Structural Analyses of CrtJ and Its B12-Binding Co-Regulators SAerR and LAerR from the Purple Photosynthetic Bacterium Rhodobacter capsulatus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Purification
2.2. Absorption Spectroscopy
2.3. DNase Footprint Analysis
2.4. Crystallization
2.5. Structure Calculations
2.6. 3D Modeling Software
3. Results
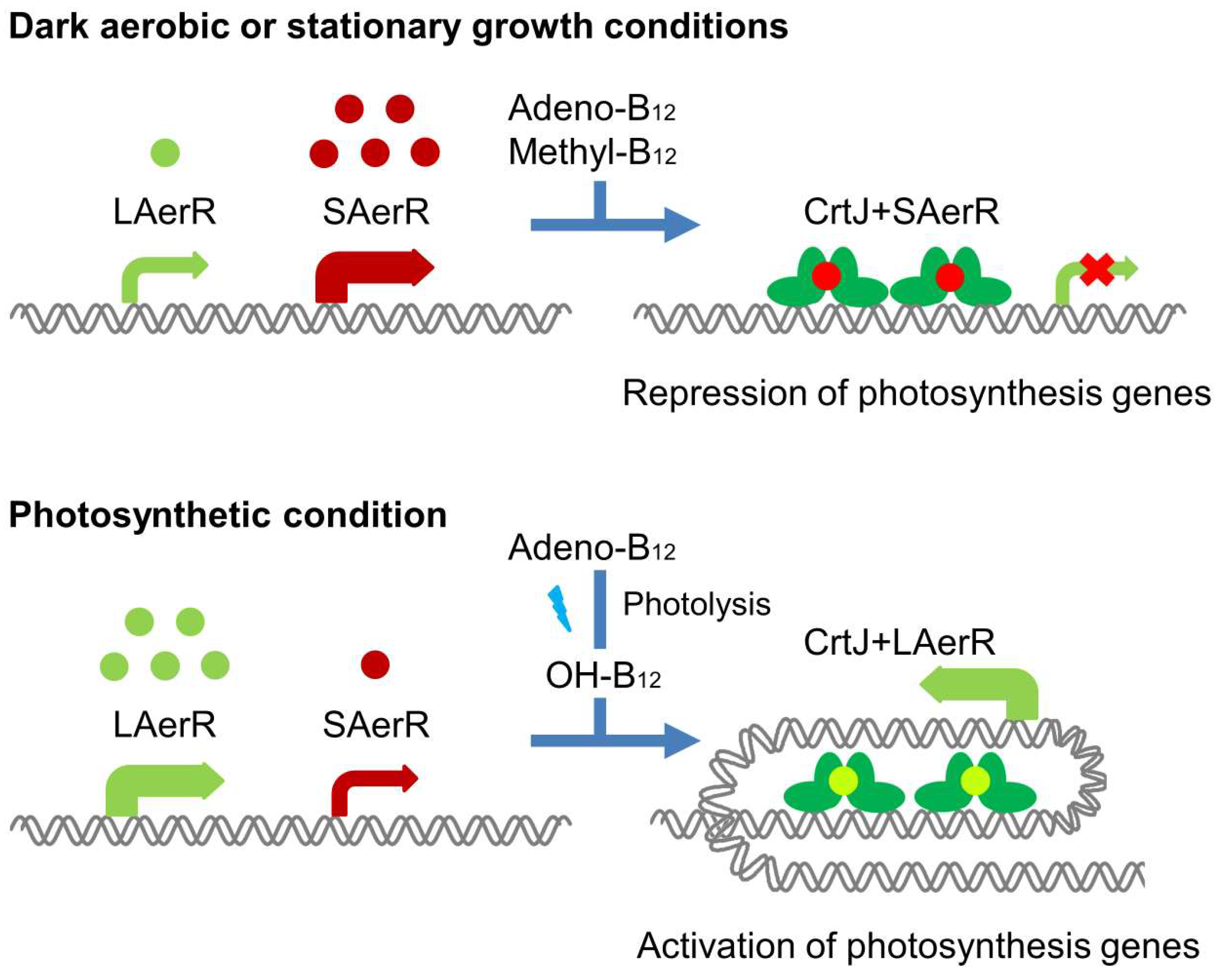
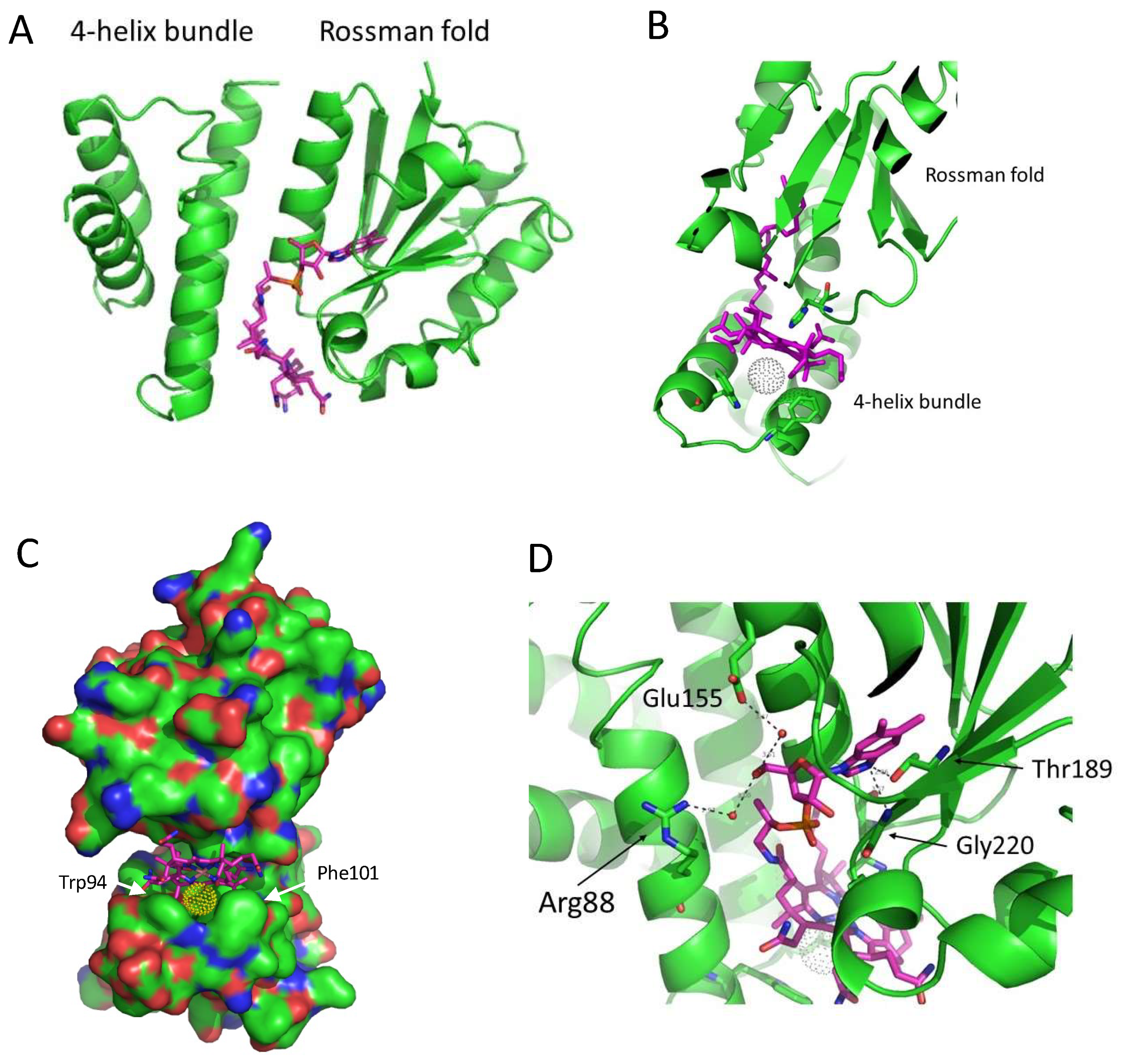
3.1. Structure of SAerR
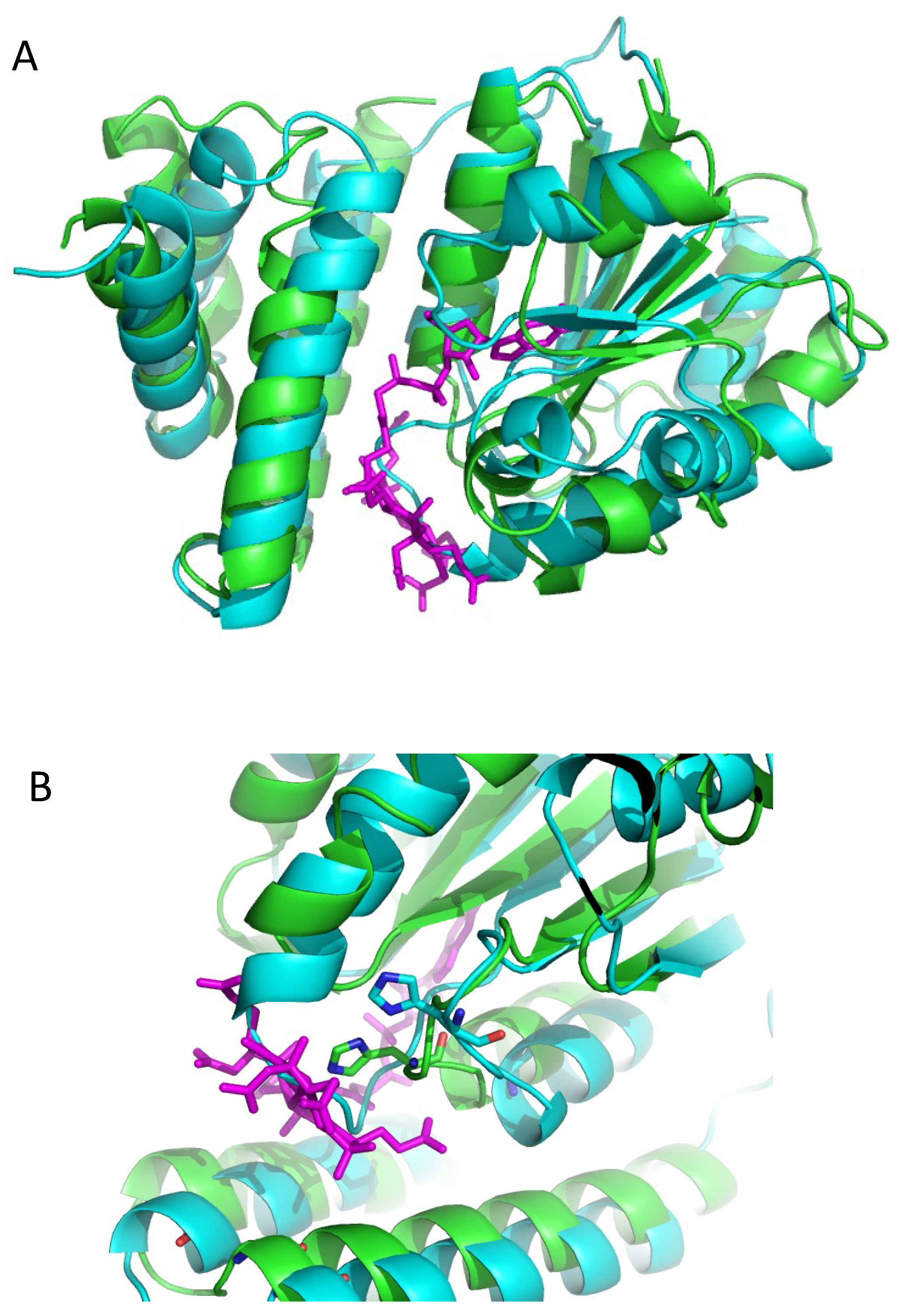
3.2. Alphafold 3D Modeling of LAerR
3.3. Alphafold 3D Modeling of CrtJ
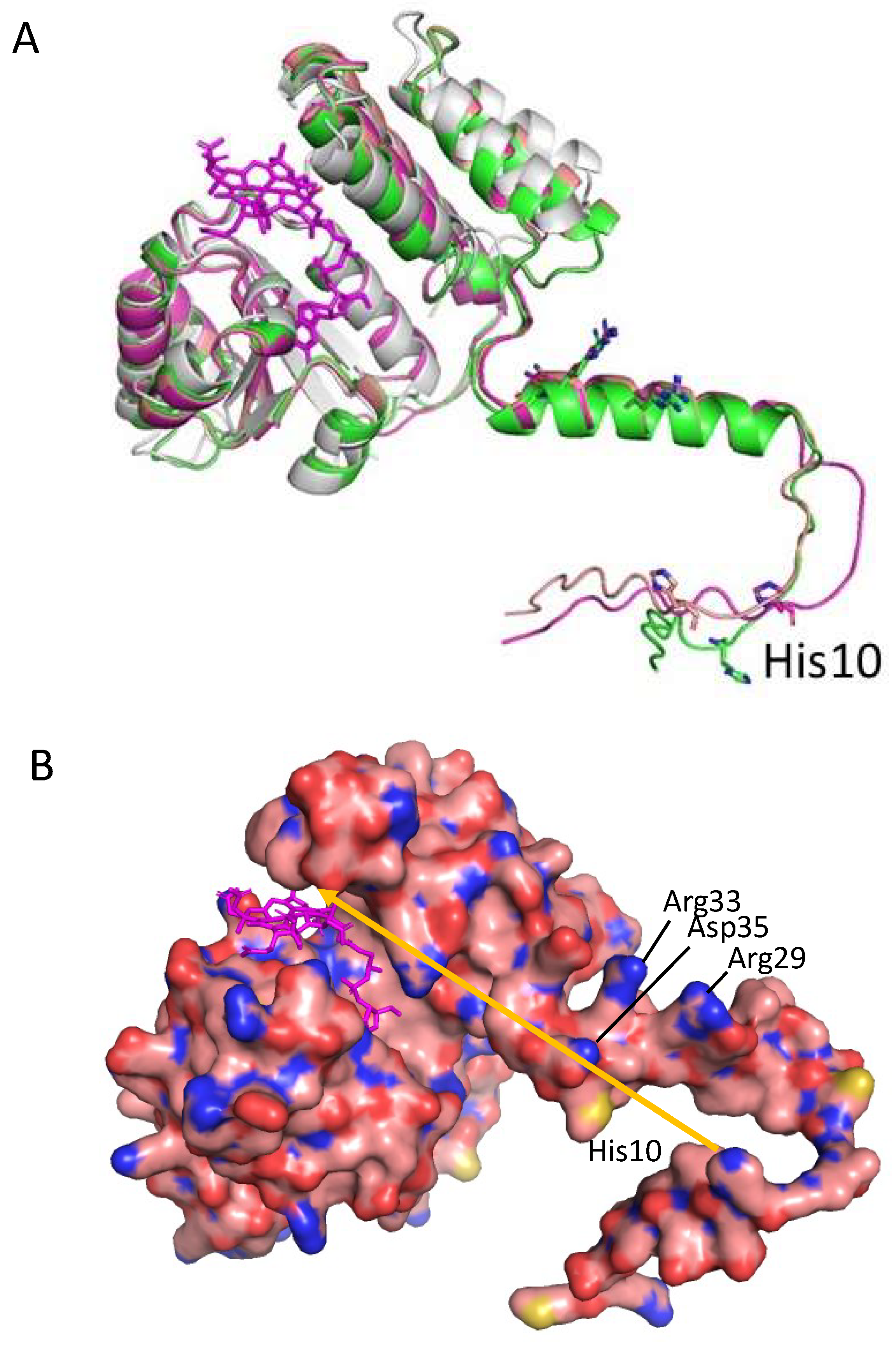
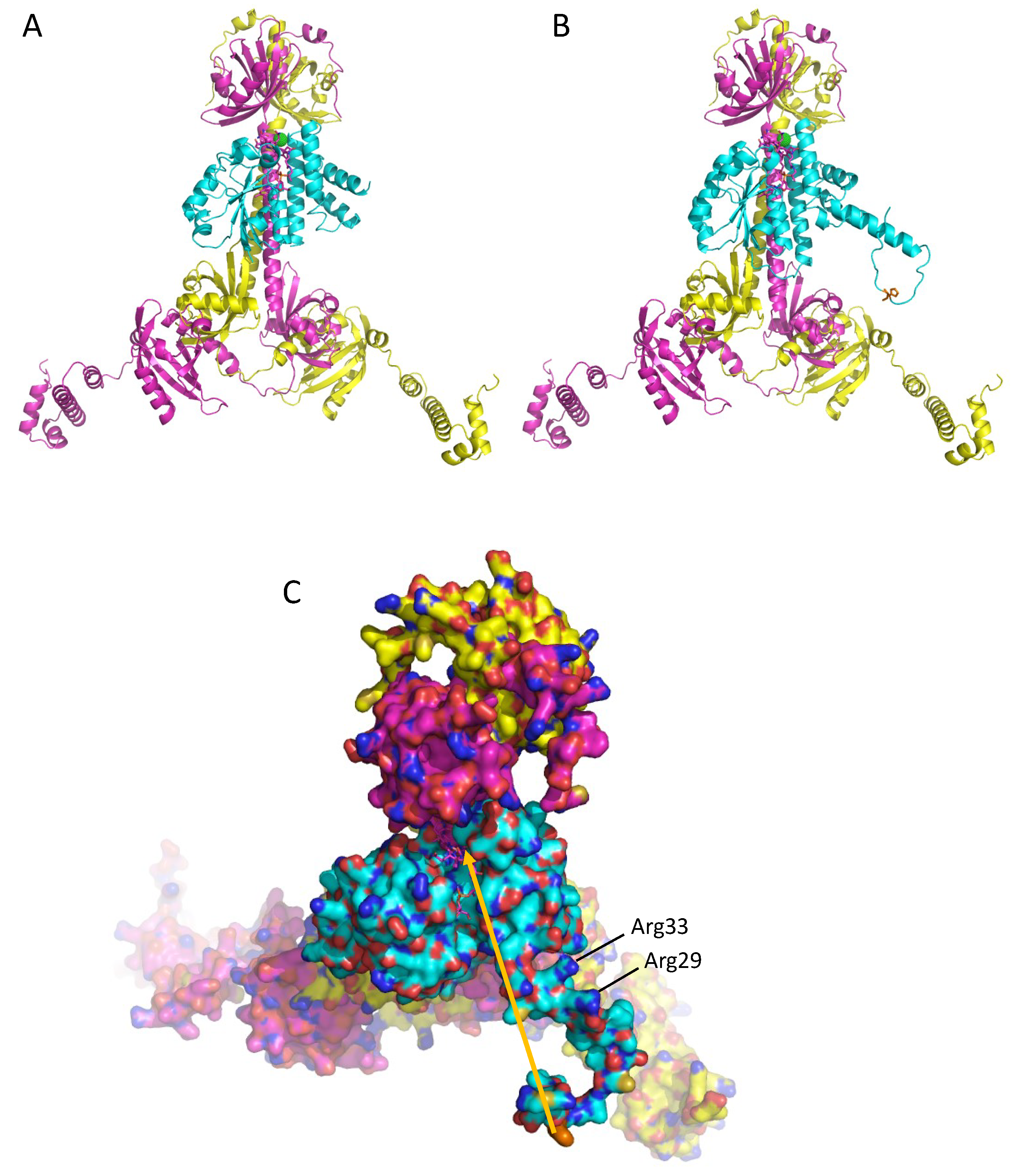
3.4. A Model of a 2CrtJ-AerR Regulatory Complex
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen-Bazire, G.W.; Sistrom, W.R.; Stanier, R.Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J. Cellular. Comp. Physiol. 1957, 49, 25–68. [Google Scholar] [CrossRef] [PubMed]
- Schindel, H.S.; Bauer, C.E. The RegA regulon exhibits variability in response to altered growth conditions and differs markedly between Rhodobacter species. Microb. Genom. 2016, 2, e000081. [Google Scholar] [CrossRef] [PubMed]
- Else, S.; Swem, L.; Swem, D.; Bauer, C.E. RegB/RegA, a highly conserved redox-responding global regulatory system. Microbio. Mol. Biol. Rev. 2004, 68, 263–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumka, J.E.; Bauer, C.E. Analysis of the FnrL regulon in Rhodobacter capsulatus reveals limited regulon overlap with orthologues from Rhodobacter sphaeroides and Escherichia coli. BMC Genom. 2015, 16, 895. [Google Scholar] [CrossRef] [Green Version]
- Bauer, C.E.; Elsen, S.; Swem, L.R.; Swem, D.L.; Masuda, S. Redox and light regulation of gene expression in photosynthetic prokaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 147–153. [Google Scholar] [CrossRef]
- Ponnampalam, S.N.; Buggy, J.J.; Bauer, C.E. Characterization of an aerobic repressor that coordinately regulates bacteriochlorophyll, carotenoid, and light harvesting-II expression in Rhodobacter capsulatus. J. Bacteriol. 1995, 177, 2990–2997. [Google Scholar] [CrossRef] [Green Version]
- Kovács, A.T.; Rákhely, G.; Kovács, K.L. Genes involved in the biosynthesis of photosynthetic pigments in the purple sulfur photosynthetic bacterium Thiocapsa roseopersicina. Appl. Environ. Microbiol. 2003, 69, 3093–3102. [Google Scholar] [CrossRef] [Green Version]
- Penfold, R.J.; Pemberton, J.M. Sequencing, chromosomal inactivation, and functional expression in Escherichia coli of ppsR, a gene which represses carotenoid and bacteriochlorophyll synthesis in Rhodobacter sphaeroides. J. Bacteriol. 1994, 176, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Kovács, A.T.; Rákhely, G.; Kovács, K.L. The PpsR regulator family. Res. Microbiol. 2005, 156, 619–625. [Google Scholar] [CrossRef]
- Fang, M.; Bauer, C.E. The vitamin B12-dependent photoreceptor AerR relieves photosystem gene repression by extending the interaction of CrtJ with photosystem promoters. mBio 2017, 8, e00261-17. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Fang, M.; Dragnea, V.; Bauer, C.E. Differing isoforms of the cobalamin binding photoreceptor AerR oppositely regulate photosystem expression. eLife 2018, 7, e39028. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, S.N.; Elsen, S.; Bauer, C.E. Aerobic repression of the Rhodobacter capsulatus bchC promoter involves cooperative interactions between CrtJ bound to neighboring palindromes. J. Biol. Chem. 1998, 273, 30757–30761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsen, S.; Ponnampalam, S.N.; Bauer, C.E. CrtJ bound to distant binding sites interacts cooperatively to aerobically repress photopigment biosynthesis and light harvesting II gene expression in Rhodobacter capsulatus. J. Biol. Chem. 1998, 273, 30762–30769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickens, D.G.; Bauer, C.E. Analysis of the puc operon promoter from Rhodobacter capsulatus. J. Bacteriol. 1998, 180, 4270–4277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Cheng, Z.; Matsuura, K.; Masuda, S.; Bauer, C.E. Evidence that altered Cis element spacing affects PpsR mediated redox control of photosynthesis gene expression in Rubrivivax gelatinosus. PLoS ONE 2015, 10, e0128446. [Google Scholar] [CrossRef]
- Masuda, S.; Dong, C.; Swem, D.; Setterdahl, A.T.; Knaff, D.B.; Bauer, C.E. Repression of photosynthesis gene expression by formation of a disulfide bond in CrtJ. Proc. Natl. Acad. Sci. USA 2002, 99, 7078–7083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Li, K.; Hammad, L.A.; Karty, J.A.; Bauer, C.E. Vitamin B12 regulates photosystem gene expression via the CrtJ antirepressor AerR in Rhodobacter capsulatus. Mol. Microbiol. 2014, 91, 649–664. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Elsen, S.; Swem, L.R.; Bauer, C.E. AerR, a second aerobic repressor of photosynthesis gene expression in Rhodobacter capsulatus. J. Bacteriol. 2002, 184, 2805–2814. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, A.J.; Bauer, C.E. Members of the PpaA/AerR antirepressor family bind cobalamin. J. Bacteriol. 2015, 197, 2694–2703. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Yamomoto, H.; Bauer, C.E. Cobalamin’s (Vitamin B12) surprising function as a photoreceptor. Trends Biochem. Sci. 2016, 41, 647–650. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Guerrero, J.M.; Polanco, M.C.; Murillo, F.J.; Padmanabhan, S.; Elías-Arnanz, M. Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc. Natl. Acad. Sci. USA 2011, 108, 7565–7570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutta, R.J.; Hardman, S.J.O.; Johannissen, L.O.; Bellina, B.; Messiha, H.L.; Ortiz-Guerrero, J.M.; Elias-Arnanz, M.; Padmanabhan, S.; Barran, P.; Scrutton, N.S.; et al. The photochemical mechanism of a B12-dependent photoreceptor protein. Nat. Commun. 2015, 6, 7907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, M.; Fernandez-Zapata, J.; Polanco, M.C.; Ortiz-Guerrero, J.M.; Chen, P.Y.; Kang, G.; Padmanabhan, S.; Elias-Arnanz, M.; Drennan, C.L. Structural basis for gene regulation by a B12-dependent photoreceptor. Nature 2015, 526, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.R.; Levy, C.; Hay, S.; Scrutton, N.S. Relating localized protein motions to the reaction coordinate in coenzyme B12-dependent enzymes. FEBS J. 2013, 280, 2997–3008. [Google Scholar] [CrossRef]
- Gomelsky, L.; Sram, J.; Moskvin, O.V.; Horne, I.M.; Dodd, H.N.; Pemberton, J.M.; McEwan, A.G.; Kaplan, S.; Gomelsky, M. Identification and in vivo characterization of PpaA, a regulator of photosystem formation in Rhodobacter sphaeroides. Microbiology 2003, 149, 377–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomelsky, M.; Kaplan, S. AppA, a redox regulator of photosystem formation in Rhodobacter sphaeroides 2.4.1, is a flavoprotein. Identification of a novel FAD binding domain. J. Biol. Chem. 1998, 273, 35319–35325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomelsky, M.; Klug, G. BLUF: A novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem. Sci. 2002, 27, 497–500. [Google Scholar] [CrossRef]
- Masuda, S.; Bauer, C.E. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell 2002, 110, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Moskvin, O.V.; Kaplan, S.; Gilles-Gonzalez, M.A.; Gomelsky, M. Novel heme-based oxygen sensor with a revealing evolutionary history. J. Biol. Chem. 2007, 282, 28740–28748. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Dragnea, V.; Feldman, G.; Hammad, L.A.; Karty, J.A.; Dann, C.E., 3rd; Bauer, C.E. Redox and light control the heme-sensing activity of AppA. mBio 2013, 4, e00563-13. [Google Scholar] [CrossRef] [Green Version]
- Gomelsky, M.; Kaplan, S. Molecular genetic analysis suggesting interactions between AppA and PpsR in regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 1997, 179, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, A.; Heintz, U.; Lindner, R.; Reinstein, J.; Shoeman, R.L.; Schlichting, I. A ternary AppA-PpsR-DNA complex mediates light regulation of photosynthesis-related gene expression. Nat. Struct. Mol. Biol. 2013, 20, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, S.; Dragnea, V.; Masuda, S.; Ybe, J.; Moffat, K.; Bauer, C. Structure of a novel photoreceptor, the BLUF domain of AppA from Rhodobacter sphaeroides. Biochemistry 2005, 44, 7998–8005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Zidek, A.; Nelson, A.W.R.; Bridgland, A.; et al. Improved protein structure prediction using potentials from deep learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wu, J.; Setterdahl, A.; Reddie, K.; Carroll, K.; Hammad, L.A.; Karty, J.A.; Bauer, C.E. Activity of the tetrapyrrole regulator CrtJ is controlled by oxidation of a redox active cysteine located in the DNA binding domain. Mol. Microbiol. 2012, 85, 734–746. [Google Scholar] [CrossRef] [Green Version]
- Willett, J.; Smart, J.L.; Bauer, C.E. RegA control of bacteriochlorophyll and carotenoid synthesis in Rhodobacter capsulatus. J. Bacteriol. 2007, 189, 7765–7773. [Google Scholar] [CrossRef] [Green Version]
- Kabsch, W. XDS. Acta Crystallographica. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Drennan, C.L.; Huang, S.; Drummond, J.T.; Matthews, R.G.; Lidwig, M.L. How a protein binds B12: A 3.0 A X-ray structure of B12-binding domains of methionine synthase. Science 1994, 266, 1669–1674. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragnea, V.; Gonzalez-Gutierrez, G.; Bauer, C.E. Structural Analyses of CrtJ and Its B12-Binding Co-Regulators SAerR and LAerR from the Purple Photosynthetic Bacterium Rhodobacter capsulatus. Microorganisms 2022, 10, 912. https://doi.org/10.3390/microorganisms10050912
Dragnea V, Gonzalez-Gutierrez G, Bauer CE. Structural Analyses of CrtJ and Its B12-Binding Co-Regulators SAerR and LAerR from the Purple Photosynthetic Bacterium Rhodobacter capsulatus. Microorganisms. 2022; 10(5):912. https://doi.org/10.3390/microorganisms10050912
Chicago/Turabian StyleDragnea, Vladimira, Giovanni Gonzalez-Gutierrez, and Carl E. Bauer. 2022. "Structural Analyses of CrtJ and Its B12-Binding Co-Regulators SAerR and LAerR from the Purple Photosynthetic Bacterium Rhodobacter capsulatus" Microorganisms 10, no. 5: 912. https://doi.org/10.3390/microorganisms10050912
APA StyleDragnea, V., Gonzalez-Gutierrez, G., & Bauer, C. E. (2022). Structural Analyses of CrtJ and Its B12-Binding Co-Regulators SAerR and LAerR from the Purple Photosynthetic Bacterium Rhodobacter capsulatus. Microorganisms, 10(5), 912. https://doi.org/10.3390/microorganisms10050912




