Subspecies Classification and Comparative Genomic Analysis of Lactobacillus kefiranofaciens HL1 and M1 for Potential Niche-Specific Genes and Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. L. kefiranofaciens Strains and Culture Conditions
2.2. Subspecies Identification
2.2.1. Phenotypic Characterization
2.2.2. Genotypic Characterization
DNA Extraction and 16S rRNA and Housekeeping Gene Sequence Analysis
ERIC-PCR
RAPD
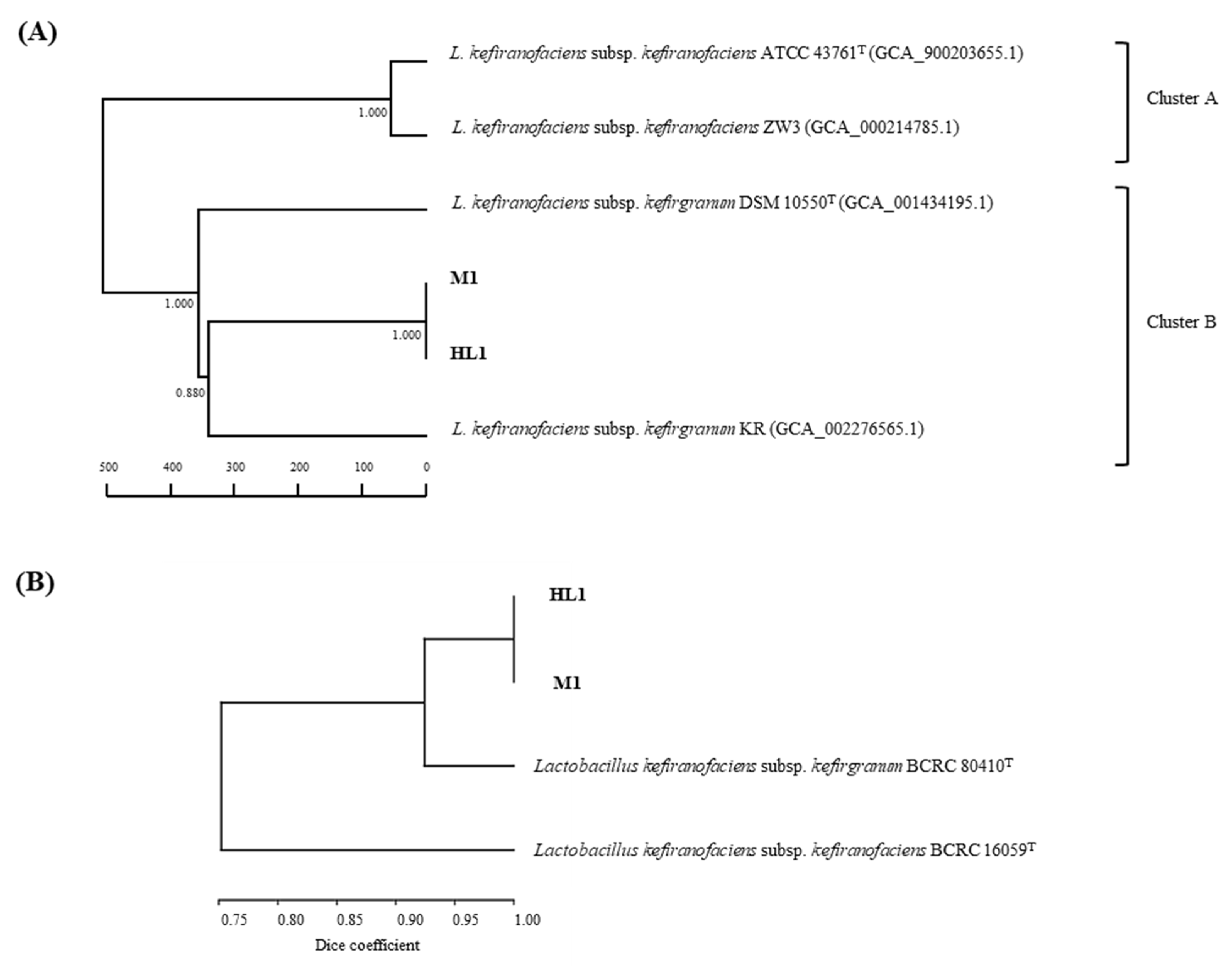
Phylogenomic Analyses
2.3. Whole Genome Sequencing, Assembly and Annotation
2.3.1. Genome Sequencing and Assembly
2.3.2. Annotation and Comparative Analysis
3. Results and Discussion
3.1. Subspecies Identification of HL1 and M1
3.1.1. Phenotypic Characterization
3.1.2. Genotypic Characterization
3.2. Comparative Genomic Analysis of HL1 and M1
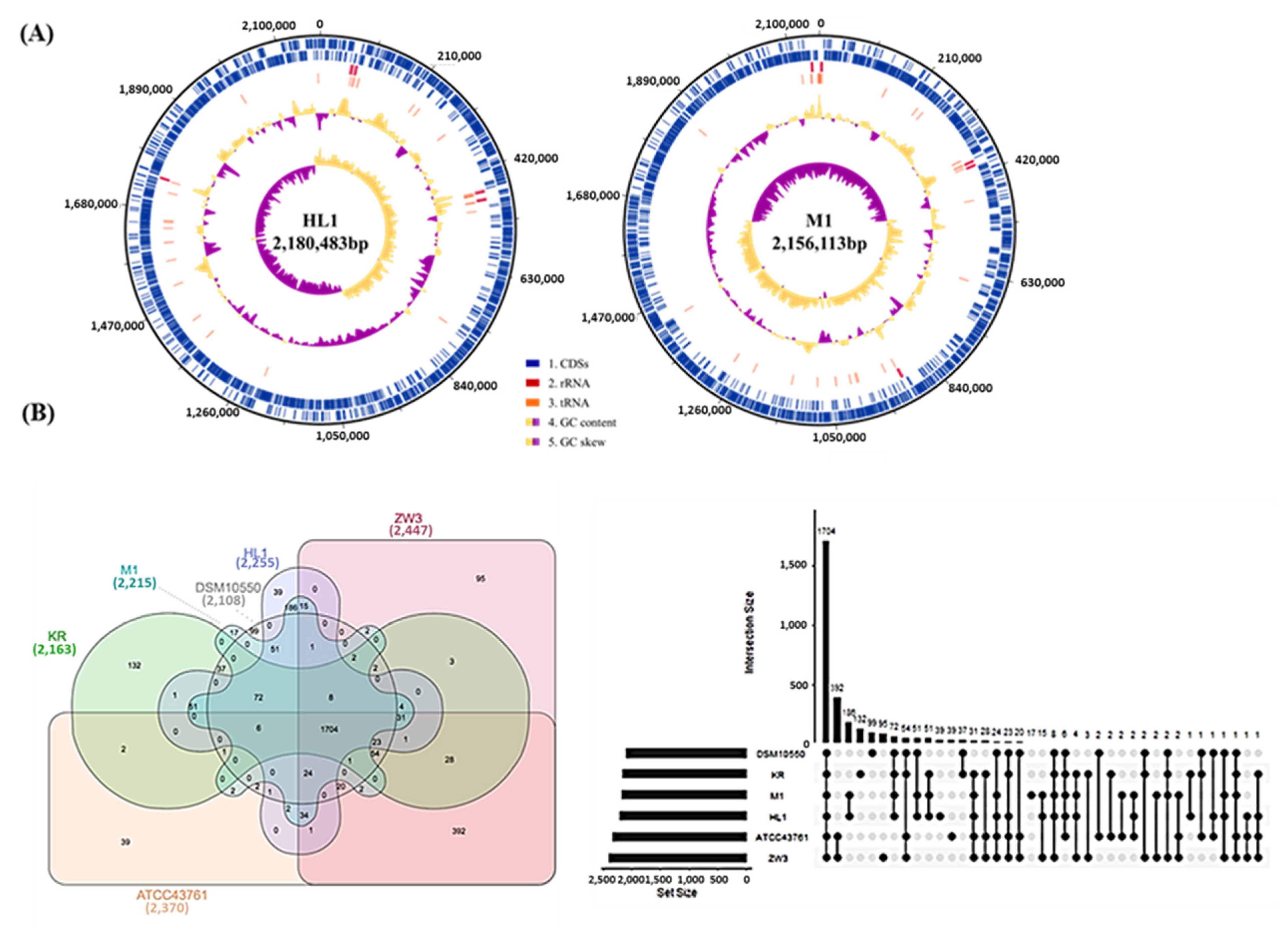
3.2.1. Genome Features
3.2.2. Whole CDS Venn Diagrams
3.3. Pan-Genome Analysis in HL1 and M1
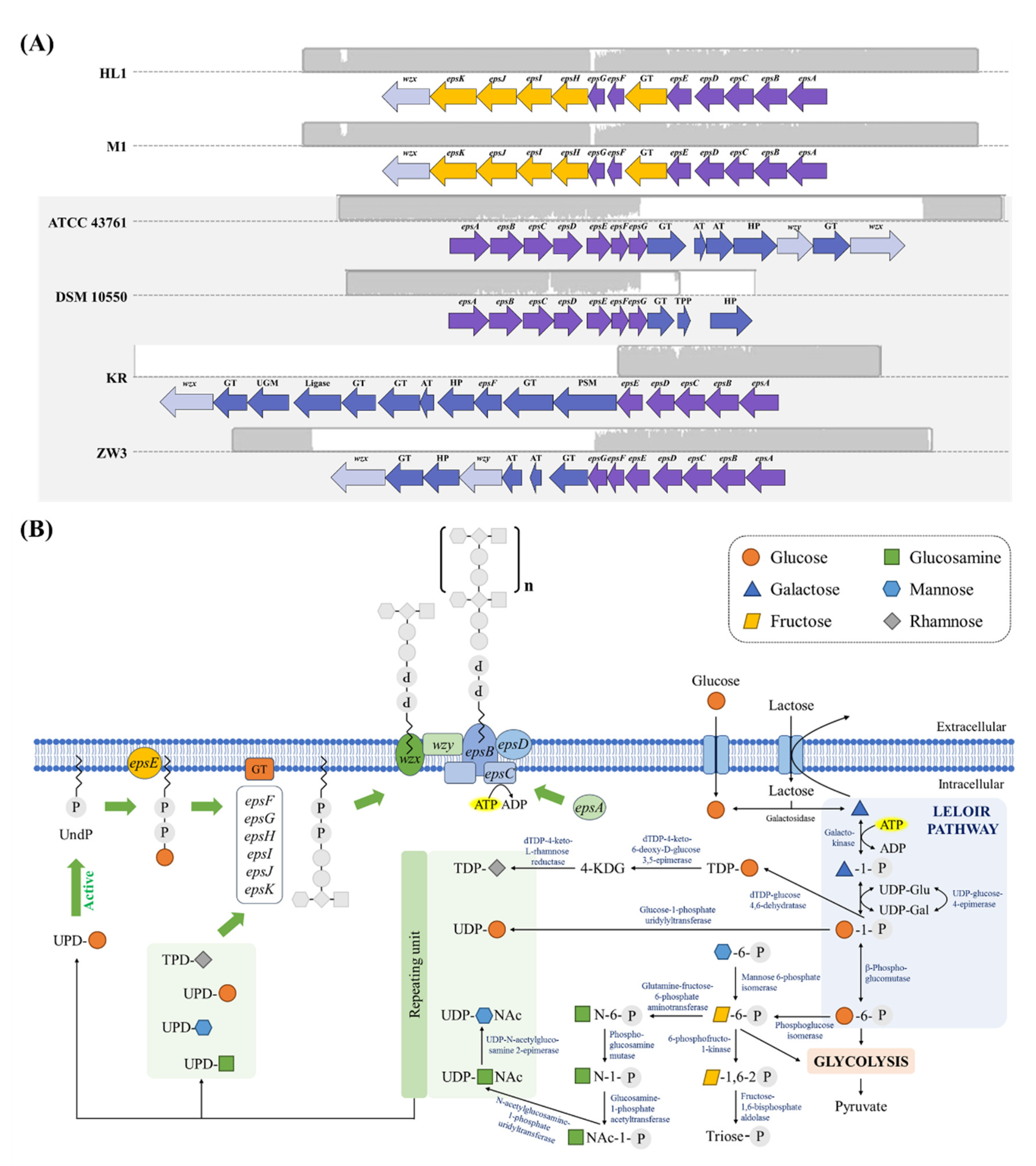
3.3.1. Polysaccharide Synthesis
The Cluster of Orthologous Groups Function of Genes in EPS Related Subsystems
Identification of the HL1 and M1EPS Biosynthetic Gene Cluster
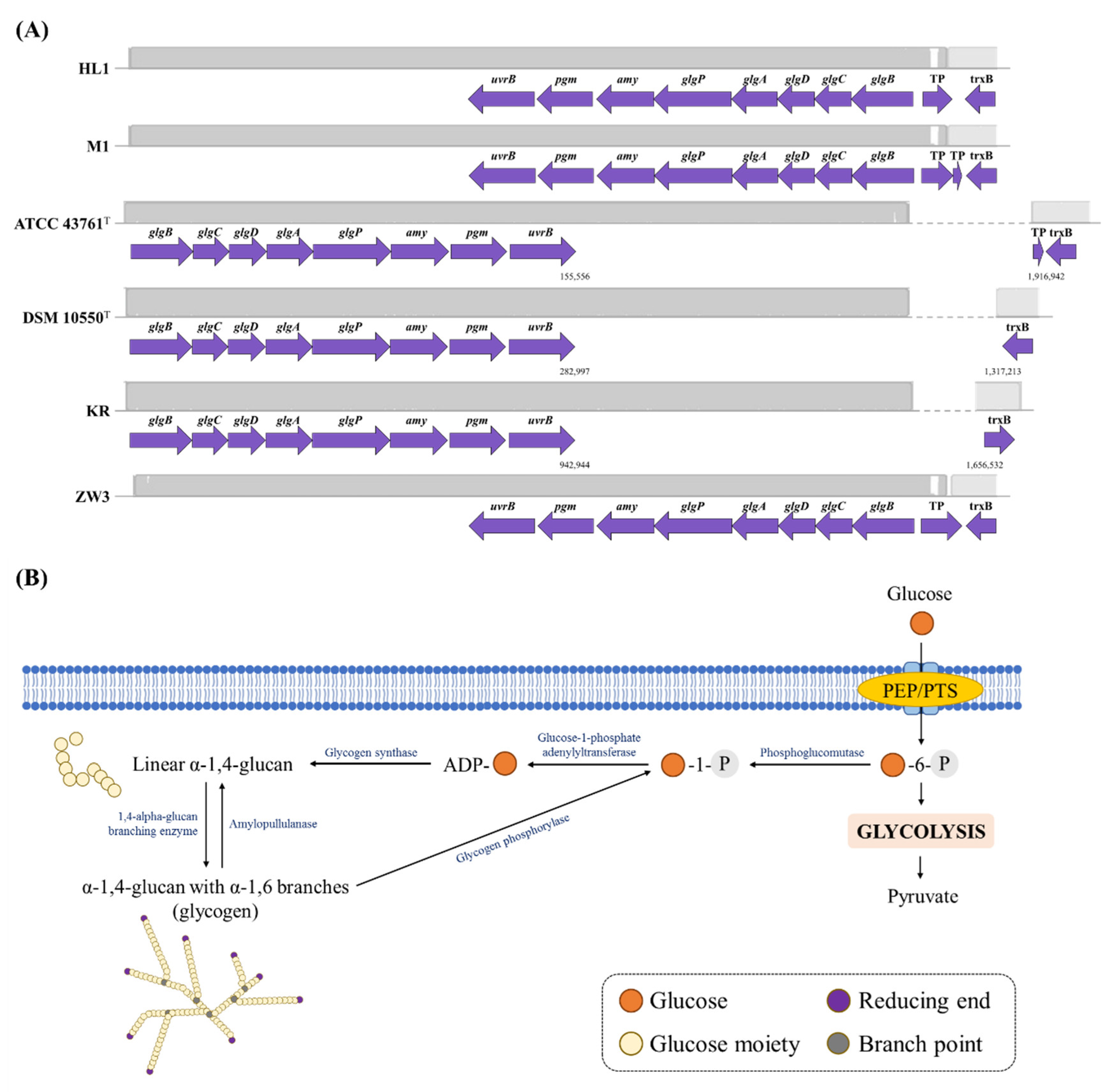
3.3.2. Glycogen Metabolism and Stress Response
3.3.3. Cell Surface Adhesins
3.4. The Unique Genes in HL1 and M1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujisawa, T.; Adachi, S.; Toba, T.; Arihara, K.; Mitsuoka, T. Lactobacillus kefiranofaciens sp. nov. isolated from kefir grains. Int. J. Syst. Bacteriol. 1988, 38, 12–14. [Google Scholar] [CrossRef]
- Wang, Y.P.; Ahmed, Z.; Feng, W.; Li, C.; Song, S.Y. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int. J. Biol. Macromol. 2008, 43, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Rimada, P.S.; Abraham, A.G. Kefiran improves rheological properties of glucono-lactone induced skim milk gels. Int. Dairy J. 2006, 16, 33–39. [Google Scholar] [CrossRef]
- Takizawa, S.; Kojima, S.; Tamura, S.; Fujinaga, S.; Benno, Y.; Nagase, T. Lactobacillus kefirgranum sp. nov. and Lactobacillus parakefir sp. nov., two new species from kefir grains. Int. J. Syst. Bacteriol. 1994, 44, 435–439. [Google Scholar] [CrossRef]
- Vancanneyt, M.; Mengaud, J.; Cleenwerck, I.; Vanhonacker, K.; Hoste, B. Reclassification of Lactobacillus kefirgranum Takizawa et al. 1994 as Lactobacillus kefiranofaciens subsp. kefirgranum subsp. nov. and emended description of L. kefiranofaciens Fujisawa et al. 1988. Int. J. Syst. Evol. Microbiol. 2004, 54, 551–556. [Google Scholar] [CrossRef]
- Chen, H.C.; Wang, S.Y.; Chen, M.J. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 2008, 25, 492–501. [Google Scholar] [CrossRef]
- Hong, W.S.; Chen, H.C.; Chen, Y.P.; Chen, M.J. Effects of kefir supernatant and lactic acid bacteria isolated from kefir grain on cytokine production by macrophage. Int. Dairy J. 2009, 19, 244–251. [Google Scholar] [CrossRef]
- Hong, W.S.; Chen, Y.P.; Chen, M.J. The antiallergic effect of kefir Lactobacilli. J. Food Sci. 2010, 75, H244–H253. [Google Scholar] [CrossRef]
- Hong, W.S.; Chen, Y.P.; Dai, T.Y.; Huang, I.N.; Chen, M.J. Effect of heat-inactivated kefir-isolated Lactobacillus kefiranofaciens M1 on preventing an allergic airway response in mice. J. Agric. Food Chem. 2011, 59, 9022–9031. [Google Scholar] [CrossRef]
- Chen, Y.P.; Hsiao, P.J.; Hong, W.S.; Dai, T.Y.; Chen, M.J. Lactobacillus kefiranofaciens M1 isolated from milk kefir grains ameliorates experimental colitis in vitro and in vivo. J. Dairy Sci. 2012, 95, 63–74. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, Y.T.; Hsieh, H.H.; Chen, M.J. Effect of Lactobacillus mali APS1 and L. kefiranofaciens M1 on obesity and glucose homeostasis in diet-induced obese mice. J. Funct. Food. 2016, 23, 580–589. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, Y.T.; Li, K.Y.; Chen, M.J. Investigating the mechanistic differences of obesity-inducing Lactobacillus kefiranofaciens M1 and anti-obesity Lactobacillus mali APS1 by microbolomics and metabolomics. Front. Microbiol. 2020, 11, 1454. [Google Scholar] [CrossRef]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of health benefits conferred by Lactobacilllus species from Kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef]
- Ho, S.T.; Hsieh, Y.T.; Wang, S.Y.; Chen, M.J. Improving effect of a probiotic mixture on memory and learning abilities in d-galactose-treated aging mice. J. Dairy Sci. 2019, 102, 1901–1909. [Google Scholar] [CrossRef]
- Pot, B.; Vandamme, P.; Kersters, K. Analysis of electrophoretic whole-organism protein fingerprints. In Chemical Methods in Prokaryotic Systematics; Goodfellow, M., O’Donnell, A.G., Eds.; John Wiley & Sons: Chichester, UK, 1994; Volume 5, pp. 493–521. [Google Scholar]
- Watanabe, K.; Fujimoto, J.; Sasamoto, M.; Dugersuren, J.; Tumursuh, T.; Demberel, S. Diversity of lactic acid bacteria and yeasts in Airag and Tarag, traditional fermented milk products of Mongolia. World J. Microbiol. Biotechnol. 2008, 24, 1313–1325. [Google Scholar] [CrossRef]
- Naser, S.M.; Dawyndt, P.; Hoste, B.; Gevers, D.; Vandemeulebroecke, K.; Cleenwerck, I.; Vancanneyt, M.; Swings, J. Identification of lactobacilli by pheS and rpoA gene sequence analyses. Int. J. Syst. Evol. Microbiol. 2007, 57, 2777–2789. [Google Scholar] [CrossRef]
- Naser, S.M.; Thompson, F.L.; Hoste, B.; Gevers, D.; Dawyndt, P.; Vancanneyt, M.; Swings, J. Application of multilocus sequence analysis (MLSA) for rapid identification of Enterococcus species based on rpoA and pheS genes. Microbiology 2005, 151, 2141–2150. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Versalovic, J.; Koeuth, T.; Lupski, J.R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef]
- Akopyanz, N.; Bukanov, N.O.; Westblom, T.U.; Kresovich, S.; Berg, D.E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992, 20, 5137–5142. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Overbeek, R.; Begley, T.; Butler, R.M.; Choudhuri, J.V.; Chuang, H.Y.; Cohoon, M.; de Crecy-Lagard, V.; Diaz, N.; Disz, T.; Edwards, R.; et al. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 2005, 33, 5691–5702. [Google Scholar] [CrossRef]
- McNaughton, A.L.; Roberts, H.E.; Bonsall, D.; de Cesare, M.; Mokaya, J.; Lumley, S.F.; Golubchik, T.; Piazza, P.; Martin, J.B.; de Lara, C.; et al. Illumina and Nanopore methods for whole genome sequencing of hepatitis B virus (HBV). Sci. Rep. 2019, 9, 7081. [Google Scholar] [CrossRef]
- Takizawa, S.; Kojima, S.; Tamura, S.; Fujinaga, S.; Benno, Y.; Nakase, T. The composition of the Lactobacillus flora in kefir grains. Syst. Appl. Microbiol. 1998, 21, 121–127. [Google Scholar] [CrossRef]
- Huptas, C.; Scherer, S.; Wenning, M. Optimized Illumina PCR-free library preparation for bacterial whole genome sequencing and analysis of factors influencing de novo assembly. BMC Res. Notes 2016, 9, 269. [Google Scholar] [CrossRef]
- Xing, Z.; Geng, W.; Li, C.; Sun, Y.; Wang, Y. Comparative genomics of Lactobacillus kefiranofaciens ZW3 and related members of Lactobacillus. spp. reveal adaptations to dairy and gut environments. Sci. Rep. 2017, 7, 12827. [Google Scholar] [CrossRef]
- Georgalaki, M.; Zoumpopoulou, G.; Anastasiou, R.; Kazou, M.; Tsakalidou, E. Lactobacillus kefiranofaciens: From isolation and taxonomy to probiotic properties and applications. Microorganisms 2021, 9, 2158. [Google Scholar] [CrossRef]
- Boels, I.C.; Beerthuyzen, M.M.; Kosters, M.H.; Van Kaauwen, M.P.; Kleerebezem, M.; De Vos, W.M. Identification and functional characterization of the Lactococcus lactis rfb operon, required for dTDP-rhamnose biosynthesis. J. Bacteriol. 2004, 186, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J.; Wolin, M.J. Cell wall polymers and phage lysis of Lactobacillus plantarum. Biochemistry 1971, 10, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Coyette, J.; Ghuysen, J.M. Structure of cell walls of Lactobacillus acidophilus strain 63 AM Gasser. Biochemistry 1970, 9, 2935–2943. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.H.; Kilpper-Bältz, R. Molecular and chemotaxonomic approaches to the classification of streptococci, enterococci and lactococci: A review. Syst. Appl. Microbiol. 1987, 10, 1–19. [Google Scholar] [CrossRef]
- Sijtsma, L.K.J.; Hellingwerf, K.J.; Wouters, T.M. Composition and phage binding capacity of cell walls isolated from Lactococcus lactis subsp. cremoris SK110 and SK112. Neth. Milk Dairy J. 1991, 45, 81–95. [Google Scholar]
- Forde, A.; Fitzgerald, G.F. Bacteriophage defence systems in lactic acid bacteria. Antonie Van Leeuwenhoek 1999, 76, 89–113. [Google Scholar] [CrossRef]
- Islam, S.T.; Lam, J.S. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can. J. Microbiol. 2014, 60, 697–716. [Google Scholar] [CrossRef]
- van Kranenburg, R.; Marugg, J.D.; van Swam, I.I.; Willem, N.J.; deVos, W.M. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol. Microbiol. 1997, 24, 387–397. [Google Scholar] [CrossRef]
- Lebeer, S.; Verhoeven, T.L.A.; Francius, G.; Schoofs, G.; Lambrichts, I.; Dufrêne, Y.; Vanderleyden, J.; De Keersmaecker, S.C.J. Identification of a gene cluster for the biosynthesis of a long, galactose-rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase. Appl. Environ. Microbiol. 2009, 75, 3554–3563. [Google Scholar] [CrossRef]
- Escalante, A.; Villegas, J.; Wacher, C.; García-Garibay, M.; Farrés, A.C. Activity of the enzymes involved in the synthesis of exopolysaccharide precursors in an overproducing mutant ropy strain of Streptococcus thermophilus. FEMS Microbiol. Lett. 2002, 209, 289–293. [Google Scholar] [CrossRef][Green Version]
- Barreto, M.; Jedlicki, E.; Holmes, D.S. Identification of a gene cluster for the formation of extracellular polysaccharide precursors in the chemolithoautotroph Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2005, 71, 2902–2909. [Google Scholar] [CrossRef]
- Looijesteijn, P.J.; Trapet, L.; de Vries, E.; Abee, T.; Hugenholtz, J. Physiological function of exopolysaccharides produced by Lactococcus lactis. Int. J. Food Microbiol. 2001, 64, 71–80. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Goh, Y.J.; Klaenhammer, T.R. A functional glycogen biosynthesis pathway in Lactobacillus acidophilus: Expression and analysis of the glg operon. Mol. Microbiol. 2013, 89, 1187–1200. [Google Scholar] [CrossRef]
- Zorzano, M.T.; Eydallin, G.; Ball, S.; Pozueta-Romero, J. Glycogen phosphorylase, the product of the glgP gene, catalyzes glycogen breakdown by removing glucose units from the nonreducing ends in Escherichia coli. J. Bacteriol. 2006, 188, 5266–5272. [Google Scholar] [CrossRef]
- Dauvillee, D.; Kinderf, I.S.; Li, Z.; Kosar-Hashemi, B.; Samuel, M.S.; Rampling, L.; Ball, S.; Morell, M.K. Role of the Escherichia coli glgX gene in glycogen metabolism. J. Bacteriol. 2005, 187, 1465–1473. [Google Scholar] [CrossRef]
- Goh, Y.J.; Klaenhammer, T.R. Insights into glycogen metabolism in Lactobacillus acidophilus: Impact on carbohydrate metabolism, stress tolerance and gut retention. Microb. Cell. Fact. 2014, 13, 94. [Google Scholar] [CrossRef]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef]
- Wang, L.; Wise, M.J. Glycogen with short average chain length enhances bacterial durability. Naturwissenschaften 2011, 98, 719–729. [Google Scholar] [CrossRef]
- Valeriano, V.D.V.; Oh, J.K.; Bagon, B.B.; Kim, H.; Kang, D.K. Comparative genomic analysis of Lactobacillus mucosae LM1 identifies potential niche-specific genes and pathways for gastrointestinal adaptation. Genomics 2019, 111, 24–33. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, K.N.; Lo, Y.M.; Chiang, M.L.; Chen, H.C.; Liu, J.R.; Chen, M.J. Investigation of microorganisms involved in biosynthesis of the kefir grain. Food Microbiol. 2012, 32, 274–285. [Google Scholar] [CrossRef]
- Mobegi, F.M.; Cremers, A.J.; de Jonge, M.I.; Bentley, S.D.; van Hijum, S.A.; Zomer, A. Deciphering the distance to antibiotic resistance for the pneumococcus using genome sequencing data. Sci. Rep. 2017, 16, 42808. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Gu, Q.; Lou, X.Y.; Zhang, X.M.; Song, D.F.; Zhang, C. Comparative genomic analysis of Lactobacillus plantarum ZJ316 reveals its genetic adaptation and potential probiotic profiles. J. Zhejiang Univ. Sci. B 2016, 17, 569–579. [Google Scholar] [CrossRef]
- Douillard, F.P.; Rasinkangas, P.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W.M. Functional identification of conserved residues involved in Lactobacillus rhamnosus strain GG sortase specificity and pilus biogenesis. J. Biol. Chem. 2014, 289, 15764–15775. [Google Scholar] [CrossRef]
- Chen, M.J.; Tang, H.Y.; Chiang, M.L. Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017, 66, 20–27. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chen, M.J. Effects of Lactobacillus kefiranofaciens M1 isolated from kefir grains on germ-free mice. PLoS ONE 2013, 8, e78789. [Google Scholar] [CrossRef]
- Kilstrup, M.; Hammer, K.; Jensen, P.R.; Martinussen, J. Nucleotide metabolism and its control in lactic acid bacteria. FEMS Microbiol. Rev. 2005, 29, 555–590. [Google Scholar] [CrossRef]
- Zuniga, M.; Perez, G.; Gonzalez-Candelas, F. Evolution of arginine deiminase (ADI) pathway genes. Mol. Phylogenet. Evol. 2002, 25, 429–444. [Google Scholar] [CrossRef]
- Galkin, A.; Kulakova, L.; Sarikaya, E.; Lim, K.; Howard, A.; Herzberg, O. Structural insight into arginine degradation by arginine deiminase, an antibacterial and parasite drug target. J. Biol. Chem. 2004, 279, 14001–14008. [Google Scholar] [CrossRef]
- Naser, S.; Thompson, F.L.; Hoste, B.; Gevers, D.; Vandemeulebroecke, K.; Cleenwerck, I.; Thompson, C.C.; Vancanneyt, M.; Swings, J. Phylogeny and identification of enterococci using atpA gene sequence analysis. J. Clin. Microbiol. 2005, 43, 2224–2230. [Google Scholar] [CrossRef]
- Bogicevic, B.; Berthoud, H.; Portmann, R.; Bavan, T.; Meile, L.; Irmler, S. Cysteine biosynthesis in Lactobacillus casei: Identification and characterization of a serine acetyltransferase. FEMS Microbiol. Lett. 2016, 363, fnw012. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, D.; Wenzel, C.; Bavan, T.; Bruggmann, R.; Berthoud, H.; Irmler, S. Transcriptional regulation of cysteine and methionine metabolism in Lactobacillus paracasei FAM18149. Front. Microbiol. 2018, 9, 126. [Google Scholar] [CrossRef] [PubMed]

| Carbon Source | HL1 | M1 | subsp. kefirgranum BCRC 80410T | subsp. kefiranofaciens BCRC 16059T |
|---|---|---|---|---|
| Amygdalin | − | − | + | − |
| Arbutin | − | − | + | − |
| Salicin | w | − | + | − |
| d-Cellobiose | − | − | + | − |
| d-Maltose | − | − | + | + |
| d-Melibiose | + | + | − | − |
| d-Sucrose | + | − | + | + |
| d-Trehalose | − | − | + | − |
| d-Raffinose | − | − | − | + |
| Gentiobiose | − | − | + | − |
| Aesculin | + | + | + | − |
| Subspecies | kefirgranum | kefiranofaciens | ||||
|---|---|---|---|---|---|---|
| Strain | HL1 | M1 | DSM 10550T | KR | ATCC 43761T | ZW3 |
| Accession No. | GCA_023674385.1 | GCA_023674405.1 | GCA_001434195.1 | GCA_002276565.1 | GCA_900103655.1 | GCA_000214785.1 |
| Genome size (Mbp) | 2.18 | 2.16 | 2.09 | 2.13 | 2.28 | 2.35 |
| Contigs | 2 | 2 | 138 | 97 | 84 | 3 |
| GC content (%) | 37.5 | 37.5 | 37.5 | 37.4 | 37.2 | 37.4 |
| CDS number | 2258 | 2208 | 2089 | 2141 | 2346 | 2423 |
| CRISPR | 7 | 7 | 6 | 2 | 1 | 1 |
| rRNA | 15 | 15 | 3 | 3 | 9 | 12 |
| COG Class | Function | Gene Number | |||||
|---|---|---|---|---|---|---|---|
| HL1 | M1 | DSM 10550T | KR | ATCC 43761T | ZW3 | ||
| L | Replication, recombination and repair | 252 | 235 | 176 | 183 | 215 | 258 |
| G | Carbohydrate transport and metabolism | 176 | 174 | 167 | 163 | 169 | 170 |
| K | Transcription | 164 | 163 | 171 | 156 | 165 | 164 |
| J | Translation, ribosomal structure and biogenesis | 151 | 151 | 154 | 154 | 154 | 154 |
| E | Amino acid transport and metabolism | 127 | 120 | 125 | 128 | 131 | 132 |
| P | Inorganic ion transport and metabolism | 120 | 119 | 120 | 118 | 125 | 125 |
| F | Nucleotide transport and metabolism | 109 | 108 | 107 | 113 | 114 | 114 |
| M | Cell wall/membrane/envelope biogenesis | 96 | 93 | 86 | 86 | 97 | 96 |
| C | Energy production and conversion | 66 | 66 | 66 | 74 | 71 | 71 |
| V | Defense mechanisms | 57 | 57 | 56 | 57 | 62 | 60 |
| I | Lipid transport and metabolism | 50 | 47 | 52 | 49 | 57 | 57 |
| H | Coenzyme transport and metabolism | 52 | 50 | 51 | 51 | 50 | 50 |
| U | Intracellular trafficking, secretion, and vesicular transport | 42 | 42 | 40 | 41 | 54 | 54 |
| O | Posttranslational modification, protein turnover, chaperones | 42 | 42 | 41 | 45 | 48 | 48 |
| T | Signal transduction mechanisms | 37 | 37 | 39 | 41 | 41 | 41 |
| D | Cell cycle control, cell division, chromosome partitioning | 35 | 35 | 35 | 34 | 36 | 35 |
| Q | Secondary metabolites biosynthesis, transport and catabolism | 10 | 10 | 10 | 10 | 13 | 13 |
| M | Cell motility | 7 | 7 | 8 | 9 | 7 | 7 |
| W | Extracellular structures | 5 | 5 | 4 | 5 | 10 | 10 |
| A | RNA processing and modification | 0 | 0 | 0 | 0 | 0 | 0 |
| B | Chromatin structure and dynamics | 0 | 0 | 0 | 0 | 0 | 0 |
| Y | Nuclear structure | 0 | 0 | 0 | 0 | 0 | 0 |
| Z | Cytoskeleton | 0 | 0 | 0 | 0 | 0 | 0 |
| S | Function unknown | 429 | 420 | 403 | 431 | 430 | 453 |
| Total | 2252 | 2200 | 2115 | 2163 | 2287 | 2360 | |
| Subcategory | HL1 | M1 | DSM 10550T | KR | ATCC 43761T | ZW3 |
|---|---|---|---|---|---|---|
| Capsular and extracellular polysaccharides | 15 | 15 | 15 | 4 | 4 | 4 |
| dTDP-rhamnose synthesis | 4 | 4 | 4 | – | – | – |
| Exopolysaccharide biosynthesis | 4 | 4 | 5 | 4 | 4 | 4 |
| Rhamnose-containing glycans | 7 | 7 | 6 | – | – | – |
| No subcategory | 7 | 7 | 7 | 7 | 7 | 7 |
| Murein hydrolases | 2 | 2 | 2 | 2 | 2 | 2 |
| Recycling of peptidoglycan amino acids | 1 | 1 | 1 | 1 | 1 | 1 |
| UDP-N-acetylmuramate from fructose-6-phosphate biosynthesis | 4 | 4 | 4 | 4 | 4 | 4 |
| Gram-Positive cell wall components | 17 | 16 | 17 | 17 | 17 | 17 |
| d-Alanyl Lipoteichoic acid biosynthesis | 3 | 3 | 3 | 3 | 3 | 3 |
| Sortase | 1 | – | 1 | 1 | 1 | 1 |
| Teichoic and lipoteichoic acids biosynthesis | 13 | 13 | 13 | 13 | 13 | 13 |
| Total | 39 | 38 | 39 | 28 | 28 | 28 |
| Locus_Tag | Start Position | End Position | ORF Length | % Identity | NCBI-Ref | Annotation |
|---|---|---|---|---|---|---|
| HL1_0556 | 539793 | 540485 | 693 | 99.86 | WP_013853943.1 | Class A sortase |
| HL1_0557 | 540610 | 542883 | 2274 | 99.74 | WP_095341978.1 | Single-stranded-DNA-specific exonuclease RecJ |
| HL1_0558 | 542989 | 543516 | 528 | 99.81 | WP_013853945.1 | Adenine phosphoribosyltransferase |
| HL1_0559 | 543772 | 546219 | 2448 | 99.80 | KRL29238.1 | Phosphoenolpyruvate synthase |
| HL1_0560 | 547467 | 546355 | 1113 | 99.91 | WP_013853947.1 | DUF871 domain-containing protein |
| HL1_0561 | 548483 | 547587 | 897 | 99.89 | WP_013853948.1 | Cysteine synthase A |
| HL1_0562 | 549284 | 548532 | 753 | 99.47 | KRL29241.1 | Homoserine O-succinyltransferase |
| HL1_0563 | 549994 | 549431 | 564 | 100.00 | KRL29242.1 | Cardiolipin synthetase |
| HL1_0564 | 550920 | 549991 | 930 | 99.68 | KRL29243.1 | Cardiolipin synthase |
| HL1_0565 | 551058 | 551549 | 492 | 99.8 | WP_095341979.1 | Lactocepin S-layer protein |
| HL1_0566 | 553917 | 551605 | 2313 | 99.87 | WP_056941039.1 | HAD-IC family P-type ATPase |
| HL1_0567 | 554068 | 555297 | 1230 | 99.76 | WP_056941040.1 | Arginine deiminase |
| HL1_0568 | 555311 | 556351 | 1041 | 99.90 | WP_013853953.1 | Ornithine carbamoyltransferase |
| HL1_0569 | 556335 | 557285 | 951 | 99.47 | KRL29248.1 | Carbamate kinase |
| HL1_0570 | 557299 | 557835 | 537 | 100.00 | WP_013853955.1 | Citrate lyase holo-[acyl-carrier protein] synthase |
| HL1_0571 | 557920 | 559083 | 1164 | 99.91 | WP_013853956.1 | GNAT family N-acetyltransferase |
| HL1_0573 | 559251 | 559865 | 615 | 100.00 | AEG40162.1 | Transcriptional regulator |
| HL1_0575 | 560614 | 561282 | 669 | 100.00 | WP_013853960.1 | Antibiotic biosynthesis monooxygenase |
| HL1_0577 | 563110 | 561755 | 1356 | 99.85 | WP_013853962.1 | Aspartate kinase |
| HL1_0578 | 564618 | 563128 | 1491 | 99.73 | WP_056941043.1 | Threonine synthase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-Y.; Chen, Y.-P.; Huang, R.-F.; Wu, Y.-L.; Ho, S.-T.; Li, K.-Y.; Watanabe, K.; Chen, M.-J. Subspecies Classification and Comparative Genomic Analysis of Lactobacillus kefiranofaciens HL1 and M1 for Potential Niche-Specific Genes and Pathways. Microorganisms 2022, 10, 1637. https://doi.org/10.3390/microorganisms10081637
Wang S-Y, Chen Y-P, Huang R-F, Wu Y-L, Ho S-T, Li K-Y, Watanabe K, Chen M-J. Subspecies Classification and Comparative Genomic Analysis of Lactobacillus kefiranofaciens HL1 and M1 for Potential Niche-Specific Genes and Pathways. Microorganisms. 2022; 10(8):1637. https://doi.org/10.3390/microorganisms10081637
Chicago/Turabian StyleWang, Sheng-Yao, Yen-Po Chen, Ren-Feng Huang, Yi-Lu Wu, Shang-Tse Ho, Kuan-Yi Li, Koichi Watanabe, and Ming-Ju Chen. 2022. "Subspecies Classification and Comparative Genomic Analysis of Lactobacillus kefiranofaciens HL1 and M1 for Potential Niche-Specific Genes and Pathways" Microorganisms 10, no. 8: 1637. https://doi.org/10.3390/microorganisms10081637
APA StyleWang, S.-Y., Chen, Y.-P., Huang, R.-F., Wu, Y.-L., Ho, S.-T., Li, K.-Y., Watanabe, K., & Chen, M.-J. (2022). Subspecies Classification and Comparative Genomic Analysis of Lactobacillus kefiranofaciens HL1 and M1 for Potential Niche-Specific Genes and Pathways. Microorganisms, 10(8), 1637. https://doi.org/10.3390/microorganisms10081637






