Abstract
The emergence and spread of β-lactams and colistin-resistant Escherichia coli in birds deserve a special concern worldwide. This study aimed to investigate the presence of β-lactams and colistin-resistant Escherichia coli strains isolated from the faeces of urban and rural pigeons in Batna, Algeria, and to characterise their molecular traits of resistance. Between March and April 2019, a total of 276 faecal droppings samples were collected in Batna, Algeria. Samples were subjected to selective isolation of β-lactams and colistin-resistant Escherichia coli. The representative colonies were then identified using Matrix-Assisted Laser Desorption-Ionization Time-of-Flight Mass Spectrometry. Antimicrobial susceptibility testing was performed using the disc diffusion method. β-lactamases, as well as mcr genes, were screened for by PCR and confirmed by sequencing. Genetic relatedness of the mcr-positive E. coli strains was determined using multi-locus sequence typing analysis. Transferability features of carbapenemase genes were assessed by conjugation experiments. Overall, thirty-five E. coli isolates were obtained only from urban pigeon samples. All carbapenem-resistant isolates harboured the blaOXA-48 gene as the only carbapenemase gene detected (n = 11), while blaESBL genes were detected in eighteen isolates. Out of the thirty-five isolates, four E. coli isolates were positive for the mcr-1 gene. The obtained mcr-1 positive E. coli isolates belonged to four STs, including ST1485, ST224, ST46, and a new ST. This study is the first to report the isolation of E. coli strains carrying the mcr-1 gene from pigeon faeces in Algeria and also the first to report the detection of blaOXA-48-positive E. coli in pigeons. Close surveillance is, therefore, urgently needed to monitor the dissemination of blaOXA-48 and mcr-1 producing E. coli strains in wildlife.
1. Introduction
Contaminated environments seem to be a leading factor in the dissemination of antibiotic resistance, as bacteria from various origins are able to mix and exchange antibiotic-resistance encoding genes [1,2]. Moreover, the role of the environment in promoting and spreading antibiotic-resistant bacteria and genes is understudied [3]. Birds are considered a good choice for monitoring urban ecosystems since they can be surveyed on a large scale, and they are easy to see and attractive to the population as well the fact that their occurrence and abundance are influenced by habitat characteristics. In addition, birds have also been postulated as potential reservoirs and vehicles of antibiotic resistance genes [4,5].
The Columba livia species of bird is common in cities in various countries and can transmit more than 30 diseases to humans via the air or their excreta [5]. Favourable environmental conditions, as well as the availability of food and the absence of predators, are the major factors implicated in the high increase in their populations in both urban and rural areas [6]. The presence of multi-drug resistant bacteria in pigeons is generally linked to faecal contamination of both human and animal origin [7]. Carriage of such bacteria in pigeon faeces has been reported in different countries, with much of the focus being on Escherichia coli species [8,9,10].
Escherichia coli is a Gram-negative bacterium which holds a special place in the microbiological world because some of them can cause severe infections in animals and humans, but they can also represent an important part of the autochthonous microbiota of many hosts. Of main concern is the possible transmission of resistant E. coli between animals and humans via various pathways, including direct contact, the food chain, or contact with animal excretion [11]. In the literature, numerous genes have been detected in E. coli species of human and animal origins that confer resistance to β-lactams (extended-spectrum β-lactamases and carbapenemase) and to colistin antibiotics. The increase in carbapenem and colistin-resistant bacteria is considered one of the most critical public health concerns since carbapenems and colistin are often used as a last-line treatment for multi-drug resistant Gram-negative bacterial infections [12,13]. The most emerged carbapenemase type worldwide is OXA-48-like enzyme variants, which are becoming the main carbapenemase type in Enterobacteriaceae worldwide, particularly in Mediterranean countries [14,15]. It was initially identified in a K. pneumoniae strain from a 54-year-old man with skin burns and a urinary tract infection from Istanbul, Turkey, in 2001 [16]. After the first identification, an outbreak of OXA-48-producing K. pneumoniae strains was described in Istanbul, Turkey, between May 2006 and January 2007, and since then, it has been rapidly diffused worldwide in different niches [14,17]. On the other hand, more recently, Liu et al. described the first plasmid-mediated colistin resistance mechanism, mcr-1, in human K. pneumoniae and E. coli recovered from provinces in China between April 2011 and November 2014 [18]. Currently, the mcr-1 gene has been found in different genera of the Enterobacteriaceae such as Klebsiella, Escherichia, and Enterobacter isolated from various sources, including animals and water samples, indicating that colistin resistance determinants have also disseminated into the environment notably both urban and rural areas [7,19]. To date, studies revealing the emergence of carbapenem and colistin-resistant bacteria isolated from pigeons are still limited.
Therefore, the aim of this study was to screen for the presence of ESBL, carbapenemase and mcr-producing E. coli isolates in faecal droppings samples from urban and rural pigeons in Batna, Algeria.
2. Materials and Methods
2.1. Sample Collection
Between March and April 2019, a total of 276 fresh faecal droppings samples from pigeons of the Columba livia species were collected at different urban area (the city of Batna) locations (n = 191), including a public park (n = 50), school (n = 50), university (n = 15), mosque (n = 2), different household residences: 800 household residences (n = 6), 1020 household residences (n = 24), 126 household residences (n = 23), 74 household residences (n = 1), nearest to a sewage treatment plant (n = 20), and as well as rural area (from animal farms (n = 85) in EL Madher locality) in Batna, eastern Algeria. Fresh faecal droppings samples were collected aseptically in sterile containers and were immediately transferred at 4 °C to the laboratory for analysis. The samples were first pooled before the isolation procedure, where each pooled sample contained two, three or five samples of the same location.
2.2. β-Lactams and Colistin-Resistant-E. coli Isolation and Bacterial Identification
The isolation of extended-spectrum-cephalosporins, carbapenems and colistin-resistant-E. coli started with a selective enrichment step in brain-heart infusion (BHI) broth with 64 μg/mL vancomycin and supplemented with one of the four different selective antibiotics as follows: (1): 2 μg/mL cefotaxime, (2): 2 μg/mL ertapenem, (3): 9 μg/mL imipenem or (4): 3 μg/mL colistin, respectively. After overnight incubation at 37 °C, ten microliters were taken from the enrichment tubes and were inoculated into selective MacConkey agar plates with the same selective antibiotic combinations [20,21]. The bacterial identification of the obtained isolates was performed by Matrix-Assisted Laser Desorption Ionization-Time of Flight mass spectrometry (MALDI-TOF MS), as previously described [22].
2.3. Antimicrobial Susceptibility Testing
Antimicrobial drug susceptibility of the obtained isolates was determined on Mueller–Hinton agar using the standard disc diffusion method, as recommended by the Antibiogram Committee of the French Society for Microbiology (CA-SFM, 2019) (https://www.sfm-microbiologie.org/wp-content/uploads/2019/02/CASFM2019_V1.0.pdf; accessed on 1 March 2019). The obtained isolates were tested for antibiotic resistance using a panel of thirteen antibiotics, including amoxicillin (20 μg), cefoxitin (30 μg), ceftazidime (30 μg), cefotaxime (30 μg), cefepime (30 μg), aztreonam (30 μg), amoxicillin-clavulanic acid (20–10 μg), ertapenem (10 μg), imipenem (10 μg), tobramycin (10 μg), gentamicin (10 μg), amikacin (30 μg), and ciprofloxacin (5 μg). The E. coli ATCC 25922 strain was used for quality control assays. The results were interpreted according to the CA-SFM, 2019, as well as the Clinical and Laboratory Standards Institute (CLSI, 2017) breakpoints.
The minimal inhibitory concentration (MIC) of colistin was performed by broth microdilution applying the criteria of the European Committee on Antimicrobial Susceptibility Testing Guidelines, 2017 (https://www.eucast.org/; accessed on 15 May 2019).
2.4. Phenotypic Detection of Extended Spectrum β-Lactamase and Carbapenemase Production
The detection of ESBL was further performed phenotypically using the double-disk diffusion method (DDST), while the phenotypic investigation of carbapenemase production was performed using the modified carba NP (MCNP) test as previously described [23].
2.5. Molecular Detection of β-Lactamases and mcr Genes
The obtained strains were tested for the presence of extended-spectrum β-lactamases (blaSHV, blaTEM, blaCTX-M) and for the most common carbapenemase genes (blaKPC, blaNDM, blaVIM, and blaOXA-48-like) using real-time PCR (qPCR) with specific primers (Table 1). The colistin resistance gene (mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, and mcr-8) was also searched for by qPCR [24,25,26]. Standard PCR and sequencing of the positive real-time PCR strains harbouring the carbapenemase or mcr genes were also performed.

Table 1.
Oligonucleotide primers and probes used for polymerase chain reaction.
2.6. Conjugation Experiment
The transferability of carbapenemase genes was determined through a conjugation experiment (broth mating method) using an azide-resistant E. coli J53 recipient strain and two donor strains. The transconjugants were selected on nutrient agar containing ertapenem (2 µg/mL) and sodium azide (200 µg/mL) [20]. The obtained transconjugants were verified by antimicrobial drug susceptibility testing and the modified carba NP test and were confirmed to have the blaOXA−48 gene by PCR.
2.7. Multilocus Sequence Typing
To determine the epidemiological relationships, MLST analysis was carried out on the mcr-1-positive E. coli isolates. Multilocus sequence typing (MLST) was performed by targeting seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) [31]. The obtained sequences were analysed through an E. coli MLST database website (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli; accessed on 15 October 2020).
2.8. Statistical Analysis
The isolation rate of the targeted drug resistant-E. coli (ESBL, carbapenemase and mcr-1-positive isolates) related to the sampling sites was analysed by performing the Pearson chi-square test using SPSS (version 26.0; SPSS, Inc., Chicago, IL, USA). The level of significance was set at a p-value < 0.05.
3. Results
3.1. Bacterial Identification and Antimicrobial Susceptibility Testing
Thirty-five E. coli isolates were identified from pigeon faeces recovered from the different urban areas, including the university (5.71%), 1020 household residences (5.71%), 800 household residences (8.57%), a public park (17.15%), and thosenearest to a sewage treatment plant (62.86%). However, no E. coli isolates were obtained from rural samples as well as other urban areas (school, mosque, 126 household residences, 74 household residences). In this context, thePearson chi-square test revealed no significant effect of the sampling site on the rate of positive isolated strains (positivity) (χ2 = 30; p = 0.314).
All the obtained isolates were resistant to amoxicillin (n = 35), however overall, twenty-four were resistant to amoxicillin-clavulanic acid (n = 24), followed by cefotaxime (n = 23), ceftazidime (n = 18), cefepime (n = 15), ertapenem (n = 14), aztreonam (n = 10), and cefoxitin (n = 2). Resistance to ciprofloxacin, tobramycin, and gentamicin was observed in twenty-two, ten, and seven isolates, respectively. Imipenem and amikacin showed excellent antibacterial activity against the obtained isolates with a susceptibility of 100%. In addition, four E. coli isolates were resistant to colistin with minimum inhibitory concentration measured at 4 µg/mL. The location of sampling points with the detection rate of AMR E. coli in various categories of sites targeted in this study was generated using Google Maps with open data (https://www.google.dz/maps; accessed on 15 December 2021) and presented in Figure 1.
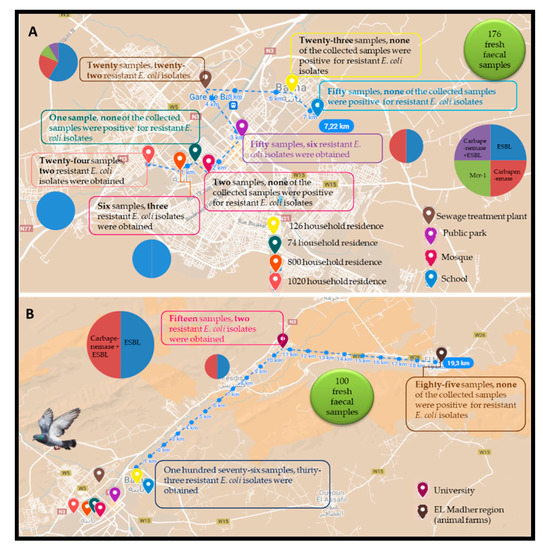
Figure 1.
Location of sampling points with the detection rate of AMR E. coli in the different sites targeted in this study. (A) 176 fresh faecal samples; (B) 100 fresh faecal samples.
3.2. Molecular Detection of ESBL, Carbapenemase and mcr Genes
The genotyping results of ESBL, carbapenemase and mcr genes among the obtained isolates are shown in Table 2. Of the 35 isolates, eighteen were ESBL producers, seven were carbapenemase producers, and four isolates were positive for carbapenemase and other β-lactamase types. Among the eighteen ESBL producing isolates, ten were found to be positive for the combination blaCTX-M−A and blaTEM gene, while eight of the obtained isolates were positive for the blaCTX-M−A gene. The eleven carbapenemase-producing isolates were blaOXA-48 positive. Among the blaOXA-48 positive E. coli, two isolates harboured the blaOXA-48, blaCTX-M−A, and blaTEM genes, one isolate of each was positive for the combination blaOXA-48 and blaTEM or blaOXA-48, blaCTX-M−A genes, respectively. The remaining seven isolates harboured only the blaOXA-48 gene. Out of 35 isolates, four E. coli isolates were positive for the mcr-1 gene.

Table 2.
Antibiotic susceptibility testing, resistance genes, and sequence types of the E. coli isolates obtained in this study.
3.3. Conjugation Experiment
Our study showed that the two tested E. coli isolates that carried the blaOXA-48 gene were successfully transferred to E. coli J53. The antimicrobial susceptibility of the obtained transconjugants (TCP21 and TCP30) showed that they were resistant to amoxicillin-clavulanic acid and ertapenem and were positive for the MCNP test. PCR results confirmed the presence of the blaOXA-48 gene in the two obtained transconjugants.
3.4. Multilocus Sequence Typing
MLST results showed that the four mcr-1 positive-E. coli isolates belonged to four different sequence types, including ST1485, ST224, ST46, and new ST.
4. Discussion
Around the world, there are significant numbers of pigeons living in close contact with humans and other animals in rural and urban areas [32]. Various studies on the contamination levels of pigeon faeces in public areas revealed that pigeon faeces represent a source of various zoonotic agents for humans and animals and have been identified as a potential source and vector that can spread antibiotic-resistant bacteria and genes [33,34,35,36,37]. In this regard, the emergence of extended-spectrum cephalosporins, carbapenem, and colistin resistance in pigeon faeces is a serious challenge worldwide, in both urban and rural areas. In our study, we report the detection of blaESBL, blaOXA-48, and mcr-1 genes from urban pigeon faeces in Algeria. These results can be explained by different contributing factors, including diverse feeding habits of urban pigeons such as sewage treatment plants and municipal solid waste dumping grounds [38]. These feeding habits of urban pigeons could lead to them being contaminated with medically important bacteria or residual antimicrobials and chemicals since they may rely on waste or nearby refuse containers as food sources [5,39]. In addition, various authors have suggested that pigeons can interact with other birds, which would facilitate the acquisition and dissemination of this resistance to other species [5].
The carriage of ESBL producers in pigeons in our study was comparable to previous studies around the world, which have reported the detection of E. coli isolates harbouring blaCTX-M genes from pigeons, including Bangladesh, France, Germany, Nicaragua, China, and Brazil [9,10,34,35,40,41]. In this study, we also detected the blaOXA-48 gene in E. coli isolates from different urban places around the city, including a sewage treatment plant, a university, and a public park. To the best of our knowledge, the two last urban areas are among the most dynamic areas in the city. The public park is the most urbanised, popular, and economically active region in the city of Batna, and it is located in an area marked by urban sprawl and overcrowding. Columba livia might favour the inter-genus or inter-species horizontal propagation of antibiotic-resistance genes because their faeces can harbour different resistant bacteria representative of the various environments that the birds recently visited [39]. Domestic pigeons do not travel long distances (maximum 5.29 km), and they have to meet their needs with what they find within the signalled distance. In this study, the sampled areas were located in environments with a high human population, close to hospitals and wastewater, known reservoirs of antibiotic-resistance genes, where the birds can access water and food that is contaminated with pharmaceutical products such as antibiotics. In this context and in the same city where our study was conducted, the first detection of the pblaOXA-48 gene was described in 2014 at Batna university hospital, then from migratory birds, community-acquired infection, currency, and more recently from hospital wastewater [20,21,42,43,44], suggesting that the detected genes in pigeon are related to their feeding mode in different locations including hospitals and wastewater or by contact with other birds such as migratory birds. From pigeons, only two studies have reported the detection of carbapenemase-producing Gram-negative bacteria worldwide. The first such study was conducted on pigeon faeces collected in Algeria and France, where the authors identified the presence of carbapenemases-encoding genes in 16 out of the 73 studied samples (13 were positive for blaOXA-58, 12 blaOXA-51-like, and eight carried the blaOXA-23 genes) [33]. The second report detected blaMUS-2, a novel variant of the chromosome-encoded blaMUS-1 associated with carbapenem resistance in Myroides odoratimimus isolates in Lebanon [45]. Importantly, in this study, we report the first detection of the mcr-1 gene in pigeons in Algeria, where colistin is considered a drug of last resort for human medicine for the treatment of infections caused by Gram-negative bacteria. However, there have only been a few reports of the mcr gene in pigeons. In agreement with our findings, a study in Qatar reported that only one E. coli isolate from pigeon faecal samples harboured the mcr-1 gene [8]. mcr-1 and mcr-3 genes have also been detected in China, with a prevalence of 13.1% and 5.1%, respectively [46]. Another study conducted in China signalled the detection of mcr-4 and mcr-5 genes with a prevalence of 17.2% and 3%, respectively [47]. In our study, the detection of the mcr-1 gene has been reported only in E. coli isolates obtained from faecal droppings samples collected near sewage treatment plants. This result can be explained by the feeding habits of urban pigeons, where a recent study has reported the detection of the mcr-1 gene with the same STs from the sewage treatment plants where our analysed pigeons fed (unpublished data), indicating that the surrounding environment could be the origin of the detected resistance genes. To the best of our knowledge, wastewater from different hospitals could be discharged into the sewage treatment plants inviting the possibility that the reported genes could be related to the hospital settings. No mcr-1 genes were found in the sampled rural area (animal farms), which may be explained by the limited or prudent use of antibiotics, particularly colistin.
The MLST results showed that the four mcr-1 positive E. coli isolates belonged to four different sequence types, including ST1485, ST224, ST46, and a new ST. In fact, these STs appear to be well adapted to animals living in rural and urban areas and have been reported worldwide, mostly in association with plasmid-mediated blaCTX-M-type genes or, similar to our study, with the mcr-1 gene. The ST224 has already been reported in isolates with the blaCTX-M gene from cats in France and Brazil [48,49], from food-producing animals (buffalo calves) in Brazil [50], and from a deer in Spain [51]. In addition, the ST224 has been reported in strains with colistin resistance from chicken meat in Algeria [52]. ST1485 E. coli isolate has already been isolated from rural dogs in Spain [53] and from birds in Chile (Andean condors) [54]. Similarly, ST46 has been previously reported in an E. coli strain with the mcr-1 gene from chicken faeces and in pets in China [55,56] and with CTX-M type ESBL from pig samples from Nigeria [57]. This suggests that pigeons could facilitate the crossover of antimicrobial resistance with other animals in the local region and contribute to the further spread of these resistance genes.
5. Conclusions
To conclude, we report here for the first time the presence of the mcr-1 gene in pigeon droppings in Algeria and also report the first detection of OXA-48-producing E. coli in pigeon droppings. This study clearly illustrates that pigeons, which live in close proximity to humans, could play a role as potential reservoirs of multi-drug-resistant bacteria, including carbapenemase and mcr producers in urban areas. Hence, risk management measures should be undertaken to limit the emergence and spreading of AMR in Algeria.
In light of these data, future studies should be conducted to identify multi-drug-resistant bacteria transmission pathways in order to understand the potential role of such birds in the spread of carbapenemase and mcr-1 genes.
Author Contributions
L.L. conceptualized, directed the study, performed the experiments and corrected the manuscript. W.C. performed the experiments, analyzed the data and wrote the original draft. E.B. performed the strains identification experiments and molecular biology experiments. Z.C. performed the molecular biology experiments and analyzed the data. M.K. and A.K. performed the sampling procedure and phonotypical experiments. J.-M.R. conceived the original idea and corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the French Government under the “Investments for the Future” program managed by the National Agency for Research (ANR) (Méditerranée-Infection10-IAHU-03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was supported by the DGRSDT of the Algerian Ministry of Higher Education and Scientific Research. The authors are grateful to Necer Abdeldjabar for the statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, J.; Huang, Y.; Rao, D.; Zhang, Y.; Yang, K. Evidence for environmental dissemination of antibiotic resistance mediated by wild birds. Front. Microbiol. 2018, 9, 745. [Google Scholar] [CrossRef]
- Aeksiri, N.; Toanan, W.; Sawikan, S.; Suwannarit, R.; Pungpomin, P.; Khieokhajonkhet, A.; Niumsup, P.R. First detection and genomic insight into mcr-1 encoding plasmid-mediated colistin-resistance gene in Escherichia coli ST101 isolated from the migratory bird species Hirundo rustica in Thailand. Microb. Drug Resist. 2019, 25, 1437–1442. [Google Scholar] [CrossRef]
- Mills, M.C.; Lee, J. The threat of carbapenem-resistant bacteria in the environment. Evidence of widespread contamination of reservoirs at a global scale. Environ. Pollut. 2019, 255, 113143. [Google Scholar] [CrossRef]
- Handrova, L.; Kmet, V. Antibiotic resistance and virulence factors of Escherichia colifrom eagles and goshawks. J. Environ. Sci. Health B 2019, 54, 605–614. [Google Scholar] [CrossRef]
- Blanco-Pena, K.; Esperon, F.; Torres-Mejia, A.M.; De la Torre, A.; De la Cruz, E.; Jiménez-Soto, M. Antimicrobial resistance genes in pigeons from public parks in Costa Rica. Zoonoses. Public Health 2017, 64, e23–e30. [Google Scholar]
- Chidamba, L.; Korsten, L. Antibiotic resistance in Escherichia coli isolates from roof-harvested rainwater tanks and urban pigeon faeces as the likely source of contamination. Environ. Monit. Assess. 2015, 187, 405. [Google Scholar] [CrossRef]
- Anyanwu, M.U.; Jaja, I.F.; Nwobi, O.C. Occurrence and characteristics of mobile colistin resistance (mcr) gene-containing isolates from the environment: A review. Int. J. Environ. Res. Public Health 2020, 17, 1028. [Google Scholar] [CrossRef] [Green Version]
- Alhababi, D.A.; Eltai, N.O.; Nasrallah, G.K.; Farg, E.A.; Al Thani, A.A.; Yassine, H.M. Antimicrobial resistance of commensal Escherichia coli isolated from food animals in Qatar. Microb. Drug Resist. 2020, 26, 420–427. [Google Scholar] [CrossRef]
- Cunha, M.P.V.; Oliveira, M.C.V.; Oliveira, M.G.X.; Menao, M.C.; Knobl, T. CTX-M- producing Escherichia coli Isolated from urban pigeons (Columba livia domestica) in Brazil. J. Infect. Dev. Ctries. 2019, 13, 1052–1056. [Google Scholar] [CrossRef]
- Guenther, S.; Grobbel, M.; Beutlich, J.; Bethe, A.; Friedrich, N.D.; Goedecke, A.; Lubke-Becker, A.; Guerra, B.; Wieler, L.H.; Ewers, C. CTX-M-15-type extended-spectrum beta-lactamases-producing Escherichia coli from wild birds in Germany. Environ. Microbiol. Rep. 2010, 2, 641–645. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 0026-2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van, D.D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar]
- Son, S.J.; Huang, R.; Squire, C.J.; Leung, I.K.H. MCR-1: A promising target for structure-based design of inhibitors to tackle polymyxin resistance. Drug Discov. Today 2019, 24, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Mairi, A.; Pantel, A.; Sotto, A.; Lavigne, J.P.; Touati, A. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 587–604. [Google Scholar] [CrossRef]
- Iovleva, A.; Doi, Y. Carbapenem-resistant Enterobacteriaceae. Clin. Lab. Med. 2017, 37, 303–315. [Google Scholar] [CrossRef]
- Poirel, L.; Héritier, C.; Tolun, V.; Nordmann, P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Carrer, A.; Poirel, L.; Eraksoy, H.; Cagatay, A.A.; Badur, S.; Nordmann, P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob. Agents Chemother. 2008, 52, 2950–2954. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Stefaniuk, E.M.; Tyski, S. Colistin resistance in Enterobacterales strains—A current view. Pol. J. Microbiol. 2019, 68, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Bendjama, E.; Loucif, L.; Chelaghma, W.; Attal, C.; Bellakh, F.Z.; Benaldjia, R.; Kahlat, I.; Meddour, A.; Rolain, J.M. First detection of an OXA-48-producing Enterobacter cloacae isolate from currency coins in Algeria. J. Glob. Antimicrob. Resist. 2020, 23, 162–166. [Google Scholar] [CrossRef]
- Cherak, Z.; Loucif, L.; Moussi, A.; Bendjama, E.; Benbouza, A.; Rolain, J.M. Emergence of Metallo-β-Lactamases and OXA-48 carbapenemase producing Gram-negative bacteria in hospital wastewater in Algeria: A potential dissemination pathway into the environment. Microb. Drug. Resist. 2021, 28, 23–30. [Google Scholar] [CrossRef]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.E.; Rolain, J.M.; Raoult, D. Ongoing revolution in bacteriology: Routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 2009, 49, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Bakour, S.; Garcia, V.; Loucif, L.; Brunel, J.M.; Gharout-Sait, A.; Touati, A.; Rolain, J.M. Rapid identification of carbapenemase-producing Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacterbaumannii using a modified Carba NP test. New Microbes New Infect. 2015, 7, 89–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabou, S.; Leangapichart, T.; Okdah, L.; Le Page, S.; Hadjadj, L.; Rolain, J.M. Real-time quantitative PCR assay with Taqman(Â) probe for rapid detection of MCR-1 plasmid-mediated colistin resistance. New Microbes New Infect. 2016, 13, 71–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly, T.D.A.; Hadjadj, L.; Hoang, V.T.; Louni, M.; Dao, T.L.; Badiaga, S.; Tissot-Dupont, H.; Raoult, D.; Rolain, J.M.; Gautret, P. Low prevalence of resistance genes in sheltered homeless population in Marseille, France, 2014–2018. Infect. Drug Resist. 2019, 12, 1139–1151. [Google Scholar] [CrossRef] [Green Version]
- Nabti, L.Z.; Sahli, F.; Ngaiganam, E.P.; Radji, N.; Mezaghcha, W.; Lupande-Mwenebitu, D.; Baron, S.A.; Rolain, J.M.; Diene, S.M. Development of real-time PCR assay allowed describing the first clinical Klebsiellapneumoniae isolate harboring plasmid-mediated colistin resistance mcr-8 gene in Algeria. J. Glob. Antimicrob. Resist. 2020, 20, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Roschanski, N.; Fischer, J.; Guerra, B.; Roesler, U. Development of a multiplex real-time PCR for the rapid detection of the predominant beta-lactamase genes CTX-M, SHV, TEM and CIT-type AmpCs in Enterobacteriaceae. PLoS ONE 2014, 9, e100956. [Google Scholar] [CrossRef]
- Yousfi, H.; Hadjadj, L.; Dandachi, I.; Lalaoui, R.; Merah, A.; Amoura, K.; Dahi, A.; Dekhil, M.; Messalhi, N.; Diene, S.M. Colistin-and carbapenem-resistant Klebsiella pneumoniae clinical_isolates: Algeria. Microb. Drug. Resist. 2019, 25, 258–263. [Google Scholar] [CrossRef]
- Diene, S.M.; Bruder, N.; Raoult, D.; Rolain, J.M. Real-time PCR assay allows detection of the New Delhi metallo-β-lactamase (NDM-1)-encoding gene in France. Int. J. Antimicrob. Agents. 2011, 37, 544–546. [Google Scholar] [CrossRef] [Green Version]
- Mathlouthi, N.; Al-Bayssari, C.; El Salabi, A.; Bakour, S.; Gwierif, S.B.; Zorgani, A.A.; Jridi, Y.; Slama, K.B.; Rolain, J.M.; Chouchani, C. Carbapenemases and extended-spectrum B-lactamases producing Enterobacteriaceae isolated from Tunisian and Libyan hospitals. J. Infect. Dev. Ctries. 2016, 10, 718–727. [Google Scholar] [CrossRef] [Green Version]
- Diancourt, L.; Passet, V.; Verhoef, J.; Grimont, P.A.; Brisse, S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 2005, 43, 4178–4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbarpour, R.; Daneshdoost, S. Identification of shiga toxin and intimin coding genes in Escherichia coli isolates from pigeons (Columba livia) in relation to phylotypes and antibiotic resistance patterns. Trop. Anim. Health Prod. 2012, 44, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Morakchi, H.; Loucif, L.; Gacemi-Kirane, D.; Rolain, J.M. Molecular characterisation of carbapenemases in urban pigeon droppings in France and Algeria. J. Glob. Antimicrob. Resist. 2017, 9, 103–110. [Google Scholar] [CrossRef]
- Ngaiganam, E.P.; Pagnier, I.; Chaalal, W.; Leangapichart, T.; Chabou, S.; Rolain, J.M.; Diene, S.M. Investigation of urban birds as source of β-lactamase-producing Gram-negative bacteria in Marseille city, France. Acta Vet. Scand. 2019, 61, 51. [Google Scholar] [CrossRef] [Green Version]
- Hasan, B.; Laurell, K.; Rakib, M.M.; Ahlstedt, E.; Hernandez, J.; Caceres, M.; Jarhult, J.D. Fecal carriage of Extended-Spectrum β-Lactamases in healthy humans, poultry, and wild birds in Leon, Nicaragua-a shared pool of bla(CTX-M) genes and possible interspecies clonal spread of Extended-Spectrum β-Lactamases-producing Escherichia coliMicrob. Drug Resist. 2016, 22, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, E.; Elmonir, W.; Suelam, I.I.A.; Mousa, W.S. Antibiogram and genetic diversity of Salmonella enterica with zoonotic potential isolated from morbid native chickens and pigeons in Egypt. J. Appl. Microbiol. 2018, 124, 1265–1273. [Google Scholar] [CrossRef]
- Borges, C.A.; Cardozo, M.V.; Beraldo, L.G.; Oliveira, E.S.; Maluta, R.P.; Barboza, K.B.; Werther, K.; Ãvila, F.A. Wild birds and urban pigeons as reservoirs for diarrheagenic Escherichia coli with zoonotic potential. J. Microbiol. 2017, 55, 344–348. [Google Scholar] [CrossRef]
- Radimersky, T.; Frolkova, P.; Janoszowska, D.; Dolejska, M.; Svec, P.; Roubalova, E.; Cikova, P.; Cizek, A.; Literak, I. Antibiotic resistance in faecal bacteria (Escherichia coli, Enterococcus spp.) in feral pigeons. J. Appl. Microbiol. 2010, 109, 1687–1695. [Google Scholar] [CrossRef]
- Da Silva, V.L.; Caçador, N.C.; Da Silva, C.S.; Fontes, C.O.; Garcia, G.D.; Nicoli, J.R.; Diniz, C.G. Occurrence of multidrug-resistant and toxic-metal tolerant enterococci in fresh feces from urban pigeons in Brazil. Microbes Environ. 2012, 27, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Hasan, B.; Islam, K.; Ahsan, M.; Hossain, Z.; Rashid, M.; Talukder, B.; Ahmed, K.U.; Olsen, B.; Abul, K.M. Fecal carriage of multi-drug resistant and extended spectrum β-lactamases producing E. coli in household pigeons, Bangladesh. Vet. Microbiol. 2014, 168, 221–224. [Google Scholar] [CrossRef]
- Yang, L.; Yang, L.; Lu, D.H.; Zhang, W.H.; Ren, S.Q.; Liu, Y.H.; Zeng, Z.L.; Jiang, H.X. Co-prevalance of PMQR and 16S rRNA methylase genes in clinical Escherichia coli isolates with high diversity of CTX-M from diseased farmed pigeons. Vet. Microbiol. 2015, 178, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Loucif, L.; Kassah-Laouar, A.; Saidi, M.; Messala, A.; Chelaghma, W.; Rolain, J.M. Outbreak of OXA-48-producing Klebsiella pneumoniae involving a sequence type 101 clone in Batna university hospital, Algeria. Antimicrob. Agents Chemother. 2016, 60, 7494–7497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loucif, L.; Chelaghma, W.; Helis, Y.; Sebaa, F.; Baoune, R.D.; Zaatout, W.; Rolain, J.M. First detection of OXA-48-producing Klebsiella pneumoniae in community-acquired urinary tract infection in Algeria. J. Glob. Antimicrob. Resist. 2018, 12, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, A.; Loucif, L.; Ayachi, A.; Guehaz, K.; Bendjama, E.; Rolain, J.M. Migratory white stork (Ciconiaciconia): A potential vector of the OXA-48-producing Escherichia coli ST38 clone in Algeria. Microb. Drug Resist. 2018, 24, 461–468. [Google Scholar] [CrossRef]
- Al-Bayssari, C.; Gupta, S.K.; Dabboussi, F.; Hamze, M.; Rolain, J.M. MUS-2, a novel variant of the chromosome-encoded β-lactamase MUS-1, from Myroidesodoratimimus. New Microbes New Infect. 2015, 7, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, L.; Wang, J.; Yassin, A.K.; Butaye, P.; Kelly, P.; Gong, J.; Guo, W.; Li, J.; Li, M.; et al. Molecular detection of colistin resistance genes (mcr-1, mcr-2 and mcr-3) in nasal/oropharyngeal and anal/cloacal swabs from pigs and poultry. Sci. Rep. 2018, 8, 3705. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, J.; Wang, J.; Butaye, P.; Kelly, P.; Li, M.; Yang, F.; Gong, J.; Yassin, A.K.; Guo, W.; et al. Newly identified colistin resistance genes, mcr-4 and mcr-5, from upper and lower alimentary tract of pigs and poultry in China. PLoS ONE 2018, 13, e0193957. [Google Scholar] [CrossRef] [Green Version]
- Dahmen, S.; Haenni, M.; Chatre, P.; Madec, J.Y. Characterization of blaCTX-MIncFII plasmids and clones of Escherichia coli from pets in France. J. Antimicrob. Chemother. 2013, 68, 2797–2801. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.M.; Sellera, F.P.; Fernandes, M.R.; Moura, Q.; Garino, F.; Azevedo, S.S.; Lincopan, N. Genomic features of a highly virulent, ceftiofur-resistant, CTX-M-8-producing Escherichia coli ST224 causing fatal infection in a domestic cat. J. Glob. Antimicrob. Resist. 2018, 15, 252–253. [Google Scholar] [CrossRef]
- Aizawa, J.; Neuwirt, N.; Barbato, L.; Neves, P.R.; Leigue, L.; Padilha, J.; Pestana de Castro, A.F.; Gregory, L.; Lincopan, N. Identification of fluoroquinolone-resistant extended-spectrum β-lactamase (CTX-M-8)-producing Escherichia coli ST224, ST2179 and ST2308 in buffalo (Bubalus bubalis). J. Antimicrob. Chemother. 2014, 69, 2866–2869. [Google Scholar] [CrossRef] [Green Version]
- Alonso, C.A.; Gonzalez-Barrio, D.; Tenorio, C.; Ruiz-Fons, F.; Torres, C. Antimicrobial resistance in faecal Escherichia coli isolates from farmed red deer and wild small mammals. Detection of a multiresistantE. coli producing extended-spectrum beta-lactamase. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Chaalal, N.; Touati, A.; Yahiaoui-Martinez, A.; Aissa, M.A.; Sotto, A.; Lavigne, J.P.; Pantel, A. Colistin-resistant Enterobacterales isolated from chicken meat in western Algeria. Microb. Drug. Resist. 2021, 27, 991–1002. [Google Scholar] [CrossRef]
- Abreu-Salinas, F.; Diaz-Jiménez, D.; Garcia-Menino, I.; Lumbreras, P.; Lopez-Beceiro, A.M.; Fidalgo, L.E.; Rodicio, M.R.; Mora, A.; Fernandez, J. High prevalence and diversity of cephalosporin-resistant Enterobacteriaceae including extraintestinal pathogenic E. coli CC648 lineage in rural and urban dogs in northwest Spain. Antibiotics 2020, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Castillo, D.; Esposito, F.; Cardoso, B.; Dalazen, G.; Moura, Q.; Fuga, B.; Fontana, H.; Cerdeira, L.; Dropa, M.; Rottmann, J.; et al. Genomic data reveal international lineages of critical priority Escherichia coli harbouring wide resistome in Andean condors (Vulturgryphus Linnaeus, 1758). Mol. Ecol. 2020, 29, 1919–1935. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Y.; Shi, X.; Wang, S.; Ren, H.; Shen, Z.; Wang, Y.; Lin, J.; Wang, S. Rapid rise of the ESBL and mcr-1 genes in Escherichia coli of chicken origin in China, 2008–2014. Emerg. Microbes Infect. 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, Z.; Zhang, Y.; Zhang, Z.; Lei, L.; Xia, Z. Increasing prevalence of ESBL-producing multidrug resistance Escherichia coli from diseased pets in Beijing, China from 2012 to 2017. Front. Microbiol. 2019, 10, 2852. [Google Scholar] [CrossRef]
- Chah, K.F.; Ugwu, I.C.; Okpala, A.; Adamu, K.Y.; Alonso, C.A.; Ceballos, S.; Nwanta, J.N.; Torres, C. Detection and molecular characterisation of extended-spectrum β-lactamase-producing enteric bacteria from pigs and chickens in Nsukka, Nigeria. J. Glob. Antimicrob. Resist. 2018, 15, 36–40. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).