Antiplanktonic and Antibiofilm Activity of Rheum palmatum Against Streptococcus oralis and Porphyromonas gingivalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Preparation of Rheum Palmatum Extract Solutions
2.3. HPLC (High-Performance Liquid Chromatography) Analysis
2.4. Investigation of the Planktonic Bacterial Growth and Metabolic Activity of S. oralis and P. gingivalis under the Influence of R. palmatum Root Extract
2.5. Investigation of R. palmatum Root Extract Effects on S. oralis and P. gingivalis Biofilms
2.6. Statistical Analysis
3. Results
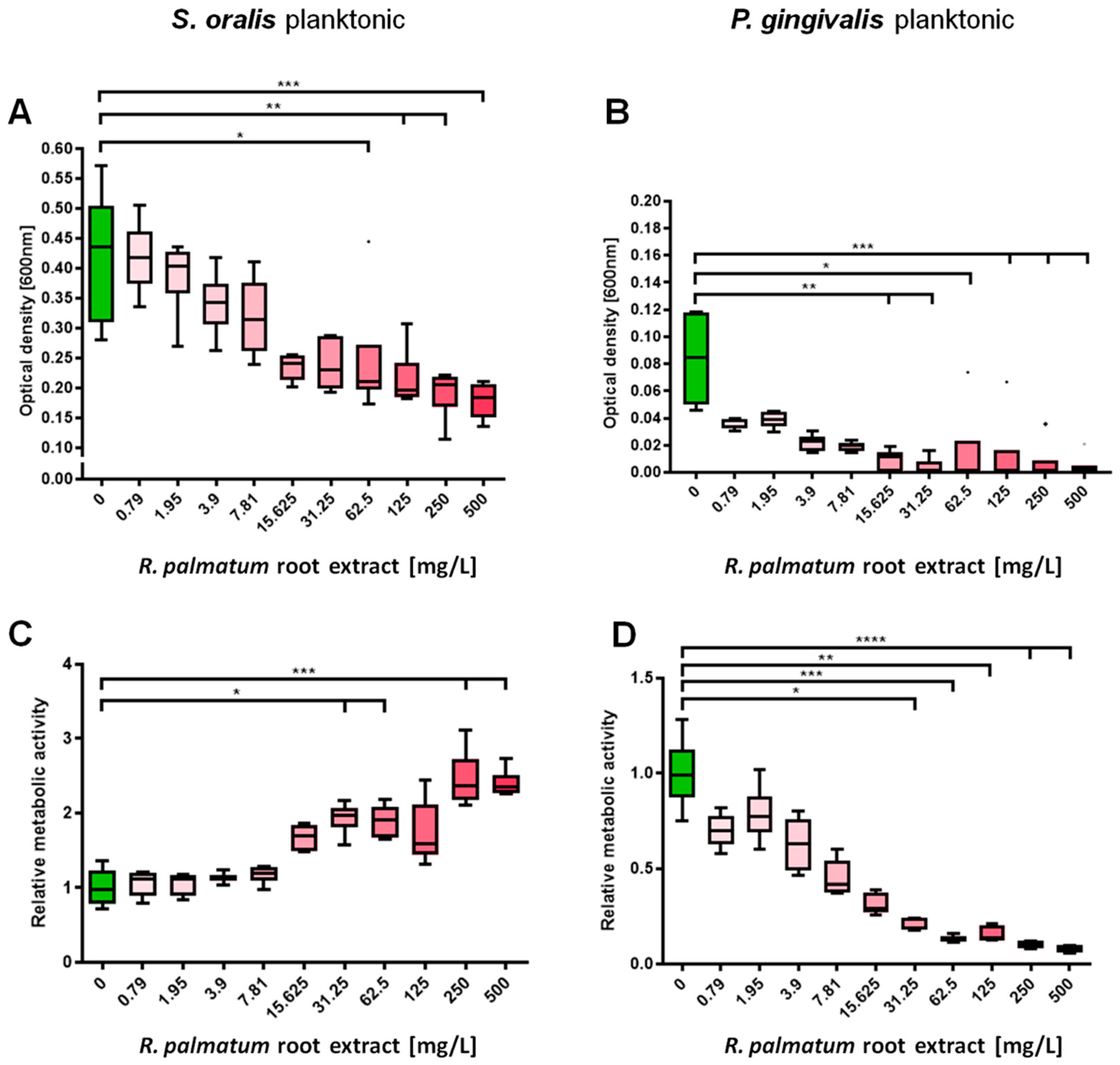
3.1. Selective Antimicrobial Effects of R. palmatum Root Extract on Planktonic S. oralis and P. gingivalis
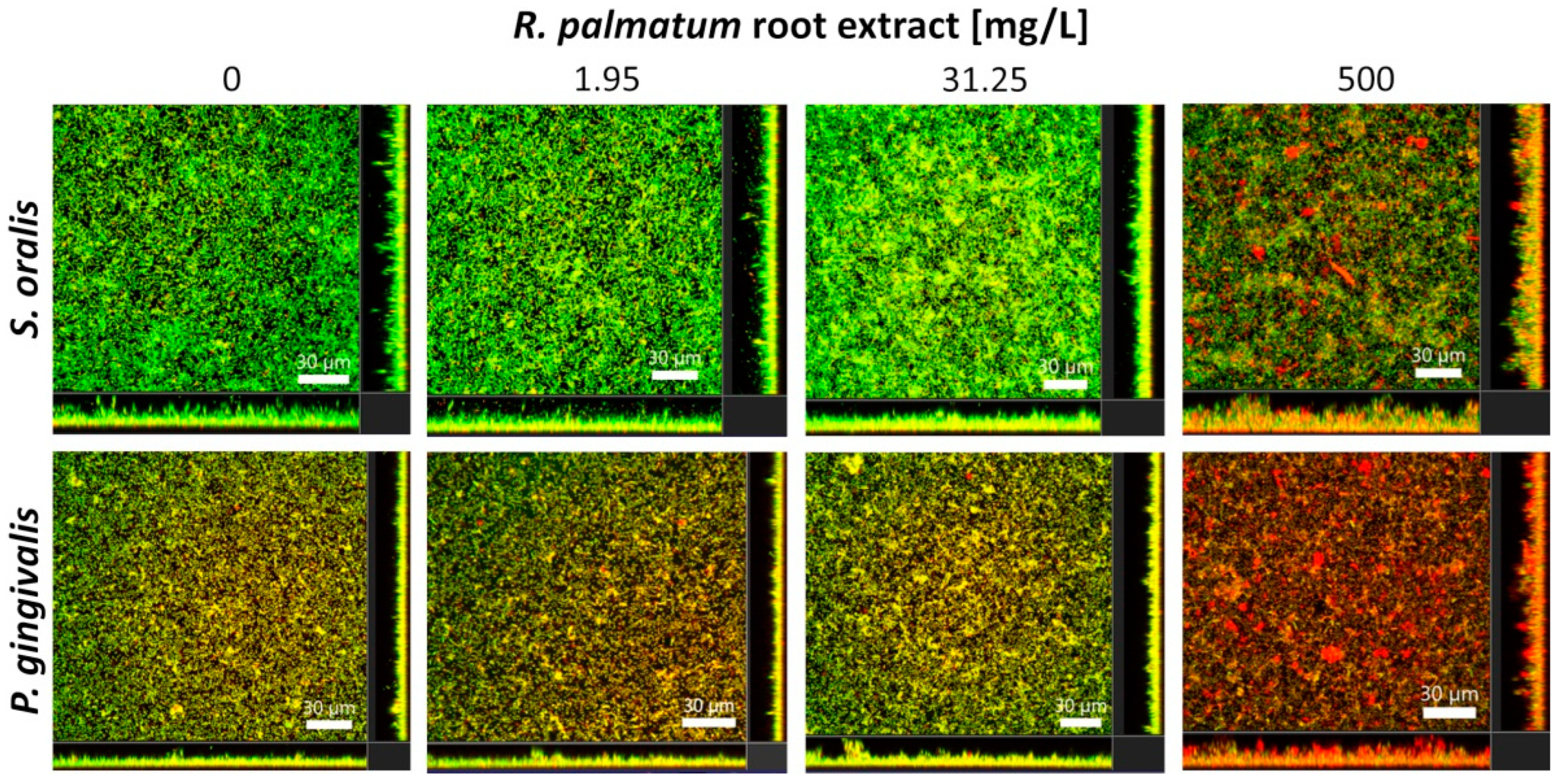
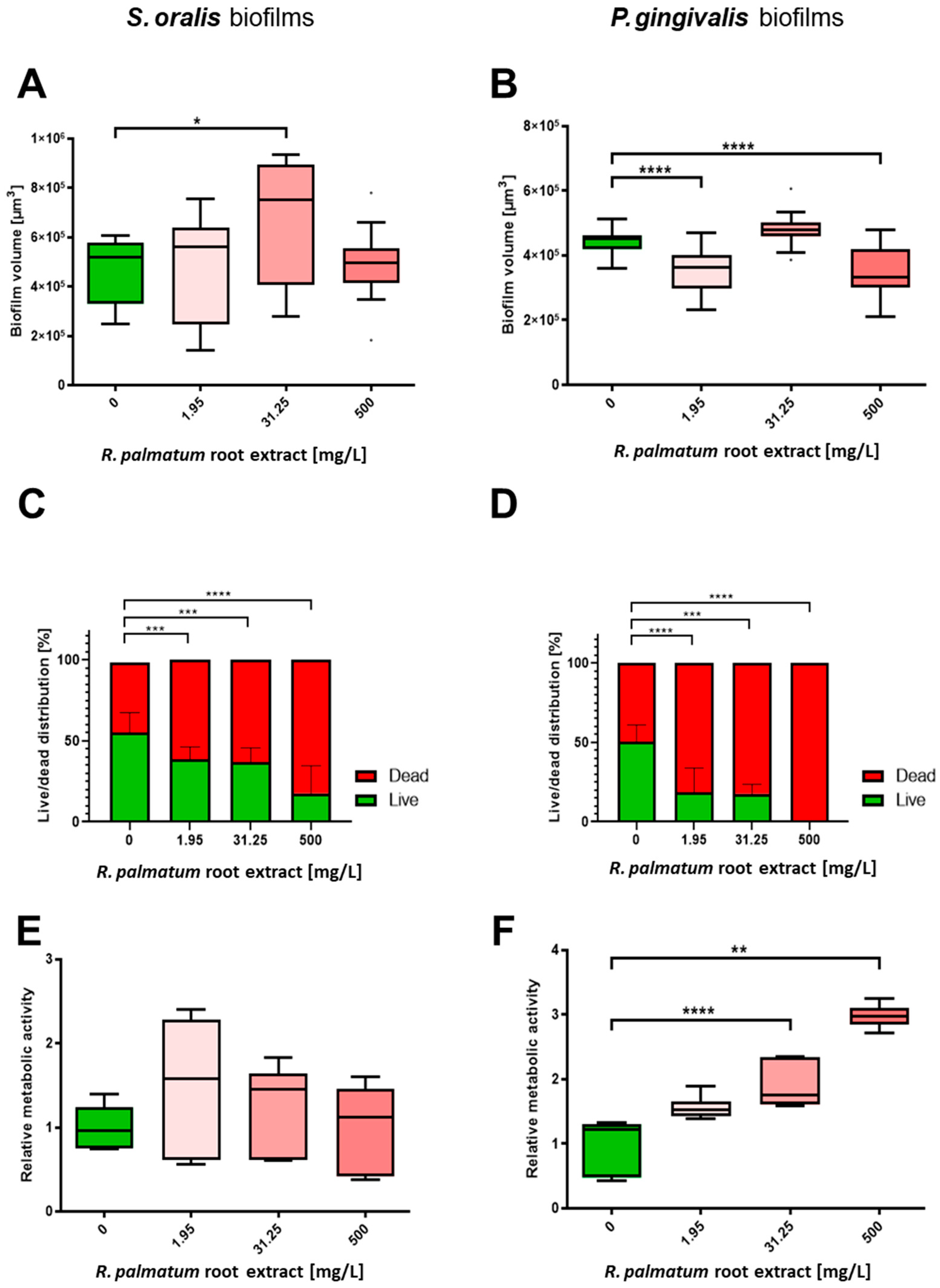
3.2. Selective Antibiofilm Activity of R. palmatum Root Extract on S. oralis and P. gingivalis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 89, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Periodontol. 2018, 89, S9–S16. [Google Scholar] [CrossRef]
- Rawlinson, A.; Vettore, M.V.; Baker, S.R.; Robinson, P.G. Periodontal treatment, psychological factors and oral health-related quality of life. J. Clin. Periodontol. 2021, 48, 226–236. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal disease: A risk factor for diabetes and cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal disease, tooth loss, and cancer risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal. Res. 2018, 53, 657–681. [Google Scholar] [CrossRef]
- Mohanty, R.; Asopa, S.J.; Joseph, M.D.; Singh, B.; Rajguru, J.P.; Saidath, K.; Sharma, U. Red complex: Polymicrobial conglomerate in oral flora: A review. J. Fam. Med. Prim. Care 2019, 8, 3480–3486. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Muzafferiy, F.; Anderson, A.C.; Wölber, J.P.; Ratka-Krüger, P.; Fretwurst, T.; Nelson, K.; Vach, K.; Hellwig, E. Shift of microbial composition of peri-implantitis-associated oral biofilm as revealed by 16S rRNA gene cloning. J. Med. Microbiol. 2018, 67, 332–340. [Google Scholar] [CrossRef]
- Duran-Pinedo, A.E.; Frias-Lopez, J. Beyond microbial community composition: Functional activities of the oral microbiome in health and disease. Microbes Infect. 2015, 17, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, P.; Gilli, F.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P.R.; Karygianni, L. The microbiome of peri-implantitis: A systematic review and meta-analysis. Microorganisms 2020, 8, 661. [Google Scholar] [CrossRef]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Waal, Y.C.D.; Eijsbouts, H.V.; Winkel, E.G.; Winkelhoff, A.J.V. Microbial Characteristics of Peri-Implantitis: A Case-Control Study. J. Periodontol. 2017, 88, 209–217. [Google Scholar] [CrossRef]
- Quirynen, M.; Teughels, W.; Soete, M.D.; Steenberghe, D.V. Topical antiseptics and antibiotics in the initial therapy of chronic adult periodontitis: Microbiological aspects. Periodontology 2000 2002, 28, 72–90. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Rhemrev, G.E.; Timmerman, M.F.; Veldkamp, I.; Winkelhoff, A.J.V.; Velden, U.V.D. Immediate effect of instrumentation on the subgingival microflora in deep inflamed pockets under strict plaque control. J. Clin. Periodontol. 2006, 33, 42–48. [Google Scholar] [CrossRef]
- Takahashi, N. Oral microbiome metabolism:from “who are they?” to “what are they doing?”. J. Dent. Res. 2015, 94, 1628–1637. [Google Scholar] [CrossRef]
- Russell, A.D. Chlorhexidine: Antibacterial action and bacterial resistance. Infection 1986, 14, 212–215. [Google Scholar] [CrossRef]
- Lachapelle, J.-M.; Castel, O.; Casado, A.; Leroy, B.; Micali, G.; Tennstedt, D.; Lambert, J. Antiseptics in the era of bacterial resistance: A focus on povidone iodine. Clin. Pract. 2013, 10, 579–592. [Google Scholar] [CrossRef]
- Hutcherson, J.A.; Sinclair, K.M.; Belvin, B.R.; Gui, Q.; Hoffman, P.S.; Lewis, J.P. Amixicile, a novel strategy for targeting oral anaerobic pathogens. Sci. Rep. 2017, 7, 10474. [Google Scholar] [CrossRef] [PubMed]
- Al-Kamel, A.; Baraniya, D.; Al-Hajj, W.A.; Halboub, E.; Abdulrab, S.; Chen, T.; Al-Hebshi, N.N. Subgingival microbiome of experimental gingivitis: Shifts associated with the use of chlorhexidine and N-acetyl cysteine mouthwashes. J. Oral Microbiol. 2019, 11, 1608141. [Google Scholar] [CrossRef] [PubMed]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Belfield, L.A.; Ashworth, A.; Casas-Agustench, P.; Raja, M.; Pollard, A.J.; Bescos, R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021, 113, 103768. [Google Scholar] [CrossRef]
- Gürgan, C.A.; Zaim, E.; Bakirsoy, I.; Soykan, E. Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse used as an adjunct to non-surgical periodontal treatment: A double-blind clinical study. J. Periodontol. 2006, 77, 370–384. [Google Scholar] [CrossRef]
- Rath, S.K.; Singh, M. Comparative clinical and microbiological efficacy of mouthwashes containing 0.2% and 0.12% chlorhexidine. Dent. Res. J. 2013, 10, 364–369. [Google Scholar]
- Nguyen, T.; Brody, H.; Radaic, A.; Kapila, Y. Probiotics for periodontal health-Current molecular findings. Periodontology 2000 2021, 87, 254–267. [Google Scholar] [CrossRef]
- Ng, E.; Tay, J.R.H.; Ong, M.M.A.; Bostanci, N.; Belibasakis, G.N.; Seneviratne, C.J. Probiotic therapy for periodontal and peri-implant health—Silver bullet or sham? Benef. Microbes 2021, 12, 215–230. [Google Scholar] [CrossRef]
- Müller-Heupt, L.K.; Vierengel, N.; Groß, J.; Opatz, T.; Deschner, J.; Loewenich, F.D.V. Antimicrobial Activity of Eucalyptus globulus, Azadirachta indica, Glycyrrhiza glabra, Rheum palmatum Extracts and Rhein against Porphyromonas gingivalis. Antibiotics 2022, 11, 186. [Google Scholar] [CrossRef]
- Liao, J.; Zhao, L.; Yoshioka, M.; Hinode, D.; Grenier, D. Effects of Japanese traditional herbal medicines (Kampo) on growth and virulence properties of Porphyromonas gingivalis and viability of oral epithelial cells. Pharm. Biol. 2013, 51, 1538–1544. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D.; et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011, 10, 497–506. [Google Scholar] [CrossRef]
- Olsen, I.; Lambris, J.D.; Hajishengallis, G. Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J. Oral Microbiol. 2017, 9, 1340085. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Nyvad, B.; Kilian, M. Comparison of the Initial Streptococcal Microflora on Dental Enamel in Caries-Active and in Caries-Inactive Individuals. Caries Res. 1990, 24, 267–272. [Google Scholar] [CrossRef]
- Listgarten, M.A.; Helldén, L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J. Clin. Periodontol. 1978, 5, 115–132. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Charalampakis, G.; Bostanci, N.; Stadlinger, B. Peri-implant infections of oral biofilm etiology. Adv. Exp. Med. Biol. 2015, 830, 69–84. [Google Scholar] [CrossRef]
- Ingendoh-Tsakmakidis, A.; Eberhard, J.; Falk, C.S.; Stiesch, M.; Winkel, A. In Vitro Effects of Streptococcus oralis Biofilm on Peri-Implant Soft Tissue Cells. Cells 2020, 9, 1226. [Google Scholar] [CrossRef]
- Gerits, E.; Verstraeten, N.; Michiels, J. New approaches to combat Porphyromonas gingivalis biofilms. J. Oral. Microbiol. 2017, 9, 1300366. [Google Scholar] [CrossRef]
- Khan, J.; Tarar, S.M.; Gul, I.; Nawaz, U.; Arshad, M. Challenges of antibiotic resistance biofilms and potential combating strategies: A review. 3 Biotech 2021, 11, 169. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Stewart, P.S. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 1996, 40, 2517–2522. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Salyers, A.; Taber, H. Bacterial Resistance to Antimicrobials: Mechanisms, Genetics, Medical Practice and Public Health; Wax, R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; Rybenkov, V.V.; Krishnamoorthy, G.; Leus, I.V. Trans-envelope multidrug efflux pumps of Gram-negative bacteria and their synergism with the outer membrane barrier. Res. Microbiol. 2018, 169, 351–356. [Google Scholar] [CrossRef]
- Inoue, T.; Nakayama, M.; Taguchi, Y.; Kano, K.; Ono, M.; Shimizu, Y.; Kuroda, T.; Ohara, N. Characterization of the tripartite drug efflux pumps of Porphyromonas gingivalis aTCC 33277. New Microbiol. 2015, 38, 101–108. [Google Scholar]
- Zhang, Q.; Liu, J.; Li, R.; Zhao, R.; Zhang, M.; Wei, S.; Ran, D.; Jin, W.; Wu, C. A Network Pharmacology Approach to Investigate the Anticancer Mechanism and Potential Active Ingredients of Rheum palmatum L. against Lung Cancer via Induction of Apoptosis. Front. Pharmacol. 2020, 11, 528308. [Google Scholar] [CrossRef]
- Seukep, A.J.; Kuete, V.; Nahar, L.; Sarker, S.D.; Guo, M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J. Pharm. Anal. 2020, 10, 277–290. [Google Scholar] [CrossRef]
- Lempp, M.; Lubrano, P.; Bange, G.; Link, H. Metabolism of non-growing bacteria. Biol. Chem. 2020, 401, 1479–1485. [Google Scholar] [CrossRef]
- Czech, L.; Mais, C.-N.; Kratzat, H.; Sarmah, P.; Giammarinaro, P.; Freibert, S.-A.; Esser, H.F.; Musial, J.; Berninghausen, O.; Steinchen, W.; et al. Inhibition of SRP-dependent protein secretion by the bacterial alarmone (p)ppGpp. Nat. Commun. 2022, 13, 1069. [Google Scholar] [CrossRef]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial metabolism and antibiotic efficacy. Cell Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Muehler, D.; Mao, X.; Czemmel, S.; Geißert, J.; Engesser, C.; Hiller, K.-A.; Widbiller, M.; Maisch, T.; Buchalla, W.; Al-Ahmad, A.; et al. Transcriptomic stress response in Streptococcus mutans following treatment with a sublethal concentration of chlorhexidine digluconate. Microorganisms 2022, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Kommerein, N.; Weigel, A.J.; Stiesch, M.; Doll, K. Plant-based oral care product exhibits antibacterial effects on different stages of oral multispecies biofilm development in vitro. BMC Oral Health 2021, 21, 170. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; Li, J.; Shin, H.D.; Du, G.; Chen, J.; Liu, L. Microbial response to environmental stresses: From fundamental mechanisms to practical applications. Appl. Microbiol. Biotechnol. 2017, 101, 3991–4008. [Google Scholar] [CrossRef] [PubMed]
- Rittershaus, E.S.; Baek, S.H.; Sassetti, C.M. The normalcy of dormancy: Common themes in microbial quiescence. Cell Host Microbe 2013, 13, 643–651. [Google Scholar] [CrossRef]
- Song, Z.M.; Zhang, J.L.; Zhou, K.; Yue, L.M.; Zhang, Y.; Wang, C.Y.; Wang, K.L.; Xu, Y. Anthraquinones as Potential Antibiofilm Agents against Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2021, 12, 709826. [Google Scholar] [CrossRef]
- Rode, D.K.H.; Singh, P.K.; Drescher, K. Multicellular and unicellular responses of microbial biofilms to stress. Biol. Chem. 2020, 401, 1365–1374. [Google Scholar] [CrossRef]
- Noiri, Y.; Okami, Y.; Narimatsu, M.; Takahashi, Y.; Kawahara, T.; Ebisu, S. Effects of chlorhexidine, minocycline, and metronidazole on Porphyromonas gingivalis strain 381 in biofilms. J. Periodontol. 2003, 74, 1647–1651. [Google Scholar] [CrossRef]
- Ding, W.-Y.; Li, Y.-H.; Lian, H.; Ai, X.-Y.; Zhao, Y.-L.; Yang, Y.-B.; Han, Q.; Liu, X.; Chen, X.-Y.; He, Z. Sub-minimum inhibitory concentrations of rhubarb water extracts inhibit Streptococcus suis biofilm formation. Front. Pharmacol. 2017, 8, 425. [Google Scholar] [CrossRef]
- Seers, C.A.; Mahmud, A.S.M.; Huq, N.L.; Cross, K.J.; Reynolds, E.C. Porphyromonas gingivalis laboratory strains and clinical isolates exhibit different distribution of cell surface and secreted gingipains. J. Oral Microbiol. 2021, 13, 1858001. [Google Scholar] [CrossRef]
- Kulik, E.M.; Thurnheer, T.; Karygianni, L.; Walter, C.; Sculean, A.; Eick, S. Antibiotic susceptibility patterns of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis strains from different decades. Antibiotics 2019, 8, 253. [Google Scholar] [CrossRef] [PubMed]
| R. palmatum Root Extract | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 0.79 | 1.95 | 3.90 | 7.8125 | 15.625 | 31.25 | 62.5 | 125 | 250 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kommerein, N.; Vierengel, N.; Groß, J.; Opatz, T.; Al-Nawas, B.; Müller-Heupt, L.K. Antiplanktonic and Antibiofilm Activity of Rheum palmatum Against Streptococcus oralis and Porphyromonas gingivalis. Microorganisms 2022, 10, 965. https://doi.org/10.3390/microorganisms10050965
Kommerein N, Vierengel N, Groß J, Opatz T, Al-Nawas B, Müller-Heupt LK. Antiplanktonic and Antibiofilm Activity of Rheum palmatum Against Streptococcus oralis and Porphyromonas gingivalis. Microorganisms. 2022; 10(5):965. https://doi.org/10.3390/microorganisms10050965
Chicago/Turabian StyleKommerein, Nadine, Nina Vierengel, Jonathan Groß, Till Opatz, Bilal Al-Nawas, and Lena Katharina Müller-Heupt. 2022. "Antiplanktonic and Antibiofilm Activity of Rheum palmatum Against Streptococcus oralis and Porphyromonas gingivalis" Microorganisms 10, no. 5: 965. https://doi.org/10.3390/microorganisms10050965
APA StyleKommerein, N., Vierengel, N., Groß, J., Opatz, T., Al-Nawas, B., & Müller-Heupt, L. K. (2022). Antiplanktonic and Antibiofilm Activity of Rheum palmatum Against Streptococcus oralis and Porphyromonas gingivalis. Microorganisms, 10(5), 965. https://doi.org/10.3390/microorganisms10050965







