Early Corticosteroid Therapy May Increase Ventilator-Associated Lower Respiratory Tract Infection in Critically Ill Patients with COVID-19: A Multicenter Retrospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Data
2.3. Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease 2019 |
| ICU | Intensive care unit |
| VA-LRTI | Ventilator-associated lower respiratory tract infection |
| VAP | Ventilator-associated pneumonia |
| VAT | Ventilator-associated tracheobronchitis |
| VFD | Ventilator-free days |
| ICU | Intensive care unit |
| LOS | Length of stay |
| MV | Mechanical ventilation |
| HFNC | High-flow nasal cannula |
| NIV | Non-invasive ventilation |
| ARDS | Acute respiratory distress syndrome |
| ECMO | Extra-corporeal membrane oxygenation |
| BSI | Bloodstream infection |
References
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Courcelle, R.; Gaudry, S.; Serck, N.; Blonz, G.; Lascarrou, J.B..; Grimald, D.; on behalf the COVADIS Study Group. Neuromuscular blocking agents (NMBA) for COVID-19 acute respiratory distress syndrome: A multicenter observational study. Crit. Care 2020, 24, 446. [Google Scholar] [CrossRef] [PubMed]
- Wicky, P.H.; Niedermann, M.S.; Timsit, J.F. Ventilator-associated pneumonia in the era of COVID-19 pandemic: How common and what is the impact? Crit. Care 2021, 25, 153. [Google Scholar] [CrossRef] [PubMed]
- Povoa, P.; Martin-Loeches, I.; Nseir, S. Secondary pneumonias in critically ill patients with COVID-19: Risk factors and outcomes. Curr. Opin. Crit. Care 2021, 27, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Lagier, D.; Platon, L.; Lambert, J.; Chow-Chine, L.; Sannini, A.; Bisbal, M.; Brun, J.-P.; Asehnoune, K.; Leone, M.; Faucher, M.; et al. Protective effect of early low-dose hydrocortisone on ventilator-associated pneumonia in the cancer patients: A propensity score analysis. Ann. Intensiv. Care 2017, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Gragueb-Chatti, I.; Lopez, A.; Hamidi, D.; Guervilly, C.; Loundou, A.; Daviet, F.; Cassir, N.; Papazian, L.; Forel, J.; Leone, M.; et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia and blood stream infections in COVID-19 patients requiring invasive mechanical ventilation: A multicenter retrospective study. Ann. Intensive Care 2021, 11, 87. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef] [Green Version]
- Alhazzani, W.; Evans, L.; Alshamsi, F.; Møller, M.H.; Ostermann, M.; Prescott, H.C.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E. Surviving Sepsis Campaign Guidelines on the Management of Adults with Coronavirus Disease 2019 (COVID-19) in the ICU. Crit. Care Med. 2021, 49, e219–e234. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [PubMed]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.-D.; Combes, A.; Dreyfuss, D.; Forel, J.M.; Guérin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallet, R.H. Ventilator Bundles in Transition: From Prevention of Ventilator-Associated Pneumonia to Prevention of Ventilator-Associated Events. Respir. Care 2019, 64, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.M.; Isacco, C.G.; et al. SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs. Microorganisms 2021, 9, 793. [Google Scholar] [CrossRef]
- Verma, H.K. Radiological and clinical spectrum of COVID-19: A major concern for public health. World J. Radiol. 2021, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]
- van Paassen, J.; Vos, J.S.; Hoekstra, E.M.; Neumann, K.M.I.; Boot, P.C.; Arbous, S.M. Corticosteroid use in COVID-19 patients: A systematic review and meta-analysis on clinical outcomes. Crit Care 2020, 24, 696. [Google Scholar] [CrossRef]
- Moreau, A.S.; Martin-Loeches, I.; Povoa, P.; Salluh, J.; Rodriguez, A.; Thille, A.W.; Santos, E.D.; Vedes, E.; Lobo, S.M.; Mégarbane, B.; et al. Impact of immunosuppression on incidence, aetiology and outcome of ventilator-associated lower respiratory tract infections. Eur. Respir. J. 2018, 51, 1701656. [Google Scholar] [CrossRef] [Green Version]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Austin, P.C.; Fine, J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat. Med. 2017, 36, 4391–4400. [Google Scholar] [CrossRef]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Geronimi, C.B.; et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, M.; Misseri, G.; Catalisano, G.; Marino, C.; Ingoglia, G.; Alessi, M.; Consiglio, E.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Nseir, S.; Martin-Loeches, I.; Povoa, P.; Metzelard, M.; Cheyron, D.D.; Lambiotte, F.; Tamion, F.; Labruyere, M.; Makris, D.; Geronimi, C.B.; et al. Relationship between ventilator-associated pneumonia and mortality in COVID-19 patients: A planned ancillary analysis of the coVAPid cohort. Crit. Care 2021, 25, 177. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, M.; Plata-Menchaca, E.P.; Nuvials, F.X.; Roca, O.; Ferrer, R. Risk factors and outcomes of ventilator-associated pneumonia in COVID-19 patients: A propensity score matched analysis. Crit. Care 2021, 25, 235. [Google Scholar] [CrossRef]
- Saade, A.; Moratelli, G.; Dumas, G.; Mabrouki, A.; Tudesq, J.-J.; Zafrani, L.; Azoulay, E.; Darmon, M. Infectious events in patients with severe COVID-19: Results of a cohort of patients with high prevalence of underlying immune defect. Ann. Intensiv. Care 2021, 11, 83. [Google Scholar] [CrossRef]
- Blonz, G.; Kouatchet, A.; Chudeau, N.; Pontis, E.; Lorber, J.; Lemeur, A.; Planche, L.; Lascarrou, J.-B.; Colin, G. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: A multicenter retrospective study in 188 patients in an un-inundated French region. Crit. Care 2021, 25, 72. [Google Scholar] [CrossRef]
- Jung, C.; Wernly, B.; Fjølner, J.; Bruno, R.R.; Dudzinski, D.; Artigas, A.; Pinto, B.B.; Schefold, J.C.; Wolff, G.; Kelm, M.; et al. Steroid use in elderly critically ill COVID-19 patients. Eur. Respir. J. 2021, 58, 2100979. [Google Scholar] [CrossRef]
- Sinha, P.; Furfaro, D.; Cummings, M.J.; Abrams, D.; Delucchi, K.; Maddali, M.V.; He, J.; Thompson, A.; Murn, M.; Fountain, J.; et al. Latent Class Analysis Reveals COVID-19-related ARDS Subgroups with Differential Responses to Corticosteroids. Am. J. Respir. Crit. Care Med. 2021, 204, 1274–1285. [Google Scholar] [CrossRef]
- Dupuis, C.; de Montmollin, E.; Buetti, N.; Goldgran-Toledano, D.; Reignier, J.; Schwebel, C.; Domitile, J.; Neuville, M.; Ursino, M.; Siami, S.; et al. Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: A multicenter cohort study of the OUTCOMEREA network. PLoS ONE 2021, 16, e02556442021. [Google Scholar] [CrossRef]
- De Backer, D.; Azoulay, E.; Vincent, J.L. Corticosteroids in severe COVID-19: A critical view of the evidence. Crit. Care 2020, 24, 627. [Google Scholar] [CrossRef]
- Croce, M.A.; Brasel, K.J.; Coimbra, R.; Adams, C.A., Jr.; Miller, P.R.; Pasquale, M.D.; McDonald, C.S.; Vuthipadadon, S.; Fabian, T.C.; Tolley, E.A. National Trauma Institute prospective evaluation of the ventilator bundle in trauma patients: Does it really work? J. Trauma Acute Care Surg. 2013, 74, 354–360; discussion 360–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlberg, J.; Chong, D.S.; Lai, W.Y. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004, 159, 229–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghamdi, I.; Hussain, I.; Alghamdi, M.; Almalki, S.; Alghamdi, M.; Elsheemy, M. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: A descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int. J. Gen. Med. 2014, 7, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefèvre, N.; Corazza, F.; Duchateau, J.; Desir, J.; Casimir, G. Sex Differences in Inflammatory Cytokines and CD99 Expression Following In Vitro Lipopolysaccharide Stimulation. Shock 2012, 38, 37–42. [Google Scholar] [CrossRef]
- Shintani, A.K.; Girard, T.; Eden, S.K.; Arbogast, P.G.; Moons, K.G.M.; Ely, E.W. Immortal time bias in critical care research: Application of time-varying Cox regression for observational cohort studies*. Crit. Care Med. 2009, 37, 2939–2945. [Google Scholar] [CrossRef] [Green Version]
- Forel, J.-M.; Voillet, F.; Pulina, D.; Gacouin, A.; Perrin, G.; Barrau, K.; Jaber, S.; Arnal, J.-M.; Fathallah, M.; Auquier, P.; et al. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit. Care 2012, 16, R65. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T.; et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020, 383, 2041–2052. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar]
- Inchingolo, A.D.; Dipalma, G.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; D’Oria, M.T.; Isacco, C.G.; Bordea, I.R.; Candrea, S.; Scarano, A.; et al. The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants. Antioxidants 2021, 10, 881. [Google Scholar] [CrossRef]
- Berton, D.C.; Kalil, A.C.; Teixeira, P.J.Z. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2014, CD006482. [Google Scholar] [CrossRef] [Green Version]
| All Population (n = 322) | No Steroid (n = 127) | Steroid (n = 195) | p Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years (+/−SD) | 64.8 (+/−10.4) | 65 (+/−10) | 64.6 (+/−10.7) | 0.71 |
| Male (%) | 219/322 (68) | 90/127 (70.9) | 129/195 (66.2) | 0.38 |
| BMI (+/−SD) | 29.7 (+/−6.2) | 29 (+/−4.8) | 30.2 (+/−6.9) | 0.081 |
| Hypertension (%) | 204/322 (63.3) | 78/127 (61.4) | 126/195 (64.6) | 0.62 |
| Diabetes mellitus (%) | 132/322 (41) | 46/127 (36.2) | 86/195 (44.1) | 0.18 |
| Cardiomyopathy (%) | 49/322 (15.2) | 19/127 (14.6) | 30/195 (15.4) | 0.94 |
| Chronic kidney disease (%) | 33/322 (10.2) | 8/127 (6.3) | 25/195 (12.8) | 0.062 |
| Immunosuppression (%) | 30/322 (9.3) | 6/127 (4.7) | 24/195 (12.3) | 0.024 |
| COPD (%) | 28/322 (8.7) | 9/127 (7.1) | 19/195 (9.74) | 0.42 |
| Neoplasia <2 years (%) | 21/322 (6.5) | 5/127 (4) | 16/195 (8.2) | 0.13 |
| Admission characteristics | ||||
| SOFA (+/−SD) | 5.6 (+/−2.4) | 5.4 (+/−2.4) | 5.7 (+/−2.5) | 0.26 |
| APACHE II (+/−SD) | 16 (+/−5.5) | 16.1 (+/−4.5) | 15.9 (+/−5.8) | 0.73 |
| PaO2/FiO2, mmHg (+/−SD) | 93.7 (+/−47.8) | 104.2 (+/−54) | 86.8 (+/−42) | 0.0014 |
| Mild ARDS (PaO2/FiO2 < 300 mmHg) | 10/322 (3.1) | 6/127 (4.7) | 4/195 (2.1) | 0.17 |
| Moderate ARDS (PaO2/FiO2 < 200 mmHg) | 89/322 (27.6) | 43/127 (33.9) | 46/195 (23.6) | 0.044 |
| Severe ARDS (PaO2/FiO2 < 100 mmHg) | 219/322 (68) | 76/127 (59.8) | 143/195 (73.3) | 0.011 |
| Ferritin, mcg/L (+/−SD), (n = 165) | 2041 (+/−1794) | 2051 (+/−1808) | 2035 (+/−1794) | 0.95 |
| CRP, mg/L (+/−SD) | 172.9 (+/−101.5) | 186.1 (+/−103.5) | 165,2 (+/−99.7) | 0.082 |
| Lymphocytes, /mcL (+/−SD) | 840 (+/−996) | 780 (+/−427) | 878 (+/−1231) | 0.39 |
| Shock (%) | 53/322 (16.4) | 18/127 (14.2) | 35/195 (18) | 0.37 |
| Prior antibiotic treatment (%) | 143/322 (44.7) | 73/127 (57.5) | 70/195 (35.8) | 0.0002 |
| Treatment | ||||
| Hydroxychloroquine (%) | 64/322 (19.9) | 59/127 (46.4) | 5/195 (2.6) | <0.0001 |
| Azythromycin (%) | 19/322 (5.9) | 15/127 (11.8) | 4/195 (2.1) | 0.0003 |
| Remdesivir (%) | 13/322 (4) | 6/127 (4.7) | 7/195 (3.6) | 0.61 |
| Immunomodulating therapies, IL6 and IL1 antagonist (%) | 12/322 (3.7) | 2/127 (1.6) | 10/195 (5.1) | 0.10 |
| ECMO (%) | 44/322 (13.7) | 12/127 (9.5) | 32/195 (16.4) | 0.076 |
| Prone positionning (%) | 271/322 (84.2) | 108/127 (86.4) | 163/195 (83.6) | 0.78 |
| Sedation, days (+/−SD) | 14.4 (+/−13.2) | 12.9 (+/−9.9) | 15.3 (+/−14.9) | 0.11 |
| Hospital admission to intubation, days (+/−SD) | 4.2 (+/−5.8) | 4.2 (+/−6.6) | 4.2 (+/−5.2) | 0.96 |
| All Population (n = 322) | No Steroid (n = 127) | Steroid (n = 195) | p Value | |
|---|---|---|---|---|
| ICU mortality (%) | 151/322 (46.9) | 51/127 (40.2) | 100/195 (51.3) | 0.051 |
| ICU Day-28 mortality (%) | 120/322 (37.3) | 40/127 (31.5) | 80/195 (41) | 0.084 |
| ICU length of stay, days (+/−SD) | 23.1 (+/−20.9) | 22.9 (+/−15.9) | 23.2 (+/−23.7) | 0.89 |
| Duration of ventilation, days (+/−SD) | 18.3 (+/−17) | 17.9 (+/−14) | 18.5 (+/−18.7) | 0.73 |
| Ventilatory-free day D28 (+/−SD) | 6.8 (+/−8.7) | 7.6 (+/−8.3) | 6.3 (+/−9) | 0.19 |
| Bloodstream infection (%) | 73/322 (22.6) | 23/127 (18.1) | 50/195 (25.6) | 0.11 |
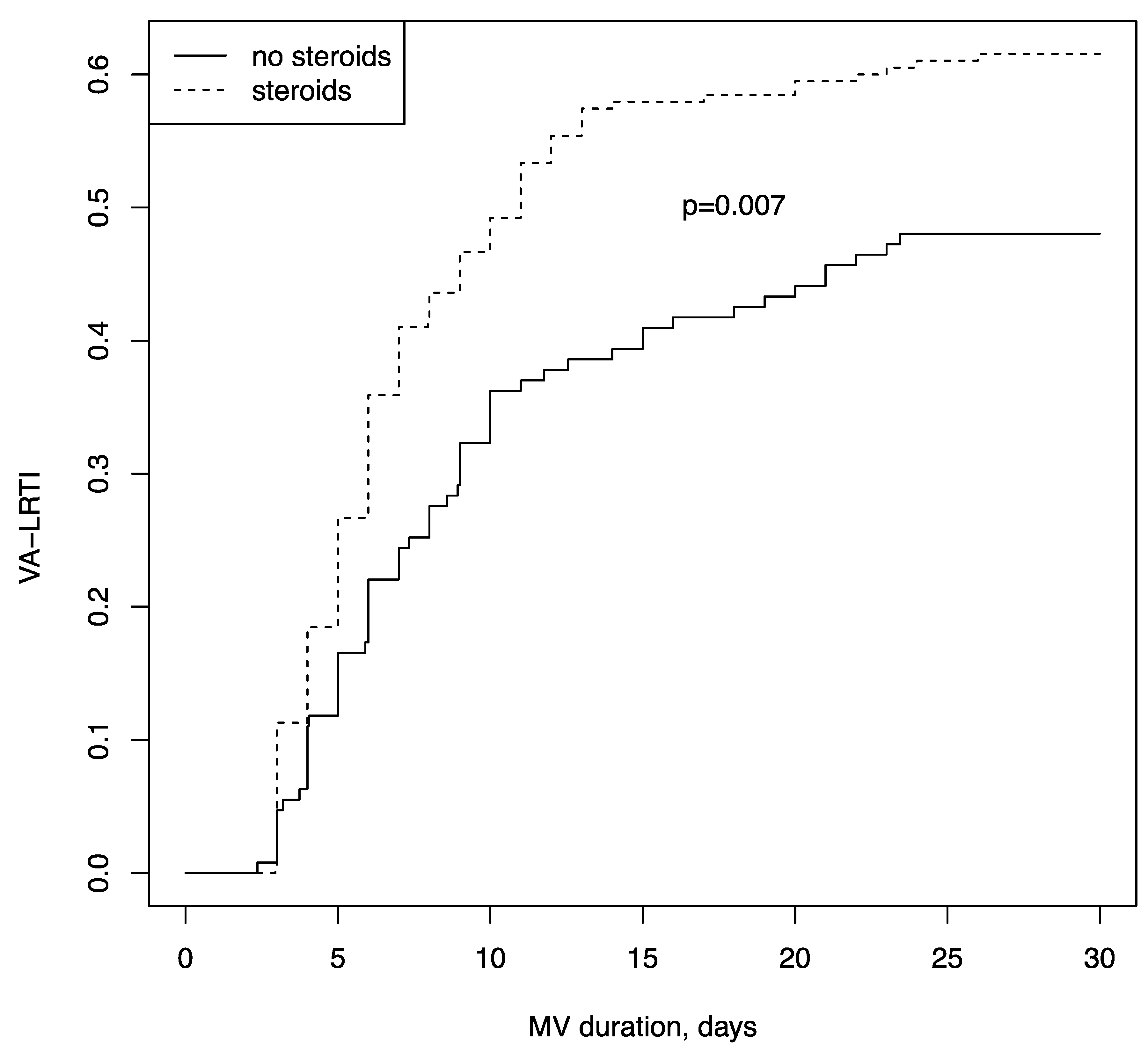
| Ventilator-associated lower respiratory tract infection (%) | 185/322 (57.5) | 62/127 (48.8) | 123/195 (63.1) | 0.011 |
| Multivariable Fine and Gray Model | ||||||
| Variables | Unadjusted Sub-hazard ratio | IC 95 | p | Adjusted Sub-hazard ratio | IC 95 | p |
| Corticosteroids | 1.61 | 1.17–2.02 | 0.003 | 1.44 | 1.05–1.98 | 0.022 |
| Male Sex | 1.74 | 1.24–2.44 | 0.002 | 1.70 | 1.21–2.39 | 0.0022 |
| Microorganisms | Non CS Group | % Isolate | % VA-LRTI | CS Group | % Isolate | % VA-LRTI | Total | % Isolate | % VA-LRTI |
|---|---|---|---|---|---|---|---|---|---|
| Gram-positive cocci | 25 | 25.8 | 34.7 | 58 | 26.4 | 37.4 | 83 | 26.2 | 36.6 |
| MSSA | 20 | 20.6 | 27.8 | 43 | 19.4 | 26.9 | 63 | 19.9 | 27.8 |
| MRSA | 1 | 1 | 1.4 | 7 | 2.0 | 2.7 | 8 | 2.5 | 3.5 |
| Streptococcus pneumoniae | 2 | 2.1 | 2.8 | 7 | 2.0 | 2.7 | 9 | 2.8 | 4.0 |
| Streptococcus agalactiae | 0 | - | - | 1 | 1 | 0.3 | 0.4 | ||
| Streptcoccus constellatus | 1 | 1 | 1.4 | 0 | - | - | 1 | 0.3 | 0.4 |
| Streptococcus dysgalactiae | 1 | 1 | 1.4 | 0 | - | - | 1 | 0.3 | 0.4 |
| Gram-negative bacilli | 72 | 74.2 | 100 | 160 | 72.7 | 103.2 | 232 | 73.2 | 102.2 |
| Pseudomonas aeruginosa | 17 | 17.5 | 23.6 | 31 | 14.1 | 20 | 48 | 15.1 | 21.1 |
| Escherichia coli | 16 | 16.5 | 22.2 | 26 | 11.8 | 16.8 | 42 | 13.2 | 18.5 |
| Enterobacter cloacae | 5 | 5.2 | 6.9 | 16 | 7.3 | 10.3 | 21 | 6.6 | 9.3 |
| Klebsiella pneumoniae | 4 | 4.1 | 5.6 | 17 | 7.7 | 11 | 21 | 6.6 | 9.3 |
| Citrobacter koresi | 4 | 4.1 | 5.6 | 11 | 5 | 7.1 | 15 | 4.7 | 6.6 |
| Proteus mirabilis | 4 | 4.1 | 5.6 | 8 | 3.6 | 5.2 | 12 | 3.8 | 5.3 |
| Serratia marcescens | 4 | 4.1 | 5.6 | 7 | 3.2 | 4.5 | 11 | 3.5 | 4.8 |
| Moraxella catarhalis | 0 | - | - | 2 | 0.9 | 1.3 | 2 | 0.6 | 0.9 |
| Klebsiella aerogenes | 2 | 2 | 2.8 | 13 | 5.9 | 8.4 | 15 | 4.7 | 6.6 |
| Klebsiella oxytoca | 4 | 4.1 | 5.6 | 2 | 0.9 | 1.3 | 6 | 1.9 | 2.6 |
| Stenotrophomonas maltophilia | 0 | - | - | 5 | 2.3 | 3.2 | 5 | 1.6 | 2.2 |
| Haemophilius influenzae | 7 | 7.2 | 9.7 | 14 | 6.4 | 9 | 21 | 6.6 | 9.3 |
| Citrobacter freundii | 1 | 1 | 1.4 | 2 | 0.9 | 1.3 | 3 | 0.9 | 1.3 |
| Morganella morganii | 0 | - | - | 3 | 1.4 | 1.9 | 3 | 0.9 | 1.3 |
| Klebsiella varicola | 0 | - | - | 2 | 0.9 | 1.3 | 2 | 0.6 | 0.9 |
| Hafnia alvei | 1 | 1 | 1.4 | 0 | - | - | 1 | 0.3 | 0.4 |
| Chryseobacterium indologenes | 1 | 1 | 1.4 | 0 | - | - | 1 | 0.3 | 0.4 |
| Proteus vulgaris | 1 | 1 | 1.4 | 0 | - | - | 1 | 0.3 | 0.4 |
| Achromobacter | 1 | 1 | 1.4 | 0 | - | - | 1 | 0.3 | 0.4 |
| Raoultella ornithinolytica | 0 | - | - | 1 | 0.5 | 0.6 | 1 | 0.3 | 0.4 |
| Gram-negative cocci | 0 | - | - | 2 | 0.9 | 1.3 | 2 | 0.6 | 0.9 |
| Neisseria meningitidis | 0 | - | - | 2 | 0.9 | 1.3 | 2 | 0.6 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesland, J.-B.; Carlier, E.; François, B.; Serck, N.; Gerard, L.; Briat, C.; Piagnerelli, M.; Laterre, P.-F.; on behalf of the COVCORVAP Collaboration Group. Early Corticosteroid Therapy May Increase Ventilator-Associated Lower Respiratory Tract Infection in Critically Ill Patients with COVID-19: A Multicenter Retrospective Cohort Study. Microorganisms 2022, 10, 984. https://doi.org/10.3390/microorganisms10050984
Mesland J-B, Carlier E, François B, Serck N, Gerard L, Briat C, Piagnerelli M, Laterre P-F, on behalf of the COVCORVAP Collaboration Group. Early Corticosteroid Therapy May Increase Ventilator-Associated Lower Respiratory Tract Infection in Critically Ill Patients with COVID-19: A Multicenter Retrospective Cohort Study. Microorganisms. 2022; 10(5):984. https://doi.org/10.3390/microorganisms10050984
Chicago/Turabian StyleMesland, Jean-Baptiste, Eric Carlier, Bruno François, Nicolas Serck, Ludovic Gerard, Charlotte Briat, Michael Piagnerelli, Pierre-François Laterre, and on behalf of the COVCORVAP Collaboration Group. 2022. "Early Corticosteroid Therapy May Increase Ventilator-Associated Lower Respiratory Tract Infection in Critically Ill Patients with COVID-19: A Multicenter Retrospective Cohort Study" Microorganisms 10, no. 5: 984. https://doi.org/10.3390/microorganisms10050984
APA StyleMesland, J.-B., Carlier, E., François, B., Serck, N., Gerard, L., Briat, C., Piagnerelli, M., Laterre, P.-F., & on behalf of the COVCORVAP Collaboration Group. (2022). Early Corticosteroid Therapy May Increase Ventilator-Associated Lower Respiratory Tract Infection in Critically Ill Patients with COVID-19: A Multicenter Retrospective Cohort Study. Microorganisms, 10(5), 984. https://doi.org/10.3390/microorganisms10050984






