Terpenes Combinations Inhibit Biofilm Formation in Staphyloccocus aureus by Interfering with Initial Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Strains
2.2. Monoterpenes and Sesquiterpenes
2.3. Minimum Inhibitory Concentration (MIC)
2.4. Minimum Inhibitory Concentration for Terpene Combinations
2.5. Biofilm Formation Inhibition Assay
2.6. Biofilm Formation Inhibition Assay for Terpene Combinations
2.7. Gene Expression Assay
2.8. Statistics
3. Results and Discussion
3.1. Minimum Inhibitory Concentration
3.2. Effect of Terpenes on Biofilm Formation
3.3. Effect of Terpene Combinations on Biofilm Formation
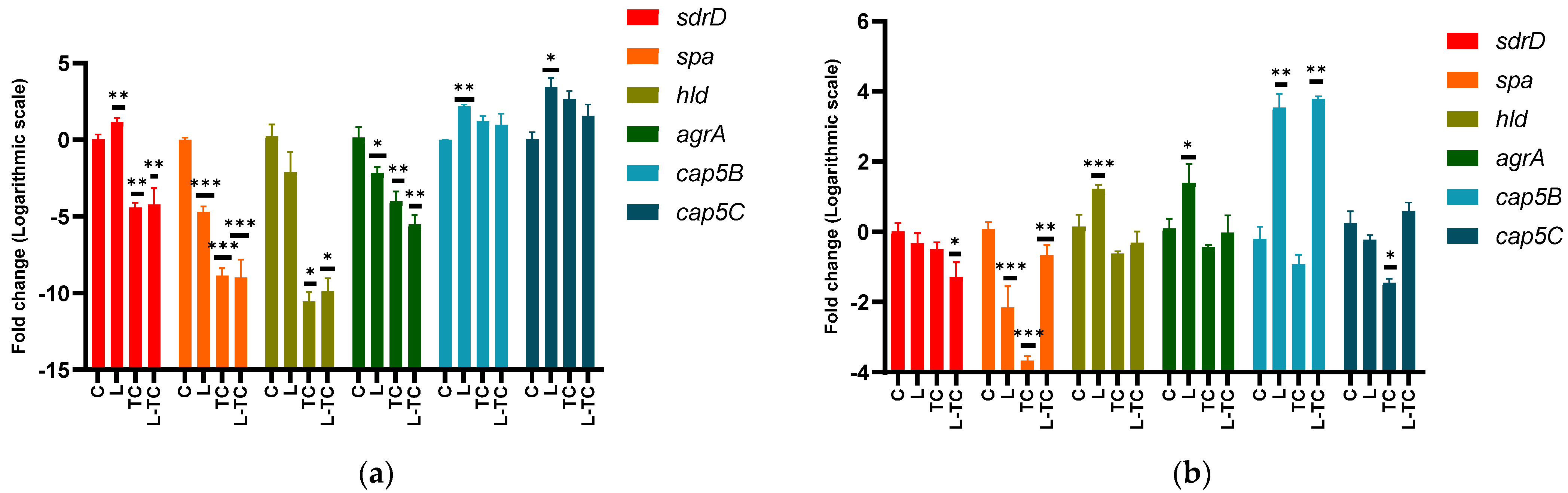
3.4. Effect of Linalool, (−)-trans-Caryophyllene and Their Combination on the Expression of Genes Associated with Biofilm Formation
3.4.1. Genes Associated with Adhesion
3.4.2. Genes Associated with Biofilm Dispersion
3.4.3. Genes Associated with the Synthesis of Capsular Polysaccharides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderl, J.N.; Zahller, J.; Roe, F.; Stewart, P.S. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2003, 47, 1251–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Bryers, J.D. Non-invasive determination of conjugative transfer of plasmids bearing antibiotic-resistance genes in biofilm-bound bacteria: Effects of substrate loading and antibiotic selection. Appl. Microbiol. Biotechnol. 2013, 97, 317–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbu, E.M.; Mackenzie, C.; Foster, T.J.; Höök, M. SdrC induces staphylococcal biofilm formation through a homophilic interaction. Mol. Microbiol. 2014, 94, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Biofilm: Adhesins, regulation, and host response. Microbiol. Spectr. 2016, 4, 529–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reffuveille, F.; Josse, J.; Vallé, Q.; Mongaret, C.; Gangloff, S.C. Staphylococcus aureus Biofilms and their Impact on the Medical Field. In The Rise of Virulence and Antibiotic Resistance in Staphylococcus aureus; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Periasamy, S.; Joo, H.S.; Duong, A.C.; Bach, T.H.L.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kırmusaoğlu, S. Staphylococcal Biofilms: Pathogenicity, Mechanism and Regulation of Biofilm Formation by Quorum-Sensing System and Antibiotic Resistance Mechanisms of Biofilm-Embedded Microorganisms. In Microbial Biofilms—Importance and Applications; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.; Chee, C.-F.; Richter, K.; Thomas, N.; Rahman, N.A.; Nathan, S. Suppression of Staphylococcus aureus biofilm formation and virulence by a benzimidazole derivative, UM-C162. Sci. Rep. 2018, 8, 2758. [Google Scholar] [CrossRef]
- Belanger, C.R.; Mansour, S.C.; Pletzer, D.; Hancock, R.E.W. Alternative strategies for the study and treatment of clinical bacterial biofilms. Emerg. Top. Life Sci. 2017, 1, 41–53. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Yong, Y.Y.; Dykes, G.A.; Choo, W.S. Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit. Rev. Microbiol. 2019, 45, 201–222. [Google Scholar] [CrossRef]
- Gomes, F.; Martins, N.; Ferreira, I.C.F.R.; Henriques, M. Anti-biofilm activity of hydromethanolic plant extracts against Staphylococcus aureus isolates from bovine mastitis. Heliyon 2019, 5, e01728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, A.D.; Vizhi, D.K.; Lavanya, K.; Kalpana, V.N.; Devi Rajeswari, V.; Babujanarthanam, R. In Vitro anti- biofilm and anti-bacterial activity of Sesbania grandiflora extract against Staphylococcus aureus. Biochem. Biophys. Rep. 2017, 12, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Erasto, P.; Viljoen, A.M. Limonene—A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.-Y.; Lim, J.H.; Hwang, S.; Lee, J.-C.; Cho, G.-S.; Kim, W.-K. Anti-ischemic and anti-inflammatory activity of (S)-cis-verbenol. Free Radic. Res. 2010, 44, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; do Carmo Vieira, M.; Ruiz, A.L.T.G.; Foglio, M.A.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Rodríguez, F.; Salinas, C.; Fernández, S.; Haim, S.; Mollerach, M.; Basualdo, W.; Castro, H.; Quiñónez, B.; Arguello, R.; Rodríguez, M.; et al. Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) clones from Paraguayan children. J. Infect. Dev. Ctries. 2020, 14, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and Antimicrobial Activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef]
- Qin, N.; Tan, X.; Jiao, Y.; Liu, L.; Zhao, W.; Yang, S.; Jia, A. RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci. Rep. 2014, 4, 5467. [Google Scholar] [CrossRef] [Green Version]
- Yuyama, K.T.; Wendt, L.; Surup, F.; Kretz, R.; Chepkirui, C.; Wittstein, K.; Boonlarppradab, C.; Wongkanoun, S.; Luangsa-ard, J.; Stadler, M.; et al. Cytochalasans Act as Inhibitors of Biofilm Formation of Staphylococcus aureus. Biomolecules 2018, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, R.; González Barrios, A.F.; Bedzyk, L.A.; Eldridge, G.R.; Pasmore, M.E.; Wood, T.K. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005, 71, 4022–4034. [Google Scholar] [CrossRef] [Green Version]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Almássy, K.; Krisch, J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 2011, 144, 480–486. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef]
- Pei, R.-S.; Zhou, F.; Ji, B.-P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Viljoen, A. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Fragr. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Gallucci, M.N.; Oliva, M.; Casero, C.; Dambolena, J.; Luna, A.; Zygadlo, J.; Demo, M. Antimicrobial combined action of terpenes against the food-borne microorganisms Escherichia coli, Staphylococcus aureus and Bacillus cereus. Flavour Fragr. J. 2009, 24, 348–354. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moleyar, V.; Narasimham, P. Antibacterial activity of essential oil components. Int. J. Food Microbiol. 1992, 16, 337–342. [Google Scholar] [CrossRef]
- Ngome, M.T.; Alves, J.G.L.F.; de Oliveira, A.C.F.; da Silva Machado, P.; Mondragón-Bernal, O.L.; Piccoli, R.H. Linalool, citral, eugenol and thymol: Control of planktonic and sessile cells of Shigella flexneri. AMB Express 2018, 8, 105. [Google Scholar] [CrossRef]
- Gao, Z.; Van Nostrand, J.D.; Zhou, J.; Zhong, W.; Chen, K.; Guo, J. Anti-listeria Activities of Linalool and Its Mechanism Revealed by Comparative Transcriptome Analysis. Front. Microbiol. 2019, 10, 2947. [Google Scholar] [CrossRef]
- Alves, S.; Duarte, A.; Sousa, S.; Domingues, F.C. Study of the major essential oil compounds of Coriandrum sativum against Acinetobacter baumannii and the effect of linalool on adhesion, biofilms and quorum sensing. Biofouling 2016, 32, 155–165. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Lee, J.-H.; Kim, S.-I.; Baek, K.-H.; Lee, J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int. J. Food Microbiol. 2015, 195, 30–39. [Google Scholar] [CrossRef]
- Vetas, D.; Dimitropoulou, E.; Mitropoulou, G.; Kourkoutas, Y.; Giaouris, E. Disinfection efficiencies of sage and spearmint essential oils against planktonic and biofilm Staphylococcus aureus cells in comparison with sodium hypochlorite. Int. J. Food Microbiol. 2017, 257, 19–25. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, J.B.; de Carvalho, R.J.; de Souza, N.T.; de Sousa Oliveira, K.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Papa, R.; Garzoli, S.; Vrenna, G.; Sabatino, M.; Sapienza, F.; Relucenti, M.; Donfrancesco, O.; Fiscarelli, E.V.; Artini, M.; Selan, L.; et al. Essential Oils Biofilm Modulation Activity, Chemical and Machine Learning Analysis—Application on Staphylococcus aureus Isolates from Cystic Fibrosis Patients. Int. J. Mol. Sci. 2020, 21, 9258. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Annous, B.A.; Ezeji, T.C.; Karcher, P.; Maddox, I.S. Biofilm reactors for industrial bioconversion processes: Employing potential of enhanced reaction rates. Microb. Cell Fact. 2005, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelfattah, A.; Hossain, M.I.; Cheng, L. High-strength wastewater treatment using microbial biofilm reactor: A critical review. World J. Microbiol. Biotechnol. 2020, 36, 75. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Kim, S.-I.; Lee, J. Inhibitory effects of the essential oils α-longipinene and linalool on biofilm formation and hyphal growth of Candida albicans. Biofouling 2017, 33, 143–155. [Google Scholar] [CrossRef]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Evaluation of antibiofilm efficacy of essential oil components β-caryophyllene, cinnamaldehyde and eugenol alone and in combination against biofilm formation and preformed biofilms of Listeria monocytogenes and Salmonella typhimurium. Lett. Appl. Microbiol. 2020, 71, 195–202. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Grapefruit essential oils inhibit quorum sensing of Pseudomonas aeruginosa. Food Sci. Technol. Int. 2019, 26, 231–241. [Google Scholar] [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Bastaki, M.; Api, A.M.; Aubanel, M.; Bauter, M.; Cachet, T.; Demyttenaere, J.C.; Diop, M.M.; Harman, C.L.; Hayashi, S.M.; Krammer, G.; et al. Dietary administration of β-caryophyllene and its epoxide to Sprague-Dawley rats for 90 days. Food Chem. Toxicol. 2020, 135, 110876. [Google Scholar] [CrossRef]
- Da Silva Oliveira, G.L.; Machado, K.C.; Machado, K.C.; da Silva, A.P.d.S.C.L.; Feitosa, C.M.; de Castro Almeida, F.R. Non-clinical toxicity of β-caryophyllene, a dietary cannabinoid: Absence of adverse effects in female Swiss mice. Regul. Toxicol. Pharmacol. 2018, 92, 338–346. [Google Scholar] [CrossRef]
- Askarian, F.; Ajayi, C.; Hanssen, A.M.; Van Sorge, N.M.; Pettersen, I.; Diep, D.B.; Sollid, J.U.; Johannessen, M. The interaction between Staphylococcus aureus SdrD and desmoglein 1 is important for adhesion to host cells. Sci. Rep. 2016, 6, 22134. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, L.; Kayama, S.; Sasaki, M.; Kato, F.; Hisatsune, J.; Tsuruda, K.; Koizumi, K.; Tatsukawa, N.; Yu, L.; Takeda, K.; et al. Inhibitory effects of antibiofilm compound 1 against Staphylococcus aureus biofilms. Microbiol. Immunol. 2016, 60, n148–n159. [Google Scholar] [CrossRef] [Green Version]
- Paulander, W.; Varming, A.N.; Bojer, M.S.; Friberg, C.; Bæk, K.; Ingmer, H. The agr quorum sensing system in Staphylococcus aureus cells mediates death of sub-population. BMC Res. Notes 2018, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Boles, B.R.; Horswill, A.R. agr-Mediated Dispersal of Staphylococcus aureus Biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, M.A.; de la Garza-Ramos, M.A.; Martínez-Ávila, C.G.C.; Gutiérrez-Díez, A.; Ojeda-Zacarías, M.; Aguirre-Arzola, V.E. Inhibición de la expresión del sistema agr de Staphylococcus aureus resistente a meticilina mediante el uso de polifenoles totales de hojas de aguacate mexicano (Persea americana var. drymifolia). Nova Sci. 2017, 9, 200–221. [Google Scholar] [CrossRef] [Green Version]
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci—An overview. Front. Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butrico, C.E.; Cassat, J.E. Quorum Sensing and Toxin Production in Staphylococcus aureus Osteomyelitis: Pathogenesis and Paradox. Toxins 2020, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Salimena, A.P.; Lange, C.C.; Camussone, C.; Signorini, M.; Calvinho, L.F.; Brito, M.A.; Borges, C.A.; Guimarães, A.S.; Ribeiro, J.B.; Mendonça, L.C.; et al. Genotypic and phenotypic detection of capsular polysaccharide and biofilm formation in Staphylococcus aureus isolated from bovine milk collected from Brazilian dairy farms. Vet. Res. Commun. 2016, 40, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Grunert, T.; Stessl, B.; Wolf, F.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M. Distinct phenotypic traits of Staphylococcus aureus are associated with persistent, contagious bovine intramammary infections. Sci. Rep. 2018, 8, 15968. [Google Scholar] [CrossRef] [Green Version]
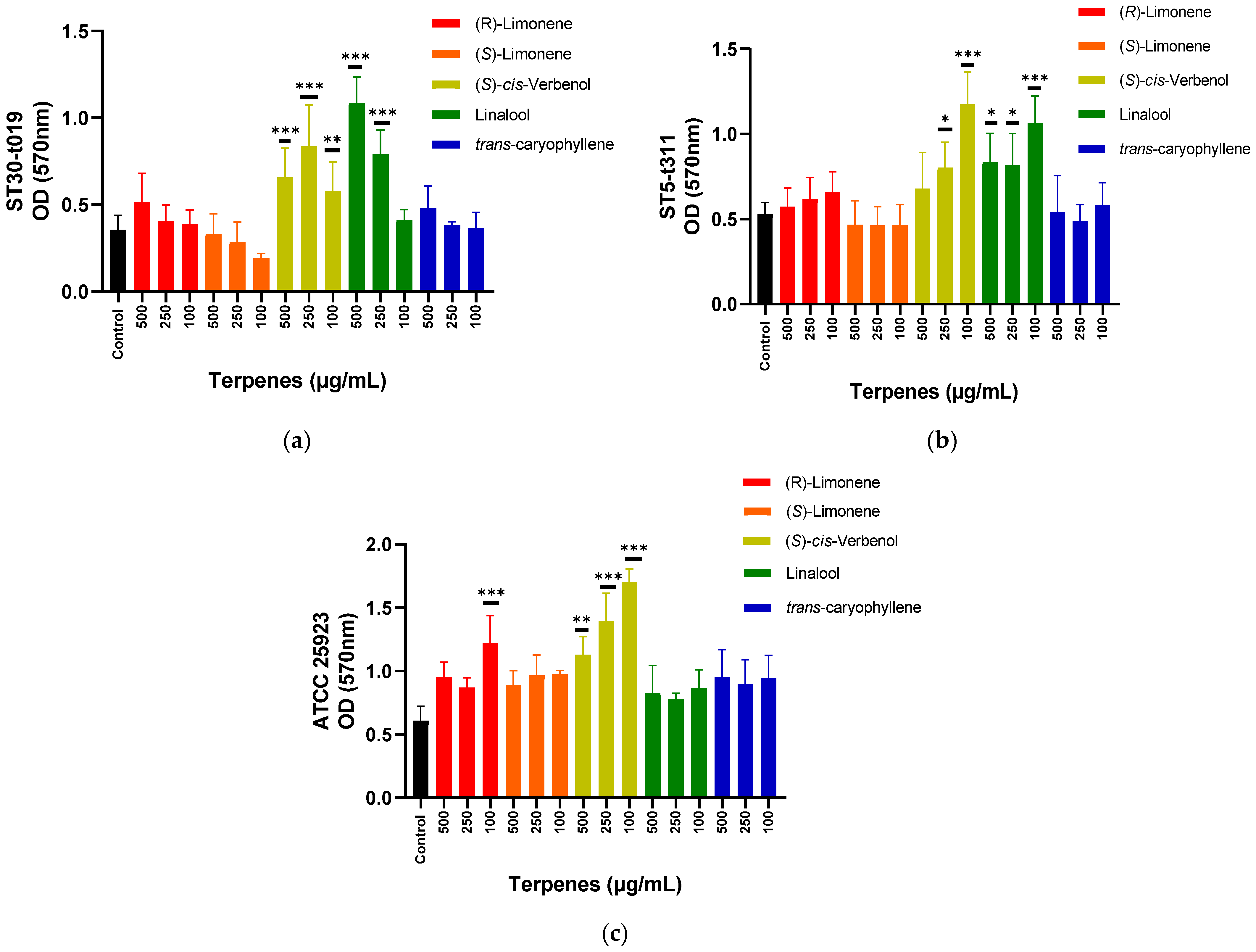
| Terpenes | MIC (mg/mL) | ||
|---|---|---|---|
| ST30-t019 | ST5-t311 | ATCC 25923 | |
| (S)-Limonene | 5 | 15 | 5 |
| (R)-Limonene | 15 | 20 | 10 |
| (−)-trans-caryophyllene | 5 | >20 | >20 |
| (S)-cis-verbenol | 20 | 7,5 | 5 |
| Linalool | 20 | 7,5 | 7.5 |
| ST5-t311 | ATCC 25923 | |||||
|---|---|---|---|---|---|---|
| Terpenes | MIC (mg/mL) | FIC | Interaction | MIC (mg/mL) | FIC | Interaction |
| (S)-(−)- Limonene/Linalool | 10 | 2 | Indifferent | 10 | 3.3 | Indifferent |
| (S)-(−)- Limonene/(S) -cis-Verbenol | 7.5 | 1.5 | Indifferent | 7.5 | 3 | Indifferent |
| (R)-(+)-Limonene/Linalool | 10 | 1.8 | Indifferent | 10 | 2.3 | Indifferent |
| (R)-(+)-Limonene/(S) -cis-Verbenol | 10 | 1.8 | Indifferent | 10 | 3 | Indifferent |
| (−)-trans-caryophyllene/Linalool | 5 | 0.9 | Additive | 5 | 0.9 | Additive |
| (−)-trans-caryophyllene/(S)-cis-Verbenol | 5 | 0.9 | Additive | 5 | 1.25 | Indifferent |
| Compounds | Concentration (μg/mL) | ST5-t311 | ATCC 25923 | ||
|---|---|---|---|---|---|
| Percentage of Inhibition | Inhibitory Effect | Percentage of Inhibition | Inhibitory Effect * | ||
| (S)-(−)- Limonene /Linalool | 500 | 80.1 | Elevated | −40.0 ** | - |
| 250 | 80.5 | Elevated | −21.0 ** | - | |
| 100 | 78.1 | Elevated | −8.4 ** | - | |
| (S)-(−)- Limonene /(S)-cis -Verbenol | 500 | 69.5 | Good | −20.7 ** | - |
| 250 | 63.7 | Good | 22.1 | Moderate | |
| 100 | 57.1 | Good | 23.4 | Moderate | |
| (R)-(+)-Limonene /Linalool | 500 | 83.8 | Elevated | 58.0 | Good |
| 250 | 83.6 | Elevated | 34.2 | Moderate | |
| 100 | 77.9 | Elevated | 29.3 | Moderate | |
| (R)-(+)-Limonene /(S)-cis-Verbenol | 500 | 73.0 | Elevated | 6.0 | Inactive |
| 250 | 81.9 | Elevated | 15.1 | Inactive | |
| 100 | 77.0 | Elevated | −21.3 ** | - | |
| (−)-trans-caryophyllene/Linalool | 500 | 88.1 | Elevated | 67.2 | Good |
| 250 | 86.6 | Elevated | 54.9 | Good | |
| 100 | 74.6 | Elevated | 9.0 | Inactive | |
| (−)-trans-caryophyllene/(S)-cis-Verbenol | 500 | 85.9 | Elevated | 64.3 | Good |
| 250 | 86.7 | Elevated | 54.3 | Good | |
| 100 | 65.0 | Good | 19.5 | Inactive | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas, C.; Florentín, G.; Rodríguez, F.; Alvarenga, N.; Guillén, R. Terpenes Combinations Inhibit Biofilm Formation in Staphyloccocus aureus by Interfering with Initial Adhesion. Microorganisms 2022, 10, 1527. https://doi.org/10.3390/microorganisms10081527
Salinas C, Florentín G, Rodríguez F, Alvarenga N, Guillén R. Terpenes Combinations Inhibit Biofilm Formation in Staphyloccocus aureus by Interfering with Initial Adhesion. Microorganisms. 2022; 10(8):1527. https://doi.org/10.3390/microorganisms10081527
Chicago/Turabian StyleSalinas, Claudia, Gladys Florentín, Fátima Rodríguez, Nelson Alvarenga, and Rosa Guillén. 2022. "Terpenes Combinations Inhibit Biofilm Formation in Staphyloccocus aureus by Interfering with Initial Adhesion" Microorganisms 10, no. 8: 1527. https://doi.org/10.3390/microorganisms10081527
APA StyleSalinas, C., Florentín, G., Rodríguez, F., Alvarenga, N., & Guillén, R. (2022). Terpenes Combinations Inhibit Biofilm Formation in Staphyloccocus aureus by Interfering with Initial Adhesion. Microorganisms, 10(8), 1527. https://doi.org/10.3390/microorganisms10081527






