Root-Associated Bacteria Are Biocontrol Agents for Multiple Plant Pests
Abstract
:1. Introduction
| Bacterial Strains | Pathogens | Pests | Active Metabolites | Reference | |
|---|---|---|---|---|---|
| Antimicrobial | Insecticidal | ||||
| Gram-positive bacteria | |||||
| Bacillus amyloliquefaciens AG1 | Various fungal pathogens | Tuta absoluta | Crude protein extract | Biosurfactant | [11,12] |
| Bacillus atrophaeus L193 | Botrytis cinerea, Monilinia laxa | Rhopalosiphum padi | 2,3-Butanediol | Biosurfactant | [13,14] |
| Bacillus subtilis PTB185 | Various fungal pathogens | Aulacorthum solani Aphis gossypii | Lipopeptides | Chitinase | [15,16] |
| Bacillus subtilis SPB1 | Fusarium solani Rhizoctonia bataticola Rhizoctonia solani | Ectomyelois ceratoniae Spodoptera littoralis | Biosurfactant | Biosurfactant | [17,18,19,20] |
| Bacillus subtilis V26 | Botrytis cinerea | Tuta absoluta | Biosurfactant | Biosurfactant | [21] |
| Bacillus thuringiensis strains | Sclerotinia sclerotiorum | Plutella xylostella | Salicylic acid, ethylene, and jasmonic acid | BT toxin | [22] |
| Bacillus thuringiensis CMB26 | Colletotrichum gloeosporioides | Pieris rapae crucivora | Lipopeptide | Lipopeptide | [23] |
| Brevibacillus laterosporus Lak1210 | Fusarium equiseti | Plutella xylostella | Chitinase | Chitinase | [24] |
| Paenibacillus elgii HOA73 | Botrytis cinerea Cladosporiumsphaerospermum | Plutella xylostella Meloidogyne incognita | Chitinase | Crude enzyme, gelatinase, chitinase | [25,26,27] |
| Paenibacillus elgii HOA73 | Botrytis cinerea, Rhizoctonia solani, Fusarium oxysporum f. sp. lycopersici | Methyl-2,3 dihydroxybenzoate (phenolic compound) | [28] | ||
| Paenibacillus elgii HOA73 | Botrytis cinerea, Rhizoctonia solani | Protocatechuic acid | [29] | ||
| Paenibacillus polymyxa BMP-11 | Various fungal pathogens | Tribolium castaneum | 1-Octen-3-ol benzothiazole | 1-Octen-3-ol benzothiazole | [30] |
| Streptomyces hydrogenans DH16 | Various fungal pathogens | Spodoptera litura Meloidogyne incognita | NA | NA | [31,32] |
| Streptomyces tanaschiensis | Saccharomyces sp. Penicillium sp. | Musca domestica Locusta migratoria | Flavensomycin | Flavensomycin | [33] |
| Gram-negative bacteria | |||||
| Photorhabdus temperata M1021 | Phytophthora capsica, Rhizoctonia solani, Corynespora cassiicola | Galleria mellonella | Benzaldehyde | Benzaldehyde | [34] |
| Pseudomonas fluorescens CHA0 | Pythium ultimum | Spodoptera littoralis Heliothis virescens Plutella xylostella Meloidogyne javanica M. incognita | Pyoluteorin, 2,4-DAPG * | Pyoluteorin, 2,4-DAPG extracellular protease, and insect toxin (Fit **) | [35,36,37,38] |
| Pseudomonas chlororaphis PCL1391 | Fusarium oxysporum f. sp. radicis-lycopersici | Spodoptera littoralis Heliothis virescens Plutella xylostella Galleria mellonella | Phenazine-1-carboxamide | Potent insect toxin, HCN, lipopeptide, Fit toxin | [38,39,40] |
| Pseudomonas chlororaphis O6 | Rhizoctonia solani Fusarium graminearum Phytophthora infestans | Meloidogyne hapla Myzus persicae | Pyrrolnitrin, phenazines | Hydrogen cyanide (HCN), Cyclic lipopeptides | [41,42,43,44] |
| Pseudomonas chlororaphis PA23 | Sclerotinia sclerotiorum | Caenorhabditis elegans | Pyrrolnitrin | Pyrrolnitrin, HCN | [45,46] |
| Serratia entomophila AB2 | Aspergillus flavus Candida albicans Fusarium oxysporum | Heliothis armigera | NA | NA | [47] |
2. The Array of Isolates and Their Products for Biocontrol of Multiple Targets
2.1. The Rhizosphere Habitat: A Significant Trait of Microbes with Multiple Biocontrol Activities
2.2. Active Products from Bacillus spp.
2.3. Paenibacillus spp. Metabolites and Enzymes
2.4. Pseudomonad Products
2.5. Streptomyces, Brevibacillus, Serratia, and Photorhabdus
3. Induction of Plant Systemic Resistance Mechanism by Biocontrol Agents
4. Secondary Metabolites and Cell Preparations for Commercial Biocontrol Formulations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Labourdette, G.; Lachaise, H.; Rieck, H.; Steiger, D. Fluopyram: Efficacy and beyond on problematic diseases. In Proceedings of the Modern Fungicides and Antifungal Compounds VI, 16th International Reinhardsbrunn Symposium, Friedrichroda, Germany, 25–29 April 2010; pp. 75–80. [Google Scholar]
- Faske, T.; Hurd, K. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to fluopyram. J. Nematol. 2015, 47, 316. [Google Scholar] [PubMed]
- Rouquie, D.; Tinwell, H.; Blanck, O.; Schorsch, F.; Geter, D.; Wason, S.; Bars, R. Thyroid tumor formation in the male mouse induced by fluopyram is mediated by activation of hepatic CAR/PXR nuclear receptors. Regul. Toxicol. Pharmacol. 2014, 70, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.K.; Khare, E.; Maheshwari, D.K. Plant growth promoting rhizobacteria: Constraints in bioformulation, commercialization, and future strategies. In Plant Growth and Health Promoting Bacteria; Microbiology Monographs 18; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 97–116. [Google Scholar]
- Bashan, Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 1998, 16, 729–770. [Google Scholar] [CrossRef]
- Cook, R.J. Take-all of wheat. Physiol. Mol. Plant Pathol. 2003, 62, 73–86. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Kang, B.R.; Han, J.H.; Kim, J.J.; Kim, Y.C. Dual biocontrol potential of the entomopathogenic fungus, Isaria javanica, for both aphids and plant fungal pathogens. Mycobiology 2018, 46, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Alfonzo, A.; Lo Piccolo, S.; Conigliaro, G.; Ventorino, V.; Burruano, S.; Moschetti, G. Antifungal peptides produced by Bacillus amyloliquefaciens AG1 active against grapevine fungal pathogens. Ann. Microbiol. 2012, 62, 1593–1599. [Google Scholar] [CrossRef]
- Khedher, S.B.; Boukedi, H.; Kilani-Feki, O.; Chaib, I.; Laarif, A.; Abdelkefi-Mesrati, L.; Tounsi, S. Bacillus amyloliquefaciens AG1 biosurfactant: Putative receptor diversity and histopathological effects on Tuta absoluta midgut. J. Invertebr. Pathol. 2015, 132, 42–47. [Google Scholar] [CrossRef]
- Rodríguez, M.; Marín, A.; Torres, M.; Béjar, V.; Campos, M.; Sampedro, I. Aphicidal activity of surfactants produced by Bacillus atrophaeus L193. Front. Microbiol. 2018, 9, 3114. [Google Scholar] [CrossRef] [Green Version]
- Navarro, L.T.; Rodríguez, M.; Martínez-Checa, F.; Montaño, A.; Cortés-Delgado, A.; Smolinska, A.; Llamas, I.; Sampedro, I. Identification of volatile organic compounds in extremophilic bacteria and their effective use in biocontrol of postharvest fungal phytopathogens. Front. Microbiol. 2021, 12, 3349. [Google Scholar]
- Cossus, L.; Roux-Dalvai, F.; Kelly, I.; Nguyen, T.T.A.; Antoun, H.; Droit, A.; Tweddell, R.J. Interactions with plant pathogens influence lipopeptides production and antimicrobial activity of Bacillus subtilis strain PTB185. Biol. Control 2021, 154, 104497. [Google Scholar] [CrossRef]
- Kahia, M.; Nguyen, T.T.A.; McCune, F.; Naasz, R.; Antoun, H.; Fournier, V. Insecticidal effect of Bacillus pumilus PTB180 and Bacillus subtilis PTB185 used alone and in combination against the foxglove aphid and the melon aphid (Hemiptera: Aphididae). Can. Entomol. 2021, 153, 726–740. [Google Scholar] [CrossRef]
- Ghribi, D.; Abdelkefi-Mesrati, L.; Boukedi, H.; Elleuch, M.; Ellouze-Chaabouni, S.; Tounsi, S. The impact of the Bacillus subtilis SPB1 biosurfactant on the midgut histology of Spodoptera littoralis (Lepidoptera: Noctuidae) and determination of its putative receptor. J. Invertebr. Pathol. 2012, 109, 183–186. [Google Scholar] [CrossRef]
- Mnif, I.; Elleuch, M.; Chaabouni, S.E.; Ghribi, D. Bacillus subtilis SPB1 biosurfactant: Production optimization and insecticidal activity against the carob moth Ectomyelois ceratoniae. Crop Prot. 2013, 50, 66–72. [Google Scholar] [CrossRef]
- Mnif, I.; Hammami, I.; Triki, M.A.; Azabou, M.C.; Ellouze-Chaabouni, S.; Ghribi, D. Antifungal efficiency of a lipopeptide biosurfactant derived from Bacillus subtilis SPB1 versus the phytopathogenic fungus, Fusarium solani. Environ. Sci. Pollut. Res. 2015, 22, 18137–18147. [Google Scholar] [CrossRef]
- Mnif, I.; Grau-Campistany, A.; Coronel-León, J.; Hammami, I.; Triki, M.A.; Manresa, A.; Ghribi, D. Purification and identification of Bacillus subtilis SPB1 lipopeptide biosurfactant exhibiting antifungal activity against Rhizoctonia bataticola and Rhizoctonia solani. Environ. Sci. Pollut. Res. 2016, 23, 6690–6699. [Google Scholar] [CrossRef]
- Ben Khedher, S.; Boukedi, H.; Laarif, A.; Tounsi, S. Biosurfactant produced by Bacillus subtilis V26: A potential biological control approach for sustainable agriculture development. Org. Agric. 2020, 10, 117–124. [Google Scholar] [CrossRef]
- Wang, M.; Geng, L.; Sun, X.; Shu, C.; Song, F.; Zhang, J. Screening of Bacillus thuringiensis strains to identify new potential biocontrol agents against Sclerotinia sclerotiorum and Plutella xylostella in Brassica campestris L. Biol. Control 2020, 145, 104262. [Google Scholar] [CrossRef]
- Kim, P.; Bai, H.; Bai, D.; Chae, H.; Chung, S.; Kim, Y.; Park, R.; Chi, Y.T. Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J. Appl. Microbiol. 2004, 97, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, L.; Eijsink, V.G.; Meadow, R.; Gåseidnes, S. A novel strain of Brevibacillus laterosporus produces chitinases that contribute to its biocontrol potential. Appl. Microbiol. Biotechnol. 2013, 97, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Kang, B.R.; Kim, Y.H.; Park, S.K. An effective and practical strategy for biocontrol of plant diseases using on-site mass cultivation of chitin-degrading bacteria. Res. Plant Dis. 2017, 23, 19–34. [Google Scholar] [CrossRef]
- Neung, S.; Nguyen, X.H.; Naing, K.W.; Lee, Y.S.; Kim, K.Y. Insecticidal potential of Paenibacillus elgii HOA73 and its combination with organic sulfur pesticide on diamondback moth, Plutella xylostella. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 181–186. [Google Scholar] [CrossRef]
- Nguyen, X.H.; Naing, K.W.; Lee, Y.S.; Jung, W.J.; Anees, M.; Kim, K.Y. Antagonistic potential of Paenibacillus elgii HOA73 against the root-knot nematode, Meloidogyne incognita. Nematology 2013, 15, 991–1000. [Google Scholar] [CrossRef]
- Lee, Y.S.; Nguyen, X.H.; Cho, J.-Y.; Moon, J.-H.; Kim, K.Y. Isolation and antifungal activity of methyl 2, 3-dihydroxybenzoate from Paenibacillus elgii HOA73. Microb. Pathog. 2017, 106, 139–145. [Google Scholar] [CrossRef]
- Nguyen, X.H.; Naing, K.W.; Lee, Y.S.; Moon, J.H.; Lee, J.H.; Kim, K.Y. Isolation and characteristics of protocatechuic acid from Paenibacillus elgii HOA73 against Botrytis cinerea on strawberry fruits. J. Basic Microbiol. 2015, 55, 625–634. [Google Scholar] [CrossRef]
- Zhao, L.-j.; Yang, X.-n.; Li, X.-y.; Wei, M.; Feng, L. Antifungal, insecticidal and herbicidal properties of volatile components from Paenibacillus polymyxa strain BMP-11. Agric. Sci. China 2011, 10, 728–736. [Google Scholar] [CrossRef]
- Kaur, T.; Manhas, R.K. Antifungal, insecticidal, and plant growth promoting potential of Streptomyces hydrogenans DH16. J. Basic Microbiol. 2014, 54, 1175–1185. [Google Scholar] [CrossRef]
- Kaur, T.; Jasrotia, S.; Ohri, P.; Manhas, R.K. Evaluation of in vitro and in vivo nematicidal potential of a multifunctional streptomycete, Streptomyces hydrogenans strain DH16 against Meloidogyne incognita. Microbiol. Res. 2016, 192, 247–252. [Google Scholar] [CrossRef]
- Craveri, R.; Giolitti, G. An antibiotic with fungicidal and insecticidal activity produced by Streptomyces. Nature 1957, 179, 1307. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Lee, I.-J.; Shin, J.-H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Schnider, U.; Keel, C.; Blumer, C.; Troxler, J.; Défago, G.; Haas, D. Amplification of the housekeeping sigma factor in Pseudomonas fluorescens CHA0 enhances antibiotic production and improves biocontrol abilities. J. Bacteriol. 1995, 177, 5387–5392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, I.A.; Shaukat, S.S. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: Importance of bacterial secondary metabolite, 2,4-diacetylpholoroglucinol. Soil Biol. Biochem. 2003, 35, 1615–1623. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Haas, D.; Heeb, S. Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root-knot nematode Meloidogyne incognita. Appl. Environ. Microbiol. 2005, 71, 5646–5649. [Google Scholar] [CrossRef] [Green Version]
- Ruffner, B.; Péchy-Tarr, M.; Ryffel, F.; Hoegger, P.; Obrist, C.; Rindlisbacher, A.; Keel, C.; Maurhofer, M. Oral insecticidal activity of plant-associated pseudomonads. Environ. Microbiol. 2013, 15, 751–763. [Google Scholar] [CrossRef]
- Chin-A-Woeng, T.F.; Bloemberg, G.V.; Mulders, I.H.; Dekkers, L.C.; Lugtenberg, B.J. Root colonization by phenazine-1-carboxamide-producing bacterium Pseudomonas chlororaphis PCL1391 is essential for biocontrol of tomato foot and root rot. Mol. Plant Microbe Interact. 2000, 13, 1340–1345. [Google Scholar] [CrossRef] [Green Version]
- Flury, P.; Vesga, P.; Péchy-Tarr, M.; Aellen, N.; Dennert, F.; Hofer, N.; Kupferschmied, K.P.; Kupferschmied, P.; Metla, Z.; Ma, Z. Antimicrobial and insecticidal: Cyclic lipopeptides and hydrogen cyanide produced by plant-beneficial Pseudomonas strains CHA0, CMR12a, and PCL1391 contribute to insect killing. Front. Microbiol. 2017, 8, 100. [Google Scholar] [CrossRef]
- Lee, J.H.; Ma, K.C.; Ko, S.J.; Kang, B.R.; Kim, I.S.; Kim, Y.C. Nematicidal activity of a nonpathogenic biocontrol bacterium, Pseudomonas chlororaphis O6. Curr. Microbiol. 2011, 62, 746–751. [Google Scholar] [CrossRef]
- Park, J.; Oh, S.; Anderson, A.; Neiswender, J.; Kim, J.C.; Kim, Y. Production of the antifungal compounds phenazine and pyrrolnitrin from Pseudomonas chlororaphis O6 is differentially regulated by glucose. Lett. Appl. Microbiol. 2011, 52, 532–537. [Google Scholar] [CrossRef]
- Kang, B.R.; Anderson, A.J.; Kim, Y.C. Hydrogen cyanide produced by Pseudomonas chlororaphis O6 exhibits nematicidal activity against Meloidogyne hapla. Plant Pathol. J. 2018, 34, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.R.; Anderson, A.J.; Kim, Y.C. Hydrogen cyanide produced by Pseudomonas chlororaphis O6 is a key aphicidal metabolite. Can. J. Microbiol. 2019, 65, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Selin, C.; Habibian, R.; Poritsanos, N.; Athukorala, S.N.; Fernando, D.; De Kievit, T.R. Phenazines are not essential for Pseudomonas chlororaphis PA23 biocontrol of Sclerotinia sclerotiorum, but do play a role in biofilm formation. FEMS Microbiol. Ecol. 2009, 71, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandi, M.; Selin, C.; Brassinga, A.K.C.; Belmonte, M.F.; Fernando, W.D.; Loewen, P.C.; De Kievit, T.R. Pyrrolnitrin and hydrogen cyanide production by Pseudomonas chlororaphis strain PA23 exhibits nematicidal and repellent activity against Caenorhabditis elegans. PLoS ONE 2015, 10, e0123184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, P.; Sen, S.K. Systemic infestation of Serratia entomophila AB2 through plant tissue inferred protection against insect pest and fungal pathogens. Afr. J. Microbiol. Res. 2013, 7, 2651–2655. [Google Scholar]
- Berlec, A. Novel techniques and findings in the study of plant microbiota: Search for plant probiotics. Plant Sci. 2012, 193, 96–102. [Google Scholar] [CrossRef]
- Spence, C.; Alff, E.; Shantharaj, D.; Bais, H. Probiotics for plants: Importance of rhizobacteria on aboveground fitness in plants. In Bacteria in Agrobiology: Plant Probiotics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–14. [Google Scholar]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Regaiolo, A.; Dominelli, N.; Andresen, K.; Heermann, R. The biocontrol agent and insect pathogen Photorhabdus luminescens interacts with plant roots. Appl. Environ. Microbiol. 2020, 86, e00891-20. [Google Scholar] [CrossRef]
- Melo, A.L.d.A.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications. A review. Crit. Rev. Biotechnol. 2016, 36, 317–326. [Google Scholar] [CrossRef]
- Keel, C. A look into the toolbox of multi-talents: Insect pathogenicity determinants of plant-beneficial pseudomonads. Environ. Microbiol. 2016, 18, 3207–3209. [Google Scholar] [CrossRef] [Green Version]
- Ruffner, B.; Péchy-Tarr, M.; Höfte, M.; Bloemberg, G.; Grunder, J.; Keel, C.; Maurhofer, M. Evolutionary patchwork of an insecticidal toxin shared between plant-associated pseudomonads and the insect pathogens Photorhabdus and Xenorhabdus. BMC Genom. 2015, 16, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mnif, I.; Ghribi, D. Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Peptide Sci. 2015, 104, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Chin-A-Woeng, T.F.; Bloemberg, G.V.; Lugtenberg, B.J. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol. 2003, 157, 503–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuel, J.; Selin, C.; Fernando, W.D.; de Kievit, T. Stringent response mutants of Pseudomonas chlororaphis PA23 exhibit enhanced antifungal activity against Sclerotinia sclerotiorum in vitro. Microbiology 2012, 158, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Arya, S.K. Antifungal and insecticidal potential of chitinases: A credible choice for the eco-friendly farming. Biocatal. Agric. Biotechnol. 2019, 20, 101289. [Google Scholar] [CrossRef]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Kim, Y.C.; Anderson, A.J. Rhizosphere pseudomonads as probiotics improving plant health. Mol. Plant Pathol. 2018, 19, 2349–2359. [Google Scholar] [CrossRef] [Green Version]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, D.S.; Teixeira, P.J. Root-exuded coumarin shapes the root microbiome. Proc. Natl. Acad. Sci. USA 2018, 115, 5629–5631. [Google Scholar] [CrossRef] [Green Version]
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.; Feussner, I.; Pieterse, C.M. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [Green Version]
- Maddula, V.; Pierson, E.; Pierson III, L. Altering the ratio of phenazines in Pseudomonas chlororaphis (aureofaciens) strain 30–84: Effects on biofilm formation and pathogen inhibition. J. Bacteriol. 2008, 190, 2759–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón, C.E.; Tienda, S.; Heredia-Ponce, Z.; Arrebola, E.; Cárcamo-Oyarce, G.; Eberl, L.; Cazorla, F.M. The compound 2-hexyl, 5-propyl resorcinol has a key role in biofilm formation by the biocontrol rhizobacterium Pseudomonas chlororaphis PCL1606. Front. Microbiol. 2019, 10, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daud, N.S.; Rosli, M.A.; Azam, Z.M.; Othman, N.Z.; Sarmidi, M.R. Paenibacillus polymyxa bioactive compounds for agricultural and biotechnological applications. Biocatal. Agric. Biotechnol. 2019, 18, 101092. [Google Scholar] [CrossRef]
- Chan, S.Y.; Liu, S.Y.; Seng, Z.; Chua, S.L. Biofilm matrix disrupts nematode motility and predatory behavior. ISME J. 2021, 15, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Nandi, M.; Berry, C.; Brassinga, A.K.C.; Belmonte, M.F.; Fernando, W.D.; Loewen, P.C.; de Kievit, T.R. Pseudomonas brassicacearum strain DF41 kills Caenorhabditis elegans through biofilm-dependent and biofilm-independent mechanisms. Appl. Environ. Microbiol. 2016, 82, 6889–6898. [Google Scholar] [CrossRef] [Green Version]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Boiteau, R.M.; Markillie, L.M.; Hoyt, D.W.; Hu, D.; Chu, R.K.; Mitchell, H.D.; Pasa-Tolic, L.; Jansson, J.K.; Jansson, C. Metabolic interactions between Brachypodium and Pseudomonas fluorescens under controlled iron-limited conditions. mSystems 2021, 6, e00580-20. [Google Scholar] [CrossRef]
- Yahya, M.; ul Islam, E.; Rasul, M.; Farooq, I.; Mahreen, N.; Tawab, A.; Irfan, M.; Rajput, L.; Amin, I.; Yasmin, S. Differential root exudation and architecture for improved growth of wheat mediated by phosphate solubilizing bacteria. Front. Microbiol. 2021, 12, 744094. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed 2020, 7, 639–659. [Google Scholar] [CrossRef]
- Bukkens, S.G.F. The nutritional value of edible insects. Ecol. Food Nutr. 1997, 36, 287–319. [Google Scholar] [CrossRef]
- Cox, G.N.; Kusch, M.; Edgar, R.S. Cuticle of Caenorhabditis elegans: Its isolation and partial characterization. J. Cell Biol. 1981, 90, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Pronk, L.J.; Bakker, P.A.; Keel, C.; Maurhofer, M.; Flury, P. The secret life of plant-beneficial rhizosphere bacteria: Insects as alternative hosts. Environ. Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théatre, A.; Cano-Prieto, C.; Bartolini, M.; Laurin, Y.; Deleu, M.; Niehren, J.; Fida, T.; Gerbinet, S.; Alanjary, M.; Medema, M.H. The surfactin-like lipopeptides from Bacillus spp.: Natural biodiversity and synthetic biology for a broader application range. Front. Bioeng. Biotechnol. 2021, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Arroyave-Toro, J.J.; Mosquera, S.; Villegas-Escobar, V. Biocontrol activity of Bacillus subtilis EA-CB0015 cells and lipopeptides against postharvest fungal pathogens. Biol. Control 2017, 114, 195–200. [Google Scholar] [CrossRef]
- Yan, F.; Li, C.; Ye, X.; Lian, Y.; Wu, Y.; Wang, X. Antifungal activity of lipopeptides from Bacillus amyloliquefaciens MG3 against Colletotrichum gloeosporioides in loquat fruits. Biol. Control 2020, 146, 104281. [Google Scholar] [CrossRef]
- Kalai-Grami, L.; Karkouch, I.; Naili, O.; Slimene, I.B.; Elkahoui, S.; Zekri, R.B.; Touati, I.; Mnari-Hattab, M.; Hajlaoui, M.R.; Limam, F. Production and identification of iturin A lipopeptide from Bacillus methyltrophicus TEB1 for control of Phoma tracheiphila. J. Basic Microbiol. 2016, 56, 864–871. [Google Scholar] [CrossRef]
- Romano, A.; Vitullo, D.; Senatore, M.; Lima, G.; Lanzotti, V. Antifungal cyclic lipopeptides from Bacillus amyloliquefaciens strain BO5A. J. Nat. Prod. 2013, 76, 2019–2025. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lim, D.J.; Noh, M.Y.; Kim, J.C.; Kim, Y.C.; Kim, I.S. Characterization of biosurfactants as insecticidal metabolites produced by Bacillus subtilis Y9. Entomol. Res. 2017, 47, 55–59. [Google Scholar] [CrossRef]
- Simionato, A.S.; Navarro, M.O.; de Jesus, M.L.; Barazetti, A.R.; da Silva, C.S.; Simões, G.C.; Balbi-Peña, M.I.; de Mello, J.C.; Panagio, L.A.; de Almeida, R.S. The effect of phenazine-1-carboxylic acid on mycelial growth of Botrytis cinerea produced by Pseudomonas aeruginosa LV strain. Front. Microbiol. 2017, 8, 1102. [Google Scholar] [CrossRef]
- Lee, J.Y.; Moon, S.S.; Hwang, B.K. Isolation and in vitro and in vivo activity against Phytophthora capsici and Colletotrichum orbiculare of phenazine-1-carboxylic acid from Pseudomonas aeruginosa strain GC-B26. Pest Manag. Sci. 2003, 59, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.R.; Sarode, P.D.; Chaudhari, B.L.; Chincholkar, S.B. Detection, isolation and identification of phenazine-1-carboxylic acid produced by biocontrol strains of Pseudomonas aeruginosa. J. Sci. Ind. Res. 2007, 66, 627–631. [Google Scholar]
- Xu, Z.; Wang, M.; Du, J.; Huang, T.; Liu, J.; Dong, T.; Chen, Y. Isolation of Burkholderia sp. HQB-1, a promising biocontrol bacteria to protect banana against Fusarium wilt through phenazine-1-carboxylic acid secretion. Front. Microbiol. 2020, 11, 3156. [Google Scholar] [CrossRef]
- Gurusiddaiah, S.; Weller, D.; Sarkar, A.; Cook, R. Characterization of an antibiotic produced by a strain of Pseudomonas fluorescens inhibitory to Gaeumannomyces graminis var. tritici and Pythium spp. Antimicrob. Agents Chemother. 1986, 29, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, C.; Su, P.; Liao, X. Control effect and possible mechanism of the natural compound phenazine-1-carboxamide against Botrytis cinerea. PLoS ONE 2015, 10, e0140380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugaiah, V.; Mathivanan, N.; Varghese, B. Purification, crystal structure and antimicrobial activity of phenazine-1-carboxamide produced by a growth-promoting biocontrol bacterium, Pseudomonas aeruginosa MML2212. J. Appl. Microbiol. 2010, 108, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Keel, C.; Schnider, U.; Maurhofer, M.; Voisard, C.; Laville, J.; Burger, U.; Wirthner, P.J.; Haas, D.; Défago, G. Suppression of root diseases by Pseudomonas fluorescens CHA0: Importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol. Plant Microbe Interact. 1992, 5, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.L.; Halbrendt, J.M.; Carta, L.K.; Skantar, A.M.; Liu, T.; Abdelnabby, H.M.; Vinyard, B.T. Toxicity of 2,4-diacetylphloroglucinol (DAPG) to plant-parasitic and bacterial-feeding nematodes. J. Nematol. 2009, 41, 274. [Google Scholar]
- Han, S.H.; Lee, S.J.; Moon, J.H.; Park, K.H.; Yang, K.Y.; Cho, B.H.; Kim, K.Y.; Kim, Y.W.; Lee, M.C.; Anderson, A.J. GacS-dependent production of 2R, 3R-butanediol by Pseudomonas chlororaphis O6 is a major determinant for eliciting systemic resistance against Erwinia carotovora but not against Pseudomonas syringae pv. tabaci in tobacco. Mol. Plant Microbe Interact. 2006, 19, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Mendis, H.C.; Thomas, V.P.; Schwientek, P.; Salamzade, R.; Chien, J.-T.; Waidyarathne, P.; Kloepper, J.; De La Fuente, L. Strain-specific quantification of root colonization by plant growth promoting rhizobacteria Bacillus firmus I-1582 and Bacillus amyloliquefaciens QST713 in non-sterile soil and field conditions. PLoS ONE 2018, 13, e0193119. [Google Scholar] [CrossRef]
- Berini, F.; Katz, C.; Gruzdev, N.; Casartelli, M.; Tettamanti, G.; Marinelli, F. Microbial and viral chitinases: Attractive biopesticides for integrated pest management. Biotechnol. Adv. 2018, 36, 818–838. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhou, Q.; Luo, H.; Xia, L.; Li, L.; Sun, M.; Yu, Z. Systemic nematicidal activity and biocontrol efficacy of Bacillus firmus against the root-knot nematode Meloidogyne incognita. World J. Microbiol. Biotechnol. 2015, 31, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, G.; Horak, I.; Jansen van Rensburg, P.J.; Claassens, S. Bacillus-based bionematicides: Development, modes of action and commercialisation. Biocontrol Sci. Technol. 2018, 28, 629–653. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Shi, X.; Wang, B.; Li, M.; Wang, Q.; Zhang, S. Antifungal effect of volatile organic compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes 2020, 8, 1674. [Google Scholar] [CrossRef]
- Massawe, V.C.; Hanif, A.; Farzand, A.; Mburu, D.K.; Ochola, S.O.; Wu, L.; Tahir, H.A.S.; Gu, Q.; Wu, H.; Gao, X. Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Park, S.K.; Hur, J.Y.; Kim, Y.C. Purification and characterization of a major extracellular chitinase from a biocontrol bacterium, Paenibacillus elgii HOA73. Plant Pathol. J. 2017, 33, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; Li, Q.; Li, S.; Chen, C.; Chen, Z.; Huang, W. In vitro antimicrobial activities and mechanism of 1-octen-3-ol against food-related bacteria and pathogenic fungi. J. Oleo Sci. 2017, 66, ess16196. [Google Scholar] [CrossRef] [Green Version]
- Herrera, J.M.; Pizzolitto, R.P.; Zunino, M.P.; Dambolena, J.S.; Zygadlo, J.A. Effect of fungal volatile organic compounds on a fungus and an insect that damage stored maize. J. Stored Prod. Res. 2015, 62, 74–80. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, J.; Nie, Q.; Huang, D.; Yu, C.; Zheng, L.; Cai, M.; Thomashow, L.S.; Weller, D.M.; Yu, Z. Volatile organic compounds from Paenibacillus polymyxa KM2501-1 control Meloidogyne incognita by multiple strategies. Sci. Rep. 2017, 7, 16213. [Google Scholar] [CrossRef]
- Dahlstrom, K.M.; McRose, D.L.; Newman, D.K. Keystone metabolites of crop rhizosphere microbiomes. Curr. Biol. 2020, 30, R1131–R1137. [Google Scholar] [CrossRef]
- Biessy, A.; Filion, M. Phenazines in plant-beneficial Pseudomonas spp.: Biosynthesis, regulation, function and genomics. Environ. Microbiol. 2018, 20, 3905–3917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-Y.; Shi, X.-C.; Chen, X.; Laborda, P.; Zhao, Y.-Y.; Liu, F.-Q.; Laborda, P. Biocontrol ability of phenazine-producing strains for the management of fungal plant pathogens: A review. Biol. Control 2021, 155, 104548. [Google Scholar] [CrossRef]
- Mazzola, M.; Fujimoto, D.K.; Thomashow, L.S.; Cook, R.J. Variation in sensitivity of Gaeumannomyces graminis to antibiotics produced by fluorescent Pseudomonas spp. and effect on biological control of take-all of wheat. Appl. Environ. Microbiol. 1995, 61, 2554–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.J.; Kim, Y.C. Biopesticides produced by plant-probiotic Pseudomonas chlororaphis isolates. Crop Prot. 2018, 105, 62–69. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Z.; Yu, L.; Hu, D.; Song, B. Investigating the antifungal activity and mechanism of a microbial pesticide Shenqinmycin against Phoma sp. Pestic. Biochem. Physiol. 2018, 147, 46–50. [Google Scholar] [CrossRef]
- Kavitha, K.; Mathiyazhagan, S.; Sendhilvel, V.; Nakkeeran, S.; Chandrasekar, G.; Dilantha Fernando, W. Broad spectrum action of phenazine against active and dormant structures of fungal pathogens and root knot nematode. Arch. Phytopathol. Plant Prot. 2005, 38, 69–76. [Google Scholar] [CrossRef]
- Meena, K.S.; Jonathan, E.; Ardhanareeswaran, N. Isolation of phenazine and its activity against root-knot nematode, Meloidogyne incognita. Indian J. Nematol. 2013, 43, 180–183. [Google Scholar]
- Gong, L.; Tan, H.; Chen, F.; Li, T.; Zhu, J.; Jian, Q.; Yuan, D.; Xu, L.; Hu, W.; Jiang, Y. Novel synthesized 2,4-DAPG analogues: Antifungal activity, mechanism and toxicology. Sci. Rep. 2016, 6, 32266. [Google Scholar] [CrossRef] [Green Version]
- Loper, J.E.; Henkels, M.D.; Rangel, L.I.; Olcott, M.H.; Walker, F.L.; Bond, K.L.; Kidarsa, T.A.; Hesse, C.N.; Sneh, B.; Stockwell, V.O. Rhizoxin analogs, orfamide A and chitinase production contribute to the toxicity of Pseudomonas protegens strain Pf-5 to Drosophila melanogaster. Environ. Microbiol. 2016, 18, 3509–3521. [Google Scholar] [CrossRef]
- Péchy-Tarr, M.; Bruck, D.J.; Maurhofer, M.; Fischer, E.; Vogne, C.; Henkels, M.D.; Donahue, K.M.; Grunder, J.; Loper, J.E.; Keel, C. Molecular analysis of a novel gene cluster encoding an insect toxin in plant-associated strains of Pseudomonas fluorescens. Environ. Microbiol. 2008, 10, 2368–2386. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Anand, A.; Chinchilla, D.; Tan, C.; Mène-Saffrané, L.; L’Haridon, F.; Weisskopf, L. Contribution of hydrogen cyanide to the antagonistic activity of Pseudomonas strains against Phytophthora infestans. Microorganisms 2020, 8, 1144. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Petrikovics, I.; Budai, M.; Kovacs, K.; Thompson, D.E. Past, present and future of cyanide antagonism research: From the early remedies to the current therapies. World J. Methodol. 2015, 5, 88. [Google Scholar] [CrossRef] [Green Version]
- Zdor, R.E. Bacterial cyanogenesis: Impact on biotic interactions. J. Appl. Microbiol. 2015, 118, 267–274. [Google Scholar] [CrossRef]
- Flaishman, M.A.; Eyal, Z.; Zilberstein, A.; Voisard, C.; Haas, D. Suppression of Septoria tritici blotch and leaf rust of wheat by recombinant cyanide-producing strains of Pseudomonas putida. Mol. Plant Microbe Interact. 1996, 9, 642–645. [Google Scholar] [CrossRef]
- Spence, C.A.; Raman, V.; Donofrio, N.M.; Bais, H.P. Global gene expression in rice blast pathogen Magnaporthe oryzae treated with a natural rice soil isolate. Planta 2014, 239, 171–185. [Google Scholar] [CrossRef]
- Nandi, M.; Selin, C.; Brawerman, G.; Fernando, W.D.; de Kievit, T.R. The global regulator ANR is essential for Pseudomonas chlororaphis strain PA23 biocontrol. Microbiology 2016, 162, 2159–2169. [Google Scholar] [CrossRef]
- Keel, C.; Voisard, C.; Berling, C.-H.; Kahr, G.; Defago, G. Iron sufficiency, a prerequisite for the suppression of tobacco black root rot by Pseudomonas fluorescens strain CHA 0 under gnotobiotic conditions. Phytopathology 1989, 79, 584–589. [Google Scholar] [CrossRef]
- Voisard, C.; Keel, C.; Haas, D.; Dèfago, G. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 1989, 8, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Lanteigne, C.; Gadkar, V.J.; Wallon, T.; Novinscak, A.; Filion, M. Production of DAPG and HCN by Pseudomonas sp. LBUM300 contributes to the biological control of bacterial canker of tomato. Phytopathology 2012, 102, 967–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broderick, K.E.; Chan, A.; Balasubramanian, M.; Feala, J.; Reed, S.L.; Panda, M.; Sharma, V.S.; Pilz, R.B.; Bigby, T.D.; Boss, G.R. Cyanide produced by human isolates of Pseudomonas aeruginosa contributes to lethality in Drosophila melanogaster. J. Infect. Dis. 2008, 197, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, K.K.; Kothamasi, D. Pseudomonas fluorescens CHA0 can kill subterranean termite Odontotermes obesus by inhibiting cytochrome c oxidase of the termite respiratory chain. FEMS Microbiol. Lett. 2009, 300, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.-Y.; Jang, E.-J.; Park, S.-Y.; Son, H.-J. Production of HCN, weed control substance, by Pseudomonas koreensis and its plant growth-promoting and termiticidal activities. J. Environ. Sci. Int. 2018, 27, 771–780. [Google Scholar] [CrossRef]
- Soenens, A.; Imperial, J. Biocontrol capabilities of the genus Serratia. Phytochem. Rev. 2020, 19, 577–587. [Google Scholar] [CrossRef]
- Ordentlich, A.; Elad, Y.; Chet, I. The role of chitinase of Serratia marcescens in biocontrol of Sclerotium rolfsii. Phytopathology 1988, 78, 84–88. [Google Scholar]
- Levenfors, J.J.; Hedman, R.; Thaning, C.; Gerhardson, B.; Welch, C.J. Broad-spectrum antifungal metabolites produced by the soil bacterium Serratia plymuthica A 153. Soil Biol. Biochem. 2004, 36, 677–685. [Google Scholar] [CrossRef]
- Matilla, M.A.; Drew, A.; Udaondo, Z.; Krell, T.; Salmond, G.P. Genome sequence of Serratia plymuthica A153, a model rhizobacterium for the investigation of the synthesis and regulation of haterumalides, zeamine, and andrimid. Genome Announc. 2016, 4, e00373-16. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Effmert, U.; Baermann, G.; Quella, M.; Piechulla, B. Impact of bacterial volatiles on phytopathogenic fungi: An in vitro study on microbial competition and interaction. J. Exp. Bot. 2022, 73, 596–614. [Google Scholar] [CrossRef]
- Wenke, K.; Wanke, D.; Kilian, J.; Berendzen, K.; Harter, K.; Piechulla, B. Volatiles of two growth-inhibiting rhizobacteria commonly engage AtWRKY18 function. Plant J. 2012, 70, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kesel, J.; Conrath, U.; Flors, V.; Luna, E.; Mageroy, M.H.; Mauch-Mani, B.; Pastor, V.; Pozo, M.J.; Pieterse, C.M.; Ton, J. The induced resistance lexicon: Do’s and don’ts. Trends Plant Sci. 2021, 26, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Ma, F.; Yang, X.; Shi, Z.; Miao, X. Novel crosstalk between ethylene-and jasmonic acid-pathway responses to a piercing–sucking insect in rice. New Phytol. 2020, 225, 474–487. [Google Scholar] [CrossRef] [Green Version]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Hua, G.K.H.; Ongena, M.; Höfte, M. Role of phenazines and cyclic lipopeptides produced by Pseudomonas sp. CMR12a in induced systemic resistance on rice and bean. Environ. Microbiol. Rep. 2016, 8, 896–904. [Google Scholar] [CrossRef]
- Audenaert, K.; Pattery, T.; Cornelis, P.; Höfte, M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant Microbe Interact. 2002, 15, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.-R.; Han, S.-H.; Zdor, R.E.; Anderson, A.J.; Spencer, M.; Yang, K.-Y.; Kim, Y.-H.; Lee, M.-C.; Cho, B.-H.; Kim, Y.-C. Inhibition of seed germination and induction of systemic disease resistance by Pseudomonas chlororaphis O6 requires phenazine production regulated by the global regulator, GacS. J. Microbiol. Biotechnol. 2007, 17, 586–593. [Google Scholar]
- Spencer, M.; Ryu, C.-M.; Yang, K.-Y.; Kim, Y.C.; Kloepper, J.W.; Anderson, A.J. Induced defence in tobacco by Pseudomonas chlororaphis strain O6 involves at least the ethylene pathway. Physiol. Mol. Plant Pathol. 2003, 63, 27–34. [Google Scholar] [CrossRef]
- Ramarathnam, R.; Fernando, W.; de Kievit, T. The role of antibiosis and induced systemic resistance, mediated by strains of Pseudomonas chlororaphis, Bacillus cereus and B. amyloliquefaciens, in controlling blackleg disease of canola. BioControl 2011, 56, 225–235. [Google Scholar] [CrossRef]
- Martin-Rivilla, H.; Garcia-Villaraco, A.; Ramos-Solano, B.; Gutierrez-Manero, F.; Lucas, J. Improving flavonoid metabolism in blackberry leaves and plant fitness by using the bioeffector Pseudomonas fluorescens N 21.4 and its metabolic elicitors: A biotechnological approach for a more sustainable crop. J. Agric. Food Chem. 2020, 68, 6170–6180. [Google Scholar] [CrossRef] [PubMed]
- Chae, D.-H.; Kim, D.-R.; Cheong, M.S.; Lee, Y.B.; Kwak, Y.-S. Investigating the induced systemic resistance mechanism of 2,4-Diacetylphloroglucinol (DAPG) using DAPG hydrolase-transgenic Arabidopsis. Plant Pathol. J. 2020, 36, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Weller, D.M. Natural plant protection by 2,4-diacetylphloroglucinol-producing Pseudomonas spp. in take-all decline soils. Mol. Plant Microbe Interact. 1998, 11, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Cofer, T.M.; Seidl-Adams, I.; Tumlinson, J.H. From acetoin to (Z)-3-hexen-1-ol: The diversity of volatile organic compounds that induce plant responses. J. Agric. Food Chem. 2018, 66, 11197–11208. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. J. Gen. Plant Pathol. 2007, 73, 35–37. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Lam, V.B.; Meyer, T.; Arias, A.A.; Ongena, M.; Oni, F.E.; Höfte, M. Bacillus cyclic lipopeptides iturin and fengycin control rice blast caused by Pyricularia oryzae in potting and acid sulfate soils by direct antagonism and induced systemic resistance. Microorganisms 2021, 9, 1441. [Google Scholar] [CrossRef]
- Park, K.; Park, Y.-S.; Ahamed, J.; Dutta, S.; Ryu, H.; Lee, S.-H.; Balaraju, K.; Manir, M.; Moon, S.-S. Elicitation of induced systemic resistance of chili pepper by iturin A analogs derived from Bacillus vallismortis EXTN-1. Can. J. Plant Sci. 2016, 96, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Pang, X.; Liu, H.; Lin, F.; Lu, F.; Bie, X.; Lu, Z.; Lu, Y. Iturin A induces resistance and improves the quality and safety of harvested cherry tomato. Molecules 2021, 26, 6905. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Kauffmann, S.; Geoffroy, P.; Fritig, B. Biological function of pathogenesis-related proteins: Four tobacco pathogenesis-related proteins are chitinases. Proc. Natl. Acad. Sci. USA 1987, 84, 6750–6754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pusztahelyi, T. Chitin and chitin-related compounds in plant–fungal interactions. Mycology 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Peng, D. Nematode chitin and application. Adv. Exp. Med. Biol. 2019, 1142, 209–219. [Google Scholar] [PubMed]
- Kilani, J.; Fillinger, S. Phenylpyrroles: 30 years, two molecules and (nearly) no resistance. Front. Microbiol. 2016, 7, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Yue, S.-J.; Liu, W.-H.; Zheng, Y.-F.; Zhang, C.-H.; Feng, T.-T.; Hu, H.-B.; Wang, W.; Zhang, X.-H. Engineering of glycerol utilization in Pseudomonas chlororaphis GP72 for enhancing phenazine-1-carboxylic acid production. World J. Microbiol. Biotechnol. 2020, 36, 49. [Google Scholar] [CrossRef]
- Aggarwal, N.; Thind, S.; Sharma, S. Role of secondary metabolites of Actinomycetes in crop protection. In Plant Growth Promoting Actinobacteria; Springer: Berlin/Heidelberg, Germany, 2016; pp. 99–121. [Google Scholar]
- Bargabus, R.; Zidack, N.; Sherwood, J.; Jacobsen, B. Characterisation of systemic resistance in sugar beet elicited by a non-pathogenic, phyllosphere-colonizing Bacillus mycoides, biological control agent. Physiol. Mol. Plant Pathol. 2002, 61, 289–298. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Jeffers, S.N. Efficacy of commercial formulation of two biofungicides for control of blue mold and gray mold of apples in cold storage. Crop Prot. 1997, 16, 629–633. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Matzen, N.; Heick, T.M.; Jørgensen, L.N. Control of powdery mildew (Blumeria graminis spp.) in cereals by Serenade® ASO (Bacillus amyloliquefaciens (former subtilis) strain QST 713). Biol. Control 2019, 139, 104067. [Google Scholar] [CrossRef]
- Kilani-Feki, O.; Khedher, S.B.; Dammak, M.; Kamoun, A.; Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Tounsi, S. Improvement of antifungal metabolites production by Bacillus subtilis V26 for biocontrol of tomato postharvest disease. Biol. Control 2016, 95, 73–82. [Google Scholar] [CrossRef]
- Liu, S.; Tang, M.H.; Cheng, J.S. Fermentation optimization of surfactin production of Bacillus amyloliquefaciens HM618. Biotechnol. Appl. Biochem. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Hur, J.Y.; Park, S.K. Biocontrol of Botrytis cinerea by chitin-based cultures of Paenibacillus elgii HOA73. Euro. J. Plant Pathol. 2019, 155, 253–263. [Google Scholar] [CrossRef]
- Park, J.Y.; Kang, B.R.; Ryu, C.M.; Anderson, A.J.; Kim, Y.C. Polyamine is a critical determinant of Pseudomonas chlororaphis O6 for GacS-dependent bacterial cell growth and biocontrol capacity. Mol. Plant Pathol. 2018, 19, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Ghribi, D.; Mnif, I.; Boukedi, H.; Kammoun, R.; Ellouze-Chaabouni, S. Statistical optimization of low-cost medium for economical production of Bacillus subtilis biosurfactant, a biocontrol agent for the olive moth Prays oleae. Afr. J. Microbiol. Res. 2011, 5, 4927–4936. [Google Scholar]
- Meena, K.R.; Sharma, A.; Kumar, R.; Kanwar, S.S. Two factor at a time approach by response surface methodology to aggrandize the Bacillus subtilis KLP2015 surfactin lipopeptide to use as antifungal agent. J. King Saud Univ. Sci. 2020, 32, 337–348. [Google Scholar] [CrossRef]
- Ghribi, D.; Abdelkefi-Mesrati, L.; Mnif, I.; Kammoun, R.; Ayadi, I.; Saadaoui, I.; Maktouf, S.; Chaabouni-Ellouze, S. Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J. Biomed. Biotechnol. 2012, 2012, 373682. [Google Scholar] [CrossRef] [Green Version]
- Philip, N.V.; Koteshwara, A.; Kiran, G.A.; Raja, S.; Subrahmanyam, V.; Chandrashekar, H.R. Statistical optimization for coproduction of chitinase and beta 1, 4-endoglucanase by chitinolytic Paenibacillus elgii PB1 having antifungal activity. Appl. Biochem. Biotechnol. 2020, 191, 135–150. [Google Scholar] [CrossRef]
- Gao, H.; Liu, M.; Liu, J.; Dai, H.; Zhou, X.; Liu, X.; Zhuo, Y.; Zhang, W.; Zhang, L. Medium optimization for the production of avermectin B1a by Streptomyces avermitilis 14-12A using response surface methodology. Bioresour. Technol. 2009, 100, 4012–4016. [Google Scholar] [CrossRef]
- Kulkarni, M.; Gorthi, S.; Banerjee, G.; Chattopadhyay, P. Production, characterization and optimization of actinomycin D from Streptomyces hydrogenans IB310, an antagonistic bacterium against phytopathogens. Biocatal. Agric. Biotechnol. 2017, 10, 69–74. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Z.; Zhang, G.; Mo, X.; Ding, X.; Xia, L.; Hu, S. A rifampicin-resistant (rpoB) mutation in Pseudomonas protegens Pf-5 strain leads to improved antifungal activity and elevated production of secondary metabolites. Res. Microbiol. 2016, 167, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.-Y.; Liu, Q.; Choi, J.-Y.; Wang, Y.; Shim, H.-J.; Xu, H.G.; Choi, G.-J.; Kim, J.-C.; Je, Y.-H. Construction of a recombinant Bacillus velezensis strain as an integrated control agent against plant diseases and insect pests. J. Microbiol. Biotechnol. 2009, 19, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mavrodi, D.V.; Yang, M.; Thomashow, L.S.; Mavrodi, O.V.; Kelton, J.; Weller, D.M. Pseudomonas synxantha 2-79 transformed with pyrrolnitrin biosynthesis genes has improved biocontrol activity against soilborne pathogens of wheat and canola. Phytopathology 2020, 110, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hu, H.; Wang, W.; Zhang, X. Genetic engineering of Pseudomonas chlororaphis GP72 for the enhanced production of 2-hydroxyphenazine. Microb. Cell Factories 2016, 15, 131. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, Z.; Yao, W.; Zhang, X.; Wang, R.; Li, P.; Yang, K.; Wang, T.; Liu, K. Metabolic engineering of Pseudomonas chlororaphis Qlu-1 for the enhanced production of phenazine-1-carboxamide. J. Agric. Food Chem. 2020, 68, 14832–14840. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, H.; Xian, M.; Huang, W. Biosynthesis and metabolic engineering of 1-hydroxyphenazine in Pseudomonas chlororaphis H18. Microb. Cell Factories 2021, 20, 235. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Siddiqui, Z.A. Glomus intraradices, Pseudomonas alcaligenes, and Bacillus pumilus: Effective agents for the control of root-rot disease complex of chickpea (Cicer arietinum L.). J. Gen. Plant Pathol. 2008, 74, 53–60. [Google Scholar] [CrossRef]
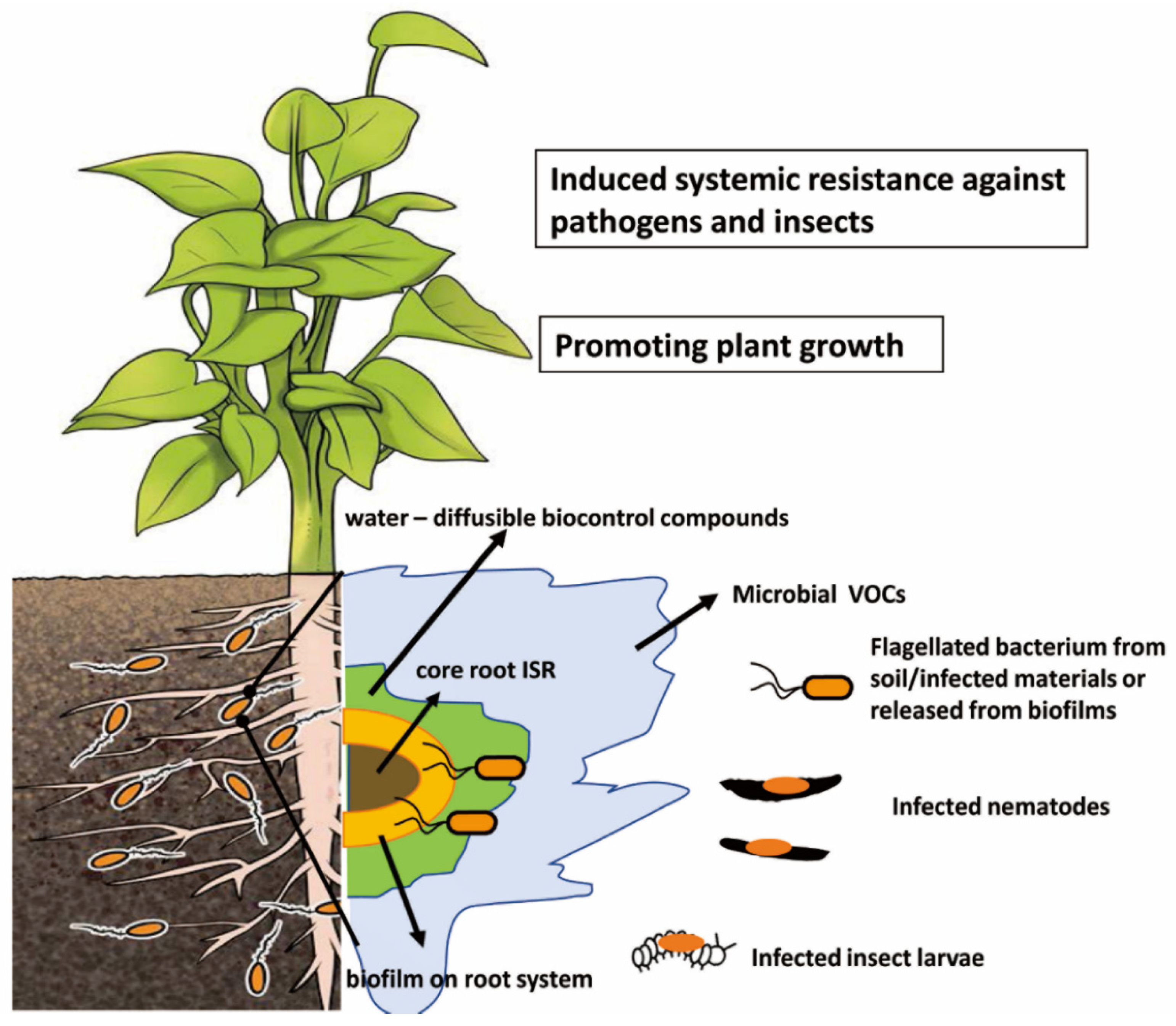
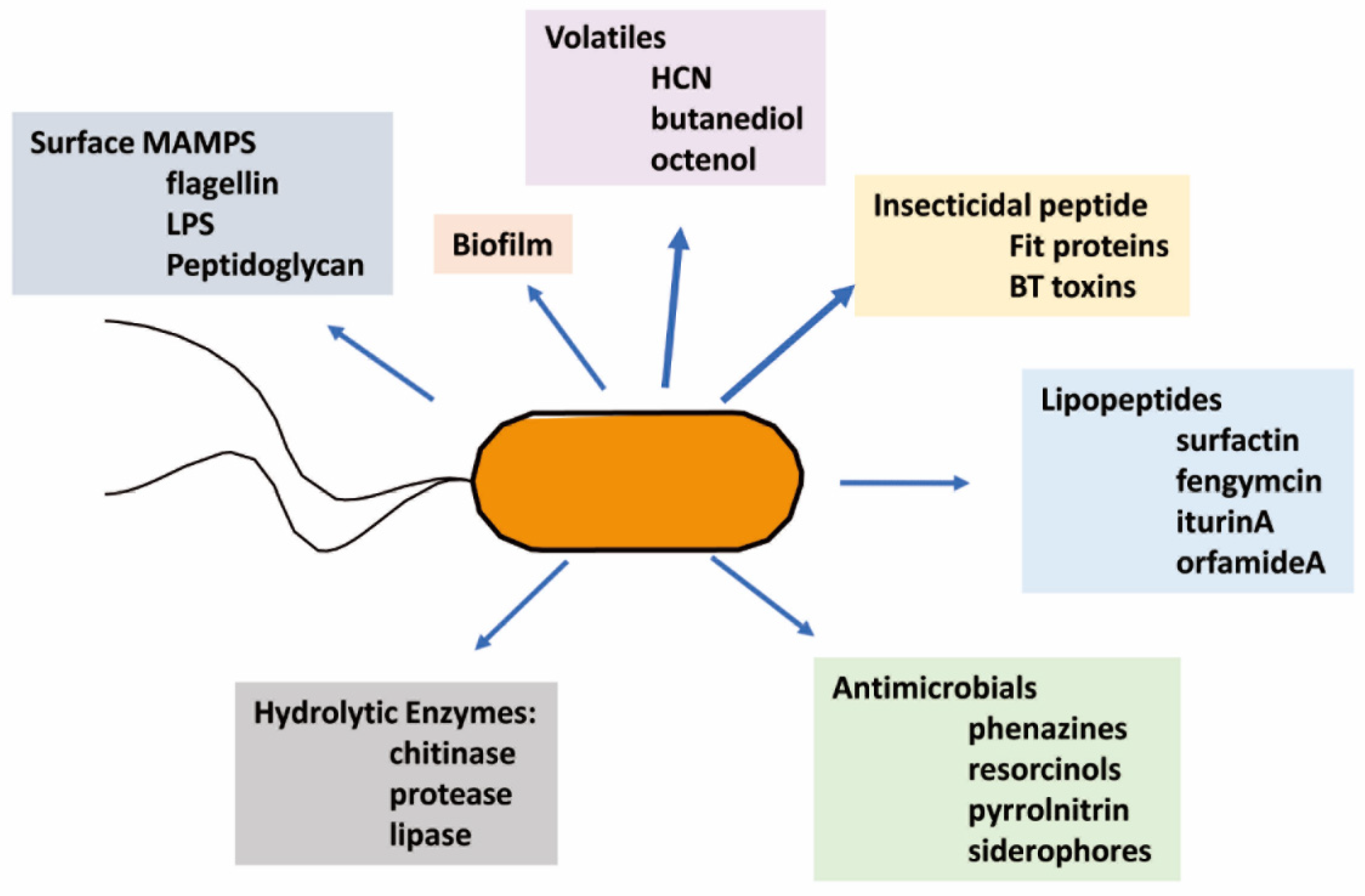
| Active Metabolite | Pathogen/Pest | MIC (ppm) | LD/LC 50 (ppm) | Inhibition | Source of Active Metabolite | Reference |
|---|---|---|---|---|---|---|
| Lipopeptide | Tuta absoluta | 180 ng/cm2 | 1st Instar larvae mortality | Bacillus amyloliquefaciens AG1 | [12] | |
| Lipopeptide | Fusarium oxysporum f. sp. lycopersici | 10 | Mycelial growth | Bacillus amyloliquefaciens BO5A | [81] | |
| Lipopeptide | Myzus persicae | 22.2 | 2nd Instar nymph mortality | Bacillus subtilis Y9 | [82] | |
| Lipopeptide | Ectomyelois ceratoniae | 152 | 3rd Instar larvae morality | Bacillus subtilis SPB1 | [18] | |
| Lipopeptide | Spodoptera littoralis | 251 ng/cm2 | 1st Instar larvae mortality | Bacillus subtilis SPB1 | [17] | |
| Lipopeptide | Fusarium solani | 3000 | Mycelial growth | Bacillus subtilis SPB1 | [19,20] | |
| Rhizoctonia bataticola | 40 | |||||
| Rhizoctonia solani | 4000 | |||||
| Lipopeptide | Colletotrichum acutatum | 32 | Mycelial growth | Bacillus subtilis EA-CB0015 | [78] | |
| Lipopeptide | Colletotrichum gloeosporioides | 36.47 | Mycelial growth | Bacillus amyloliquefaciens MG3 | [79] | |
| Lipopeptide | Phoma tracheiphila | 47.5 | Mycelial growth | Bacillus methyltrophicus TEB1 | [80] | |
| Phenazine-1-carboxylic acid (PCA) | Botrytis cinerea | 25 | Mycelial growth | Pseudomonas aeruginosa LV | [83] | |
| PCA | Botrytis cinerea | 50 | ||||
| Colletotrichum orbiculare | 5 | Mycelial growth | Pseudomonas aeruginosa GC-B26 | [84] | ||
| Phytophthora capsici | 5 | |||||
| Pythium ultimum | 5 | |||||
| PCA | Sclerotium rolfsii | 29 | Mycelial growth | Pseudomonas aeruginosa | [85] | |
| Fusarium oxysporum | 40 | |||||
| Colletotrichum falcatum | 50 | |||||
| PCA | Fusarium oxysporum | 1.56 | Mycelial growth | Burkholderia sp.HQB-1 | [86] | |
| Colletotrichum gloeosporioides | 6.13 | |||||
| Botrytis cinerea | 1.56 | |||||
| Curvularia fallax | 3.13 | |||||
| PCA | Gaeumannomyces graminis var. tritici | 1 | Mycelial growth | Pseudomonas fluorescens 2–79 | [87] | |
| Rhizoctonia solani | 1 | |||||
| Cochliobolus sativus | 1–3 | |||||
| Pythium aristosporum | 1 | |||||
| Pythium ultimum | 25–30 | |||||
| Pythium uttimum var. sporangiifurum | 80–100 | |||||
| Fusarium sp. | 25–30 | |||||
| Phenazine-1-carboxamide (PCN) | Botrytis cinerea | 108.12 * | Mycelial growth | Pseudomonas aeruginosa | [88] | |
| PCN | Rhizoctonia solani | 5 | Mycelial growth | Pseudomonas aeruginosa MML2212 | [89] | |
| 1-Octen-3-ol | Tribolium castaneum | 16.75 | Adult mortality | Paenibacillus polymyxa BMP-11 | [30] | |
| Benzothiazole | Tribolium castaneum | 3.5 | Adult mortality | Paenibacillus polymyxa BMP-11 | [30] | |
| 2,4-Diacetylphloroglucinol (2,4-DAPG) | Fusarium oxysporum f. sp. lycopersici | 16 | Mycelial growth | Pseudomonas fluorescens CHA0 | [90] | |
| Gaeumannomyces graminis var. tritici | 16–32 | |||||
| Pythium ultimum | 64 | |||||
| Rhizoctonia solani | 32–64 | |||||
| 2,4-DAPG | Xiphinema americanum | 8.3 | Adult mortality | Pseudomonas fluorescens | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Anderson, A.J.; Kim, Y.C. Root-Associated Bacteria Are Biocontrol Agents for Multiple Plant Pests. Microorganisms 2022, 10, 1053. https://doi.org/10.3390/microorganisms10051053
Lee JH, Anderson AJ, Kim YC. Root-Associated Bacteria Are Biocontrol Agents for Multiple Plant Pests. Microorganisms. 2022; 10(5):1053. https://doi.org/10.3390/microorganisms10051053
Chicago/Turabian StyleLee, Jang Hoon, Anne J. Anderson, and Young Cheol Kim. 2022. "Root-Associated Bacteria Are Biocontrol Agents for Multiple Plant Pests" Microorganisms 10, no. 5: 1053. https://doi.org/10.3390/microorganisms10051053
APA StyleLee, J. H., Anderson, A. J., & Kim, Y. C. (2022). Root-Associated Bacteria Are Biocontrol Agents for Multiple Plant Pests. Microorganisms, 10(5), 1053. https://doi.org/10.3390/microorganisms10051053






