Biochemical Diversity, Pathogenicity and Phylogenetic Analysis of Pseudomonas viridiflava from Bean and Weeds in Northern Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates, Culture Conditions and Phenotypic Characterization
2.2. 16S rDNA Sequencing and ARDRA (Amplified Ribosomal DNA Restriction Analysis
2.3. Detection of Pathogenicity Islands
2.4. Pathogenicity Assays
2.5. Phylogenetic Analysis
3. Results and Discussion
3.1. Identification and Biochemical Characterization of the Isolates
3.2. Occurrence of the Bacterium in Weeds and Bean Samples
3.3. PAI Distribution, Pectinolysis Activity, and Pathogenicity Tests
3.4. Phylogenetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, J.M.; Cheesmur, G.J.; Welham, F.V.; Henshall, W.R. Bacterial blight of kiwifruit. Ann. Appl. Biol. 1988, 112, 91–105. [Google Scholar] [CrossRef]
- Yildiz, H.N.; Aysan, Y.; Sahin, F.; Cinar, O. Potential inoculum sources of tomato stem and pith necrosis caused by Pseudomonas viridiflava in the Eastern Mediterranean Region of Turkey. Z. Pflanzenkrankh. Pflanzenschutz 2004, 111, 380–387. [Google Scholar]
- Popović, T.; Ivanović, Ž.; Ignjatov, M. First report of Pseudomonas viridiflava causing pith necrosis of tomato (Solanum lycopersicum) in Serbia. Plant Dis. 2015, 99, 1033. [Google Scholar] [CrossRef]
- Martín-Sanz, A.; Palomo, J.L.; Pérez de la Vega, M.; Caminero, C. First report of bacterial blight caused by Pseudomonas viridiflava on pea in Spain. Plant Dis. 2010, 94, 128. [Google Scholar] [CrossRef]
- Heydari, A.; Khodakaramian, G.; Zafari, D. Characterization of Pseudomonas viridiflava causing alfalfa root rot disease in Hamedan Province of Iran. J. Plant Pathol. Microbiol. 2012, 3, 135. [Google Scholar] [CrossRef]
- Almeida, I.M.G.; Maciel, K.W.; Rodrigues Neto, J.; Beriam, L.O.S. Pseudomonas viridiflava in imported carrot seeds. Australas. Plant Dis. Notes 2013, 8, 17–19. [Google Scholar] [CrossRef][Green Version]
- Al-Karablieh, N.; Mutlak, I.; Al-Dokh, A. Isolation and identification of Pseudomonas viridiflava, the causal agent of fruit rotting of Cucumis sativus. J. J. Agri. Sci. 2017, 13, 79–91. [Google Scholar]
- Choi, O.; Lee, Y.; Kang, B.; Kim, S.; Bae, J.; Kim, J. Bacterial shoot blight of sweet crab apple caused by Pseudomonas viridiflava. For. Pathol. 2020, 50, e12603. [Google Scholar] [CrossRef]
- Bophela, K.N.; Petersen, Y.; Bull, C.T.; Coutinho, T.A. Identification of Pseudomonas isolates associated with bacterial canker of stone fruit trees in the Western Cape, South Africa. Plant Dis. 2020, 104, 882–892. [Google Scholar] [CrossRef]
- Canik Orel, D. Biocontrol of bacterial diseases with beneficial bacteria in lettuce. Intern. J. Agric. Nat. Sci. 2020, 13, 108–117. [Google Scholar]
- Goumans, D.E.; Chatzaki, A.K. Characterization and host range evaluation of Pseudomonas viridiflava from melon, blite, tomato, chrysanthemum and eggplant. Eur. J. Plant Pathol. 1998, 104, 181–188. [Google Scholar] [CrossRef]
- Goss, E.M.; Kreitman, M.; Bergelson, J. Genetic diversity, recombination and cryptic clades in Pseudomonas viridiflava infecting natural populations of Arabidopsis thaliana. Genetics 2005, 169, 21–35. [Google Scholar] [CrossRef]
- González, A.J.; Rodicio, M.R.; Mendoza, M.C. Identification of an emergent and atypical Pseudomonas viridiflava lineage causing bacteriosis in plants of agronomic importance in a Spanish region. Appl. Environ. Microbiol. 2003, 69, 2936–2941. [Google Scholar] [CrossRef]
- González, A.J.; Rodicio, M.R. Pseudomonas viridiflava causing necrotic leaf spots and defoliation on Hebe spp. in Northern Spain. Plant Dis. 2006, 90, 830. [Google Scholar] [CrossRef]
- Myung, I.S.; Lee, Y.K.; Lee, S.W.; Kim, W.G.; Shim, H.S.; Ra, D.S. A new disease, bacterial leaf spot of rape, caused by atypical Pseudomonas viridiflava in South Korea. Plant Dis. 2010, 94, 1164. [Google Scholar] [CrossRef]
- Anzai, Y.; Kim, H.; Park, J.; Wakabayashi, H.; Oyaizu, H. Phylogenetic affiliation of the Pseudomonads based on 16S rRNA sequence. Int. J. Syst. Evol. Microbiol. 2000, 50, 1563–1589. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kasai, H.; Arnold, D.L.; Jackson, R.W.; Vivian, A.; Harayama, S. Phylogeny of the genus Pseudomonas: Intrageneric structure reconstructed from the nucleotide sequences of gyrB and rpoD genes. Microbiology 2000, 146, 2385–2394. [Google Scholar] [CrossRef]
- Mulet, M.; Lalucat, J.; García-Valdés, E. DNA sequence-based analysis of the Pseudomonas species. Environ. Microbiol. 2010, 12, 1513–1530. [Google Scholar]
- Araki, H.; Tian, D.; Goss, E.M.; Jakob, K.; Halldorsdottir, S.S.; Kreitman, M.; Bergelson, J. Presence/absence polymorphism for alternative pathogenicity islands in Pseudomonas viridiflava, a pathogen of Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5887–5892. [Google Scholar] [CrossRef]
- Liao, C.H.; Hung, H.Y.; Chatterjee, A.K. An extracellular pectate lyase is the pathogenicity factor of the soft-rotting bacterium Pseudomonas viridiflava. Mol. Plant-Microbe Interact. 1988, 1, 199–206. [Google Scholar] [CrossRef]
- Liao, C.H.; McCallus, D.E.; Fett, W.F. Molecular characterization of two gene loci required for production of the key pathogenicity factor pectate lyase in Pseudomonas viridiflava. Mol. Plant-Microbe Interact. 1994, 7, 391–400. [Google Scholar] [CrossRef]
- Gitaitis, R.; Macdonald, G.; Torrance, R.; Hartley, R.; Sumner, D.R.; Gay, J.D.; Johnson, W.C., III. Bacterial streak and bulb rot of sweet onion: II. Epiphytic survival of Pseudomonas viridiflava in association with multiple weed hosts. Plant Dis. 1998, 82, 935–938. [Google Scholar] [CrossRef][Green Version]
- Basavand, E.; Khodaygan, P. Common water-plantain, a new host of Pseudomonas viridiflava in rice fields in Iran. J. Plant Pathol. 2020, 102, 913. [Google Scholar] [CrossRef]
- Ryu, E. A simple method of differentiation between gram-positive and gram-negative organisms without staining. Kitasato Arch. Exp. Med. 1940, 17, 58–63. [Google Scholar]
- Hugh, R.; Leifson, E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various Gram negative bacteria. J. Bacteriol. 1953, 66, 24–26. [Google Scholar] [CrossRef]
- Lelliott, R.A.; Billing, E.; Hayward, A.C. A determinative scheme for fluorescent plant pathogenic bacteria. J. Appl. Bacteriol. 1966, 29, 470–478. [Google Scholar] [CrossRef]
- Jansing, H.; Rudolph, K. A sensitive and quick test for determination of bean seed infestation by Pseudomonas syringae pv. phaseolicola. Z. Pflanzenkrankh. Pflanzenschutz 1990, 97, 42–55. [Google Scholar]
- Schaad, N.W.; Jones, J.B.; Chun, W. Laboratory Guide for Identification of Plant-Pathogenic Bacteria, 3rd ed.; CPL APS Press: St. Paul, MN, USA, 2001; p. 398. [Google Scholar]
- Noval, C. Medios de cultivo y pruebas de diagnóstico. In Manual de Laboratorio. Diagnóstico de Hongos, Bacterias y Nematodos Fitopatógenos; MAPA: Madrid, Spain, 1991; pp. 379–410. [Google Scholar]
- Goszczynska, T.; Serfortein, J. Milk-Tween agar, a semiselective medium for isolation and differentiation of Pseudomonas syringae pv. syringae, Pseudomonas syringae pv. phaseolicola and Xanthomonas axonopodis pv. phaseoli. J. Microbiol. Methods 1998, 32, 65–72. [Google Scholar] [CrossRef]
- Edwards, U.; Rogall, T.; Blöcker, H.; Emde, M.; Böttger, E.C. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.Y.; Legard, D.E.; Hunter, J.E.; Burr, T.J. Modified bean pod assay to detect strains of Pseudomonas syringae pv. syringae that cause bacterial brown spot of snap bean. Plant Dis. 1989, 73, 149–423. [Google Scholar] [CrossRef]
- Sarkar, S.F.; Guttman, D.S. Evolution of the core genome of Pseudomonas syringae, a highly clonal, endemic plant pathogen. Appl. Environ. Microbiol. 2004, 70, 1999–2012. [Google Scholar] [CrossRef]
- Hwang, M.S.H.; Morgan, R.L.; Sarkar, S.F.; Wang, P.W.; Guttman, D.S. Phylogenetic characterization of virulence and resistence phenotypes of Pseudomonas syringae. Appl. Environ. Microbiol. 2005, 71, 5182–5191. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000; p. 333. [Google Scholar]
- Billing, E. Pseudomonas viridiflava (Burkholder, 1930; Clara, 1934). J. Appl. Bacteriol. 1970, 33, 492–500. [Google Scholar] [CrossRef]
- Wilkie, J.P.; Dye, D.W.; Watson, D.R.W. Further hosts of Pseudomonas viridiflava. N. Z. J. Agric. Res. 1973, 16, 315–323. [Google Scholar] [CrossRef]
- Sarris, P.F.; Trantas, E.A.; Mpalantinaki, E.; Ververidis, F.; Goumas, D.E. Pseudomonas viridiflava, a multi host plant pathogen with significant genetic variation at the molecular level. PLoS ONE 2012, 7, e36090. [Google Scholar] [CrossRef]
- Aysan, Y.; Uygur, S. Epiphytic survival of Pseudomonas viridiflava, causal agent of pith necrosis of tomato, on weeds in Turkey. J. Plant Pathol. 2005, 87, 135–139. [Google Scholar]
- Mariano, R.D.; McCarter, S.M. Epiphytic survival of Pseudomonas viridiflava on tomato and selected weed species. Microb. Ecol. 2004, 26, 47–58. [Google Scholar] [CrossRef]
- Jakob, K.; Kniskern, J.M.; Bergelson, J. The role of pectate lyase and the jasmonic acid defense response in Pseudomonas viridiflava virulence. Mol. Plant-Microbe Interact. 2007, 20, 146–158. [Google Scholar] [CrossRef]
- Bartoli, C.; Berge, O.; Monteil, C.L.; Guilbaud, C.; Balestra, G.M.; Varvaro, L.; Jones, C.; Dangl, J.L.; Baltrus, D.A.; Sands, D.C.; et al. The Pseudomonas viridiflava phylogroups in the P. syringae species complex are characterized by genetic variability and phenotypic plasticity of pathogenicity-related traits. Environ. Microbiol. 2014, 16, 2301–2315. [Google Scholar] [CrossRef]
- Yin, H.; Cao, L.; Xie, M.; Chen, Q.; Qiu, G.; Zhou, J.; Wu, L.; Wang, D.; Liu, X. Bacterial diversity based on 16S rRNA and gyrB genes at Yinshan mine, China. Syst. Appl. Microbiol. 2008, 31, 302–311. [Google Scholar] [CrossRef]
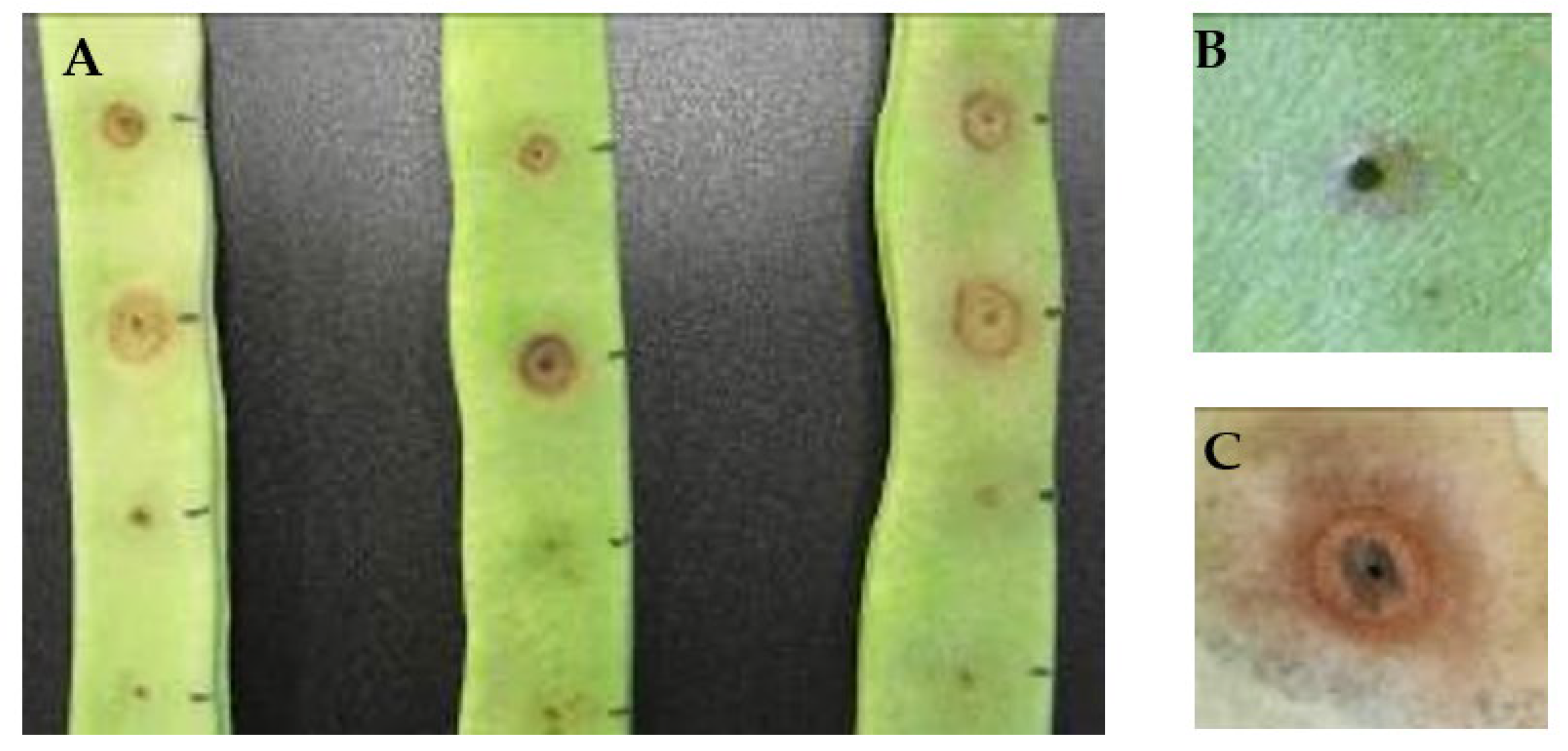
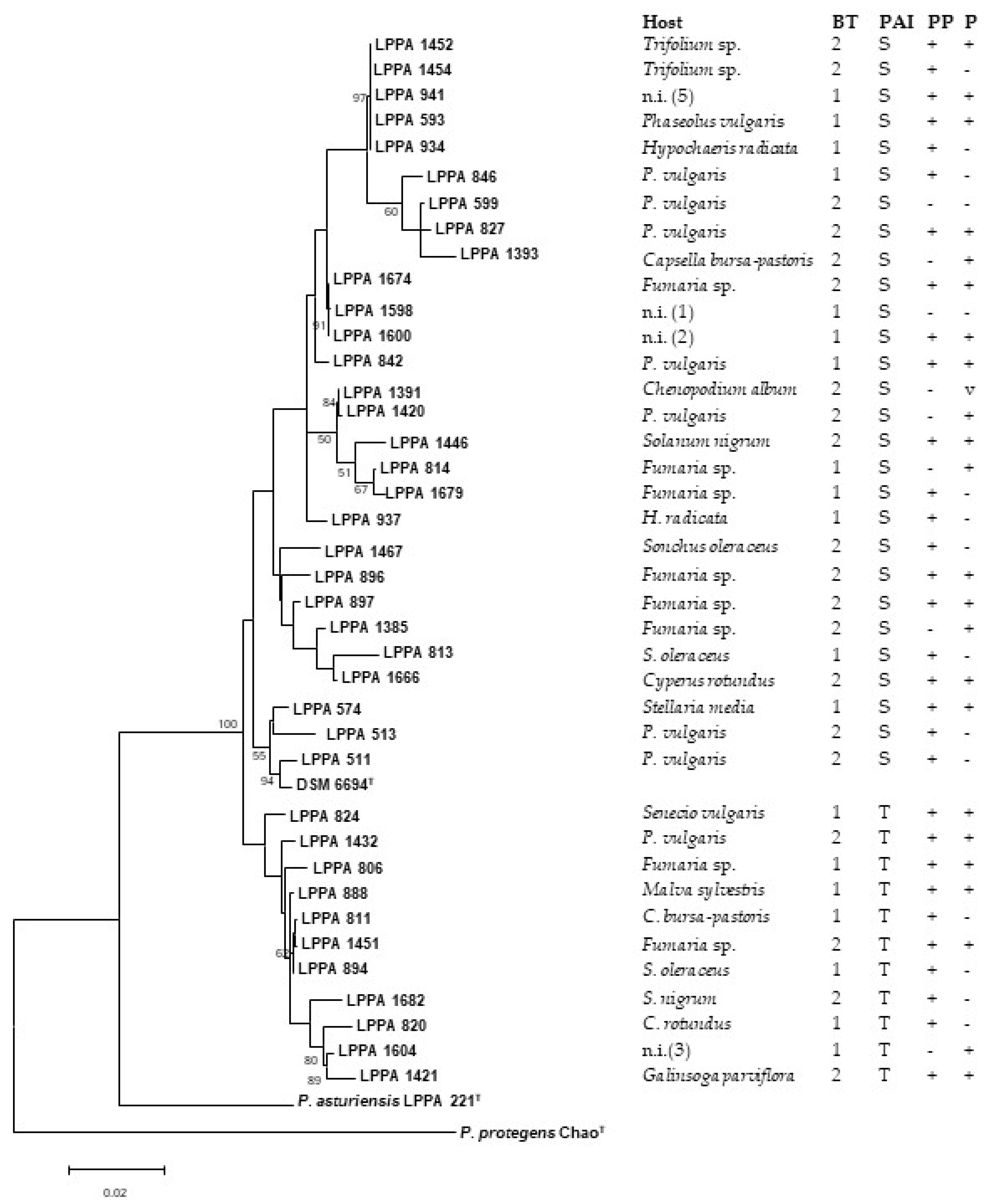
| Year | Site | Isolate | Host | BT | BP | PAI | PP | P |
|---|---|---|---|---|---|---|---|---|
| 2007 | Carbajal | LPPA 511 | Phaseolus vulgaris | 2 | 29 | S | + | − |
| LPPA 574 | Stellaria media | 1 | 2 | S | + | + | ||
| 2007 | Bárcena | LPPA 513 | P. vulgaris | 2 | 25 | S | + | − |
| 2007 | Pontigon | LPPA 1598 a | n.i. | 1 | 13 | S | − | − |
| LPPA 1600 a | n.i. | 1 | 2 | S | + | + | ||
| LPPA 1604 | n.i. | 1 | 12 | T | − | + | ||
| 2008 | Carbajal | LPPA 593 | P. vulgaris | 1 | 3 | S | + | + |
| LPPA 842 | P. vulgaris | 1 | 3 | S | + | + | ||
| 2008 | Anleo | LPPA 599 | P. vulgaris | 2 | 30 | S | + | − |
| 2008 | Busto 1 | LPPA 820 | Cyperus rotundus | 1 | 5 | T | + | − |
| 2008 | Constancios | LPPA 806 | Fumaria sp. | 1 | 8 | T | + | + |
| LPPA 824 | Senecio vulgaris | 1 | 4 | T | + | + | ||
| 2008 | Ronda | LPPA 811 | Capsella bursa-pastoris | 1 | 10 | T | + | − |
| LPPA 813 | Sonchus oleraceus | 1 | 4 | S | + | − | ||
| LPPA 814 | Fumaria sp. | 1 | 12 | S | − | + | ||
| 2008 | Argüelles | LPPA 827 | P. vulgaris | 2 | 23 | S | + | + |
| 2008 | Yerbo | LPPA 846 | P. vulgaris | 1 | 11 | S | + | − |
| 2009 | Busto 1 | LPPA 1420 | P. vulgaris | 2 | 33 | S | − | + |
| LPPA 888 | Malva sylvestris | 1 | 6 | T | + | + | ||
| LPPA 891 | S. oleraceus | 1 | 2 | T | + | − | ||
| LPPA 894 | S. oleraceus | 1 | 1 | T | + | − | ||
| LPPA 896 b | Fumaria sp. | 2 | 24 | S | + | + | ||
| LPPA 897 b | Fumaria sp. | 2 | 17 | S | + | + | ||
| LPPA 1674 | Fumaria sp. | 2 | 27 | S | + | + | ||
| LPPA 1676 | Fumaria sp. | 2 | 15 | S | + | + | ||
| LPPA 1679 | Fumaria sp. | 1 | 7 | S | + | − | ||
| LPPA 934 c | Hypochaeris radicata | 1 | 1 | S | + | − | ||
| LPPA 935 c | H. radicata | 1 | 2 | S | + | − | ||
| LPPA 937 c | H. radicata | 1 | 1 | S | + | − | ||
| LPPA 1421 | Galinsoga parviflora | 2 | 19 | T | + | + | ||
| LPPA1665 d | C. rotundus | 2 | 28 | S | + | − | ||
| LPPA 1666 d | C. rotundus | 2 | 15 | S | + | + | ||
| LPPA 1671 | C. rotundus | 1 | 9 | S | + | − | ||
| LPPA 1417 | Solanum nigrum | 2 | 14 | S | + | + | ||
| LPPA 1680 | S. nigrum | 2 | 15 | T | + | − | ||
| LPPA 1682 | S. nigrum | 2 | 14 | T | + | − | ||
| LPPA 939 e | n.i. | 1 | 1 | S | + | + | ||
| LPPA 941 e | n.i. | 1 | 1 | S | + | + | ||
| 2009 | Busto 2 | LPPA 1385 | Fumaria sp. | 2 | 16 | S | − | + |
| LPPA 1391 | Chenopodium album | 2 | 31 | S | + | v | ||
| LPPA 1393 f | C. bursa-pastoris | 2 | 32 | S | − | + | ||
| LPPA 1394 f | C. bursa-pastoris | 2 | 16 | S | − | + | ||
| 2009 | Yerbo | LPPA 1432 | P. vulgaris | 2 | 26 | T | + | + |
| 2009 | Ronda | LPPA 1446 | S. nigrum | 2 | 18 | S | + | + |
| LPPA 1451 | Fumaria sp. | 2 | 21 | T | + | + | ||
| LPPA 1452 g | Trifolium sp. | 2 | 22 | S | + | + | ||
| LPPA 1454 g | Trifolium sp. | 2 | 14 | S | + | − | ||
| 2009 | Constancios | LPPA 1467 | S. oleraceus | 2 | 20 | S | + | − |
| Test | Total (N = 48) | BT1 (N = 23) | BT2 (N = 25) |
|---|---|---|---|
| Levan | 58.3 | 0 | 100 |
| Oxidase | 0 | 0 | 0 |
| Potato rot | 81.2 | 87 | 76 |
| Arginine | 0 | 0 | 0 |
| Tobacco | 100 | 100 | 100 |
| Oxidative | 100 | 100 | 100 |
| Esculin | 100 | 100 | 100 |
| Sucrose | 0 | 0 | 0 |
| Casein | 93.75 | 87 | 100 |
| Tween80 | 50 | 60.8 | 40 |
| Gelatin | 91.6 | 82.6 | 100 |
| Mannitol | 97.9 | 100 | 96 |
| Erythritol | 89.5 | 91.3 | 88 |
| Sorbitol | 97.9 | 100 | 96 |
| M-inositol | 95.8 | 100 | 92 |
| Adonitol | 2 | 4.3 | 0 |
| D-Tartrate | 29.1 | 21.7 | 36 |
| L-Lactate | 79.1 | 82.6 | 76 |
| Trigonelline | 97.9 | 95.6 | 100 |
| Betaine | 87.5 | 95.6 | 80 |
| Homoserine | 2 | 4.3 | 0 |
| Quinate | 100 | 100 | 100 |
| Xylose | 100 | 100 | 100 |
| Lactose | 0 | 0 | 0 |
| Gene | m | n | S | π | D |
|---|---|---|---|---|---|
| gyrB | 40 | 610 | 54 | 0.025542 | 0.812835 |
| rpoD | 40 | 882 | 63 | 0.019617 | 0.605882 |
| gltA | 40 | 958 | 51 | 0.015371 | 0.813580 |
| gyrB + rpoD + gltA | 40 | 2450 | 168 | 0.019432 | 0.760686 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Sanz, A.M.; Rodicio, M.R.; González, A.J. Biochemical Diversity, Pathogenicity and Phylogenetic Analysis of Pseudomonas viridiflava from Bean and Weeds in Northern Spain. Microorganisms 2022, 10, 1542. https://doi.org/10.3390/microorganisms10081542
Fernández-Sanz AM, Rodicio MR, González AJ. Biochemical Diversity, Pathogenicity and Phylogenetic Analysis of Pseudomonas viridiflava from Bean and Weeds in Northern Spain. Microorganisms. 2022; 10(8):1542. https://doi.org/10.3390/microorganisms10081542
Chicago/Turabian StyleFernández-Sanz, Ana M., M. Rosario Rodicio, and Ana J. González. 2022. "Biochemical Diversity, Pathogenicity and Phylogenetic Analysis of Pseudomonas viridiflava from Bean and Weeds in Northern Spain" Microorganisms 10, no. 8: 1542. https://doi.org/10.3390/microorganisms10081542
APA StyleFernández-Sanz, A. M., Rodicio, M. R., & González, A. J. (2022). Biochemical Diversity, Pathogenicity and Phylogenetic Analysis of Pseudomonas viridiflava from Bean and Weeds in Northern Spain. Microorganisms, 10(8), 1542. https://doi.org/10.3390/microorganisms10081542







