0.87) | 0.74 (0.64–0.86) | |
| <200 | 0.89 (0.82–0.98) | 0.92 (0.83–1.02) | |
| HIV RNA viral load | Undetectable | 1.00 (ref) | 1.00 (ref) |
| Detectable | 0.49 (0.44–0.54) | 0.61 (0.54–0.69) | |
| Number of comorbidities | 0 | 1.00 (ref) | 1.00 (ref) |
| 1 | 1.51 (1.38–1.65) | 1.28 (1.16–1.43) | |
| 2 | 2.02 (1.82–2.24) | 1.58 (1.39–1.78) | |
| 3 | 2.31 (2.04–2.63) | 1.58 (1.36–1.84) | |
| ≥4 | 2.52 (2.25–2.82) | 1.58 (1.37–1.83) | |
| SARS-CoV-2 diagnosis | No previous SARS-CoV-2 diagnosis | 1.00 (ref) | 1.00 (ref) |
| Previous SARS-CoV-2 diagnosis | 0.56 (0.51–0.63) | 0.58 (0.51–0.65) |
| Total n = 13,662 | Unvaccinated PLWH n = 4596 | Vaccinated PLWH n = 9066 | ||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | p | |
| SARS-CoV-2 diagnosis | 616 (4.5) | 437 (9.5) | 179 (2.0) | <0.001 |
| COVID-19 hospital admissions | 10 (1.6) | 10 (2.3) | 0 (0) | <0.001 |
| COVID-19 ICU admissions | 6 (1.0) | 6 (1.4) | 0 (0) | <0.001 |
| COVID-19 deaths | 10 (1.6) | 10 (2.3) | 0 (0) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomah, D.K.; Llibre, J.M.; Díaz, Y.; Moreno, S.; Aceiton, J.; Bruguera, A.; Gutiérrez-Macià, M.; Imaz, A.; Suanzes, P.; Navarro, G.; et al. SARS-CoV-2 Vaccination Coverage and Factors Associated with Low Uptake in a Cohort of People Living with HIV. Microorganisms 2022, 10, 1666. https://doi.org/10.3390/microorganisms10081666
Nomah DK, Llibre JM, Díaz Y, Moreno S, Aceiton J, Bruguera A, Gutiérrez-Macià M, Imaz A, Suanzes P, Navarro G, et al. SARS-CoV-2 Vaccination Coverage and Factors Associated with Low Uptake in a Cohort of People Living with HIV. Microorganisms. 2022; 10(8):1666. https://doi.org/10.3390/microorganisms10081666
Chicago/Turabian StyleNomah, Daniel Kwakye, Josep Maria Llibre, Yesika Díaz, Sergio Moreno, Jordi Aceiton, Andreu Bruguera, Maria Gutiérrez-Macià, Arkaitz Imaz, Paula Suanzes, Gemma Navarro, and et al. 2022. "SARS-CoV-2 Vaccination Coverage and Factors Associated with Low Uptake in a Cohort of People Living with HIV" Microorganisms 10, no. 8: 1666. https://doi.org/10.3390/microorganisms10081666
APA StyleNomah, D. K., Llibre, J. M., Díaz, Y., Moreno, S., Aceiton, J., Bruguera, A., Gutiérrez-Macià, M., Imaz, A., Suanzes, P., Navarro, G., Orti, A., Miro, J. M., Casabona, J., Reyes-Urueña, J., & the PISCIS Study Group. (2022). SARS-CoV-2 Vaccination Coverage and Factors Associated with Low Uptake in a Cohort of People Living with HIV. Microorganisms, 10(8), 1666. https://doi.org/10.3390/microorganisms10081666








