Chicken Gut Microbiota Responses to Dietary Bacillus subtilis Probiotic in the Presence and Absence of Eimeria Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic and Isolation of E. tenella
2.2. Experimental Design
2.3. Sample Collection, Extraction of DNA and PCR Amplification
2.4. MiSeq Sequencing Analyses
2.5. Statistical Analysis
3. Results
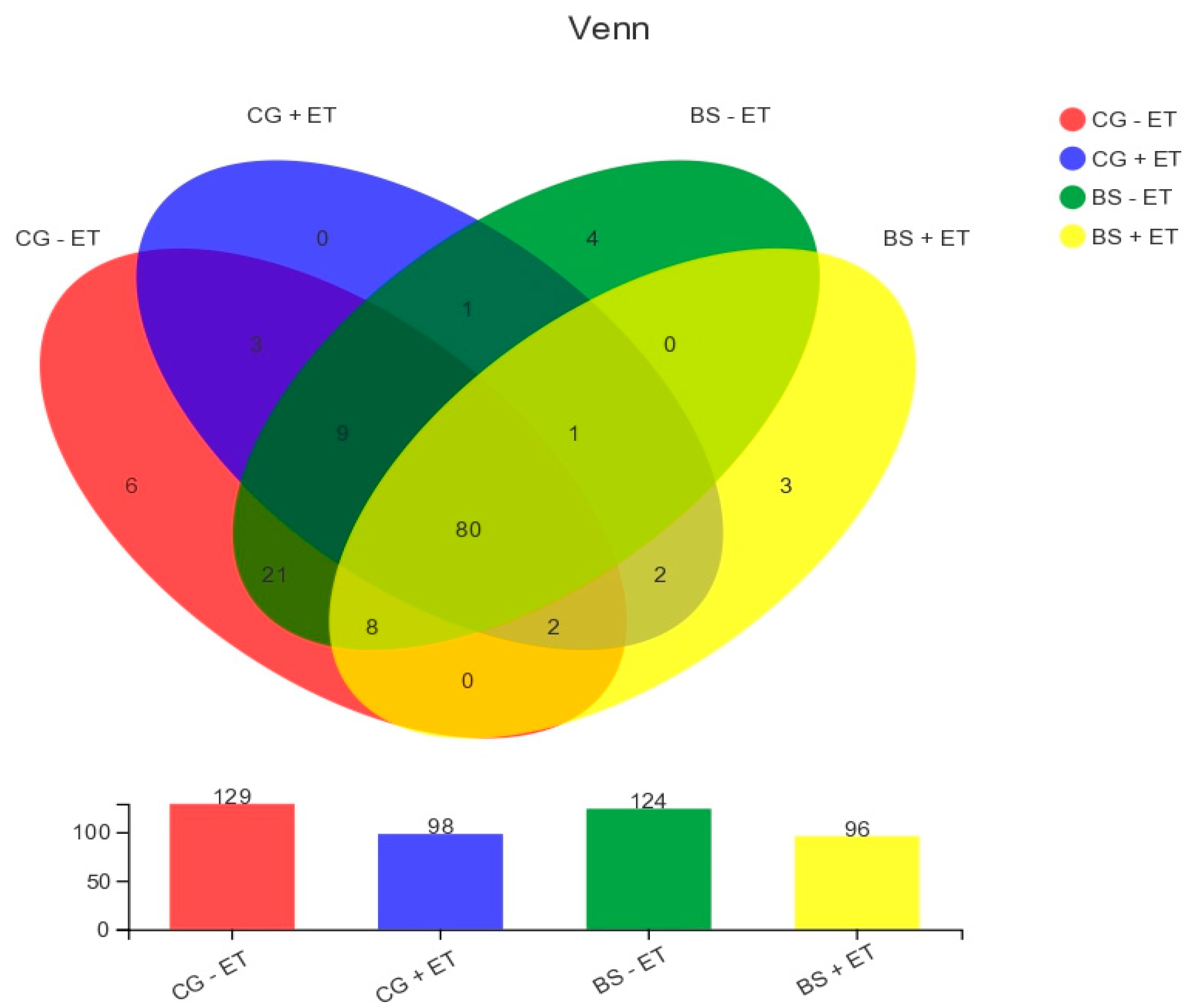
3.1. Quality Control Determination of MiSeq Sequencing
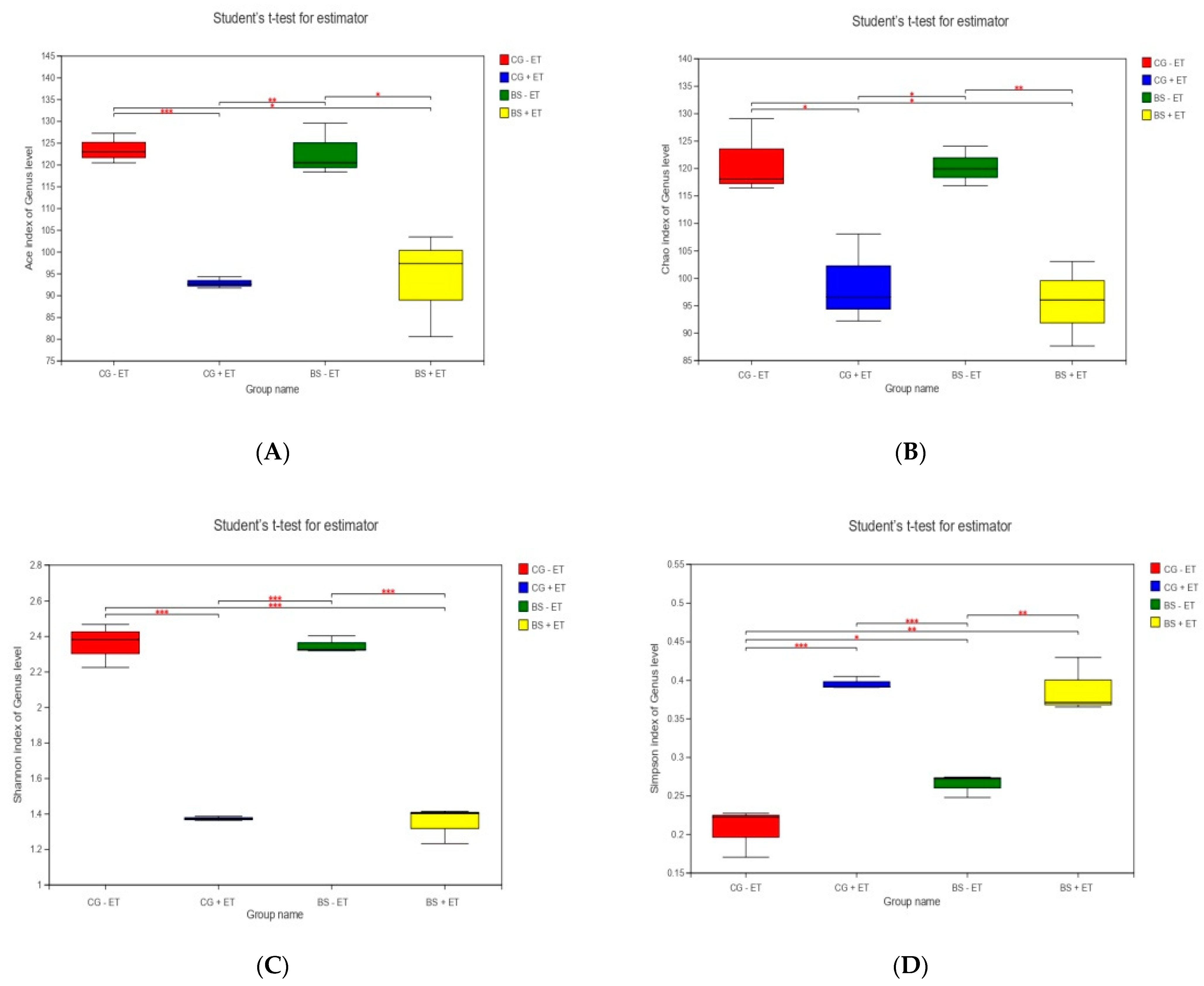
3.2. Alpha Diversity
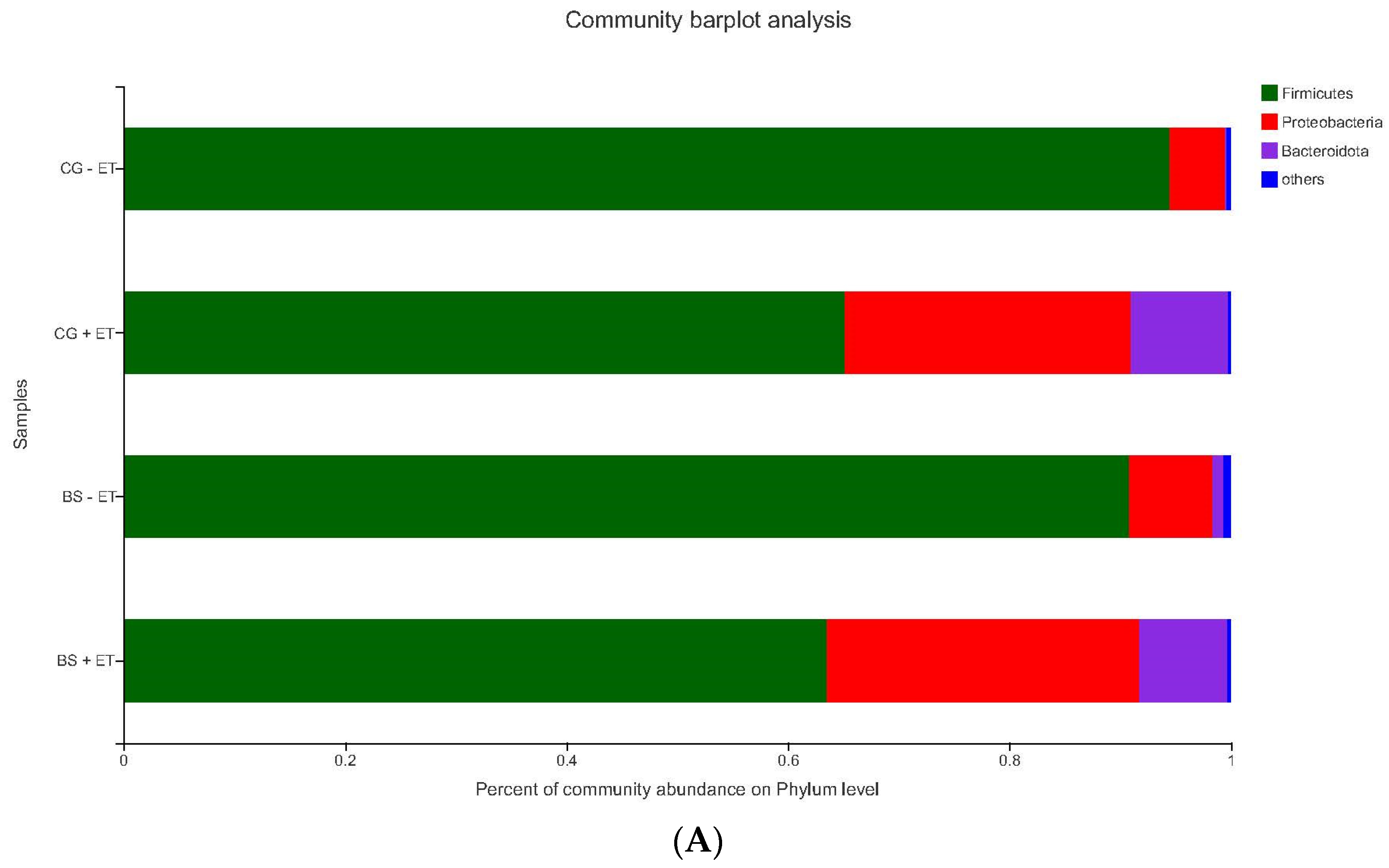
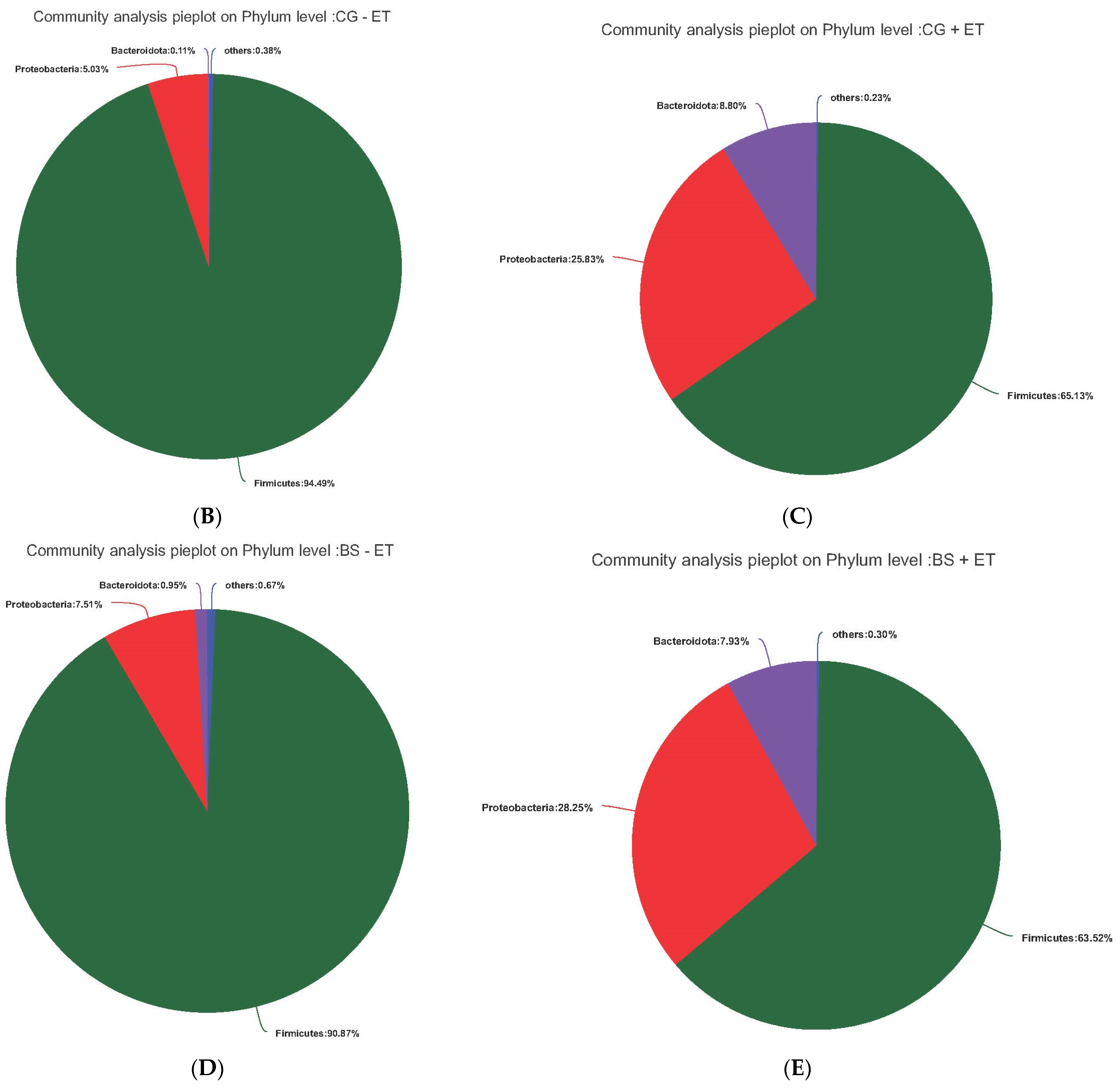
3.3. Effects of Treatments on Bacterial Abundances at Phylum Level
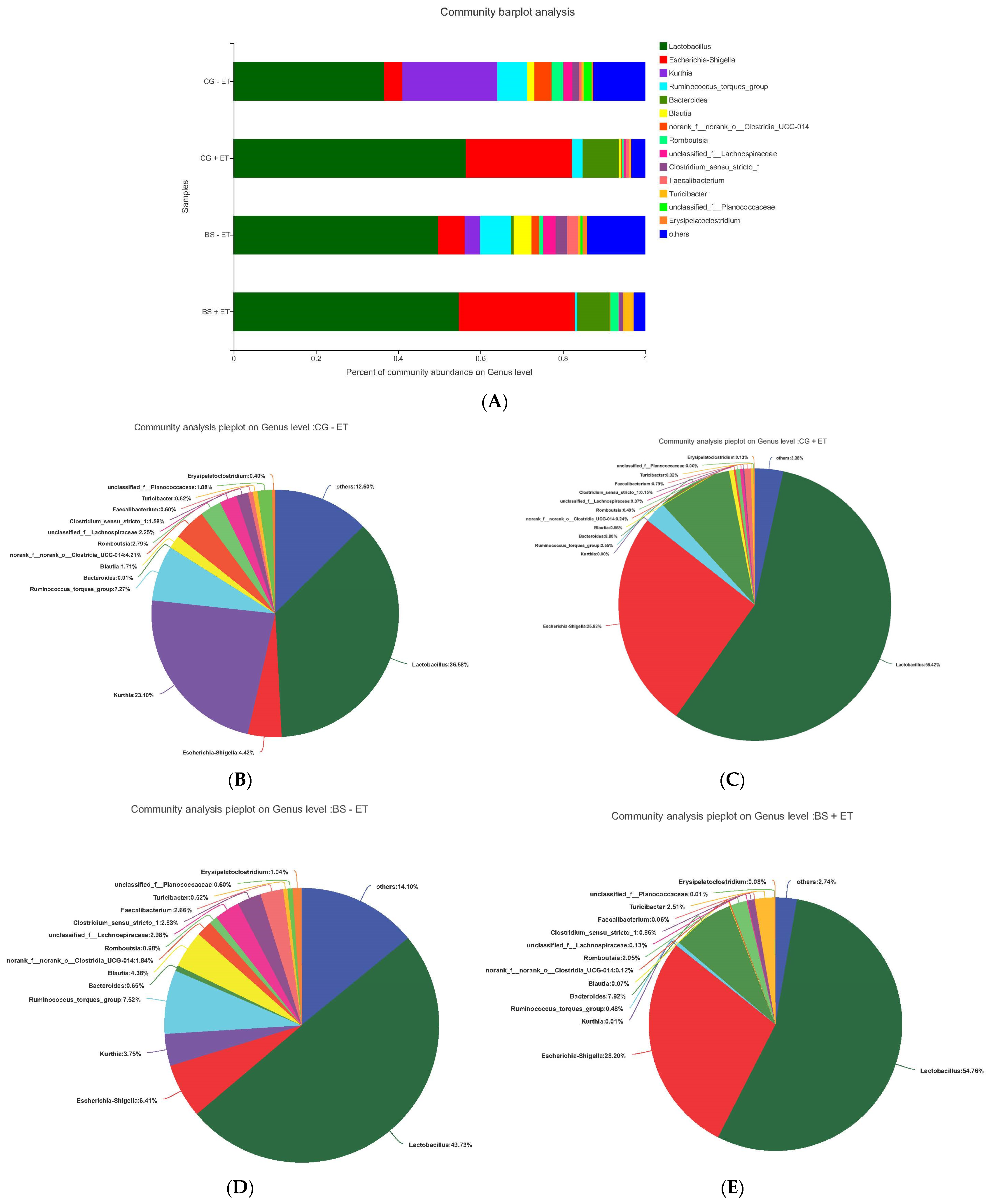
3.4. Effects of Treatments on Bacterial Abundances at Genus Level
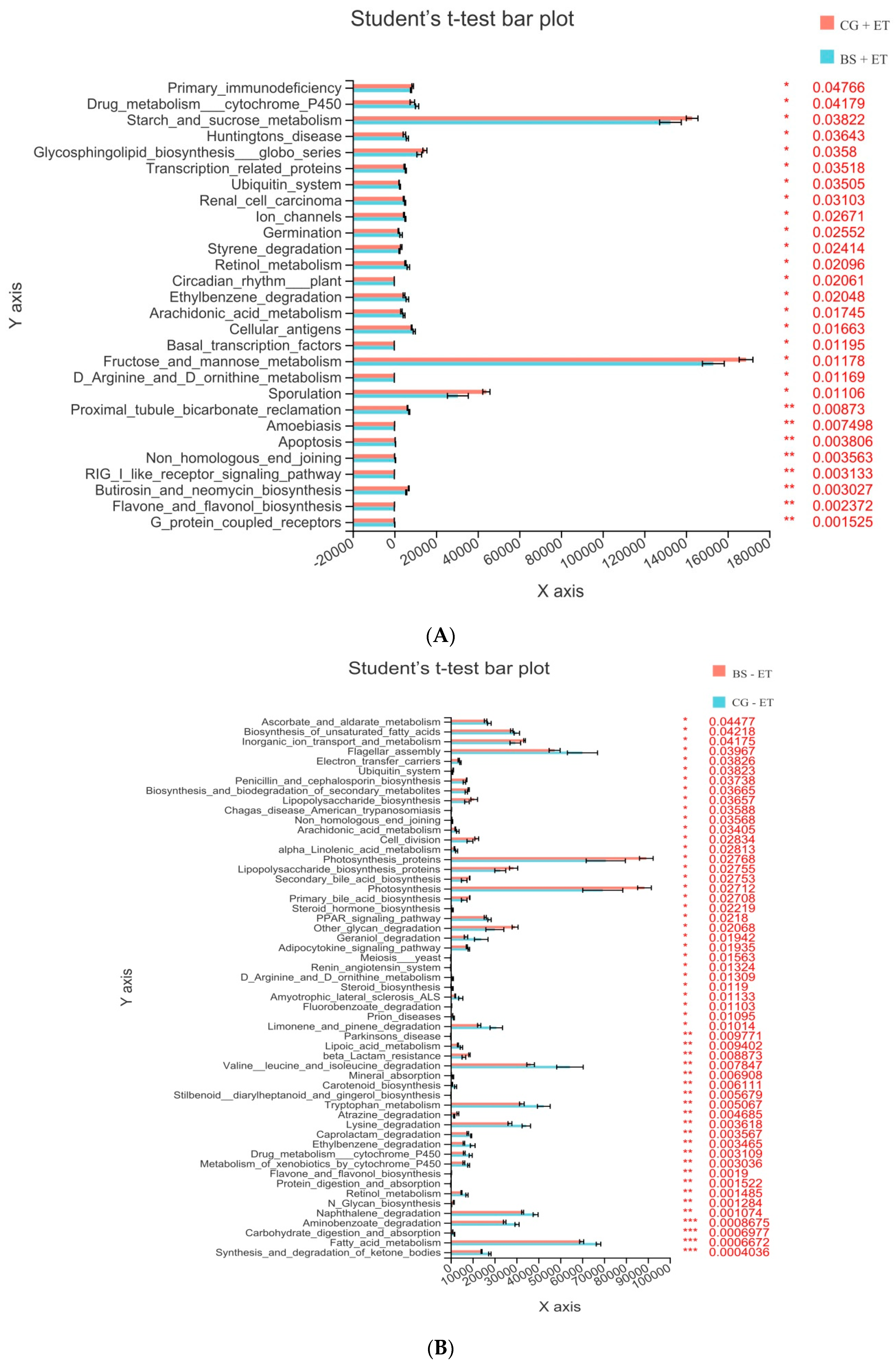
3.5. Predicted Functions of Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.; Lu, M.; Lillehoj, H.S.J.V. Coccidiosis: Recent progress in host immunity and alternatives to antibiotic strategies. Vaccines 2022, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.P.; McKenzie, M.E. Poultry Coccidiosis: Diagnostic and Testing Procedures; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Chapman, H.; Jeffers, T.; Williams, R. Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci. 2010, 89, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.A.; Iqbal, Z.; Abbas, R.Z.; Khan, M.N. Anticoccidial activity of herbal complex in broiler chickens challenged with Eimeria tenella. Parasitology 2012, 139, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Colwell, D.; Gilleard, J. Botanicals: An alternative approach for the control of avian coccidiosis. Worlds Poult. Sci. J. 2012, 68, 203–215. [Google Scholar] [CrossRef]
- Chapman, H. Practical use of vaccines for the control of coccidiosis in the chicken. Worlds Poult. Sci. J. 2000, 56, 7–20. [Google Scholar] [CrossRef]
- Patwardhan, B.; Gautam, M. Botanical immunodrugs: Scope and opportunities. Drug Discov. Today 2005, 10, 495–502. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K.J.A. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (direct-fed microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: A systematic review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Oyetayo, V.; Oyetayo, F. Potential of probiotics as biotherapeutic agents targeting the innate immune system. Afr. J. Biotech. 2005, 4, 123–127. [Google Scholar]
- Amara, A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.I.; Ahmad, N.; Miah, M.A. Comparative analysis of body weight and serum biochemistry in broilers supplemented with some selected probiotics and antibiotic growth promoters. J. Adv. Vet. Anim. Res. 2017, 4, 288–294. [Google Scholar] [CrossRef]
- Xu, L.; Fan, Q.; Zhuang, Y.; Wang, Q.; Gao, Y.; Wang, C. Bacillus coagulans enhance the immune function of the intestinal mucosa of yellow broilers. Braz. J. Poul. Sci. 2017, 19, 115–122. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Z.; Wang, Y.; Li, W. Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol. Biochem. 2010, 36, 501–509. [Google Scholar] [CrossRef]
- Lee, S.; Ingale, S.; Kim, J.; Kim, K.; Lokhande, A.; Kim, E.; Kwon, I.; Kim, Y.; Chae, B. Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim. Feed Sci. Technol. 2014, 188, 102–110. [Google Scholar] [CrossRef]
- Li, C.L.; Wang, J.; Zhang, H.J.; Wu, S.G.; Hui, Q.R.; Yang, C.B.; Fang, R.J.; Qi, G.H. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 2019, 9, 1968. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.T.; Lin, S.Y.; Yang, T.Y.; Chou, C.K.; Liu, H.C.; Lu, J.J.; Wang, B.; Chen, S.Y.; Lien, T.F. Effects of Bacillus coagulans ATCC 7050 on growth performance, intestinal morphology, and microflora composition in broiler chickens. Anim. Produc. Sci. 2012, 52, 874–879. [Google Scholar] [CrossRef]
- Jacquier, V.; Nelson, A.; Jlali, M.; Rhayat, L.; Brinch, K.; Devillard, E. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poul. Sci. 2019, 98, 2548–2554. [Google Scholar] [CrossRef]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current sampling methods for gut microbiota: A call for more precise devices. Front. Cell. Infect. Microbiol. 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, Y.; Gui, G.; Li, J.; Wang, J.; Li, D. Microbial community and short-chain fatty acid profile in gastrointestinal tract of goose. Poult. Sci. 2018, 97, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS ONE 2017, 12, e0188634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memon, F.; Yang, Y.; Lv, F.; Soliman, A.; Chen, Y.; Sun, J.; Wang, Y.; Zhang, G.; Li, Z.; Xu, B. Effects of probiotic and Bidens pilosa on the performance and gut health of chicken during induced Eimeria tenella infection. J. Appl. Microbiol. 2021, 131, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Memon, F.U.; Yang, Y.; Leghari, I.H.; Lv, F.; Soliman, A.M.; Zhang, W.; Si, H. Transcriptome Analysis Revealed Ameliorative Effects of Bacillus Based Probiotic on Immunity, Gut Barrier System, and Metabolism of Chicken under an Experimentally Induced Eimeria tenella Infection. Genes 2021, 12, 536. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magoč, T.; Salzberg, S.L.J.B. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Meth. 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Tian, Y.; Cao, Y.; Li, J.; Guo, H.; Su, Y.; Tian, Y.; Wang, C.; Wang, T.; Zhang, L. Probiotic properties of Lactobacillus paracasei subsp. paracasei L1 and its growth performance-promotion in chicken by improving the intestinal microflora. Front. Physiol. 2019, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Madlala, T.; Okpeku, M.; Adeleke, M. Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: A review. Parasites 2021, 28, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Farnell, Y.Z.; Kiess, A.S.; Peebles, E.D.; Wamsley, K.G.; Zhai, W. Effects of Bacillus subtilis and coccidial vaccination on cecal microbial diversity and composition of Eimeria-challenged male broilers. Poult. Sci. 2019, 98, 3839–3849. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xi, Y.; Xia, Y.; Wu, T.; Zhao, D.; Zhang, Z.; Ding, B. Dietary Lactobacillus fermentum and Bacillus coagulans Supplementation Modulates Intestinal Immunity and Microbiota of Broiler Chickens Challenged by Clostridium perfringens. Front. Vet. Sci. 2021, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lin, L.; Zheng, L.; Tang, H.; Fan, X.; Xue, N.; Li, M.; Liu, M.; Li, X. Cecal microbiome profile altered by Salmonella enterica, serovar Enteritidis inoculation in chicken. Gut Pathog. 2018, 10, 34. [Google Scholar] [CrossRef]
- Chen, H.-L.; Zhao, X.-Y.; Zhao, G.-X.; Huang, H.-B.; Li, H.-R.; Shi, C.-W.; Yang, W.-T.; Jiang, Y.-L.; Wang, J.-Z.; Ye, L.-P. Dissection of the cecal microbial community in chickens after Eimeria tenella infection. J. Parasites Vectors 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Pajarillo, E.A.B.; Chae, J.P.; Kim, I.H.; Yang, D.S.; Kang, D.-K. Effects of Bacillus subtilis CSL2 on the composition and functional diversity of the faecal microbiota of broiler chickens challenged with Salmonella Gallinarum. J. Anim. Sci. Biotech. 2017, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Adalsteinsdottir, S.A.; Magnusdottir, O.K.; Halldorsson, T.I.; Birgisdottir, B.E. Towards an individualized nutrition treatment: Role of the gastrointestinal microbiome in the interplay between diet and obesity. Curr. Obes. Rep. 2018, 7, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.A.B.; Kim, S.H.; Lee, J.-Y.; Valeriano, V.D.V.; Kang, D.-K. Quantitative proteogenomics and the reconstruction of the metabolic pathway in Lactobacillus mucosae LM1. Korean J. Anim. Sci. Resour. 2015, 35, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell Biosci. 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Magruder, M.; Edusei, E.; Zhang, L.; Albakry, S.; Satlin, M.J.; Westblade, L.F.; Malha, L.; Sze, C.; Lubetzky, M.; Dadhania, D.M. Gut commensal microbiota and decreased risk for Enterobacteriaceae bacteriuria and urinary tract infection. Gut Microbes 2020, 12, 1805281. [Google Scholar] [CrossRef]
- Gerritsen, J.; Hornung, B.; Ritari, J.; Paulin, L.; Rijkers, G.T.; Schaap, P.J.; de Vos, W.M.; Smidt, H. A comparative and functional genomics analysis of the genus Romboutsia provides insight into adaptation to an intestinal lifestyle. bioRxiv 2019, 845511. [Google Scholar] [CrossRef] [Green Version]
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.-D.; Delzenne, N.M.; Muccioli, G.G.; Clément, K.; Cani, P.D. From correlation to causality: The case of Subdoligranulum. Gut Microbes 2020, 12, 1849998. [Google Scholar] [CrossRef] [PubMed]
- Noriega, B.S.; Sanchez-Gonzalez, M.A.; Salyakina, D.; Coffman, J. Understanding the impact of omega-3 rich diet on the gut microbiota. Case Rep. Med. 2016, 2016, 3089303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. WJG 2011, 17, 1519. [Google Scholar] [CrossRef]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The genus Enterococcus: Between probiotic potential and safety concerns—An update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, O.N.; Ulyanova, V.V.; Yarullina, D.R.; Gataullin, I.G. Secretome of intestinal bacilli: A natural guard against pathologies. Front. Microbiol. 2017, 8, 1666. [Google Scholar] [CrossRef]


| Sample | Sequence Number | Base Number | Mean Length | Good’s Coverage |
|---|---|---|---|---|
| CG − ET_1 | 50120 | 21,043,414 | 419.86 | 0.99 |
| CG − ET_2 | 47657 | 20,114,212 | 422.06 | 0.99 |
| CG − ET_3 | 49749 | 20,880,643 | 419.71 | 0.99 |
| CG + ET_1 | 39661 | 16,889,906 | 425.85 | 0.99 |
| CG + ET_2 | 38967 | 16,588,260 | 425.70 | 0.99 |
| CG + ET_3 | 41425 | 17,642,574 | 425.89 | 0.99 |
| BS − ET_1 | 42819 | 17,960,932 | 419.46 | 0.99 |
| BS − ET_2 | 47562 | 19,942,902 | 419.30 | 0.99 |
| BS − ET_3 | 47540 | 19,961,785 | 419.89 | 0.99 |
| BS + ET_1 | 41516 | 17,708,570 | 426.54 | 0.99 |
| BS + ET_2 | 42939 | 18,320,028 | 426.65 | 0.99 |
| BS + ET_3 | 41109 | 17,562,370 | 427.21 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memon, F.U.; Yang, Y.; Zhang, G.; Leghari, I.H.; Lv, F.; Wang, Y.; Laghari, F.; Khushk, F.A.; Si, H. Chicken Gut Microbiota Responses to Dietary Bacillus subtilis Probiotic in the Presence and Absence of Eimeria Infection. Microorganisms 2022, 10, 1548. https://doi.org/10.3390/microorganisms10081548
Memon FU, Yang Y, Zhang G, Leghari IH, Lv F, Wang Y, Laghari F, Khushk FA, Si H. Chicken Gut Microbiota Responses to Dietary Bacillus subtilis Probiotic in the Presence and Absence of Eimeria Infection. Microorganisms. 2022; 10(8):1548. https://doi.org/10.3390/microorganisms10081548
Chicago/Turabian StyleMemon, Fareed Uddin, Yunqiao Yang, Geyin Zhang, Imdad Hussain Leghari, Feifei Lv, Yuhan Wang, Farooque Laghari, Farooque Ahmed Khushk, and Hongbin Si. 2022. "Chicken Gut Microbiota Responses to Dietary Bacillus subtilis Probiotic in the Presence and Absence of Eimeria Infection" Microorganisms 10, no. 8: 1548. https://doi.org/10.3390/microorganisms10081548
APA StyleMemon, F. U., Yang, Y., Zhang, G., Leghari, I. H., Lv, F., Wang, Y., Laghari, F., Khushk, F. A., & Si, H. (2022). Chicken Gut Microbiota Responses to Dietary Bacillus subtilis Probiotic in the Presence and Absence of Eimeria Infection. Microorganisms, 10(8), 1548. https://doi.org/10.3390/microorganisms10081548






