Evaluation of the Kinetics of Antibody Response to COVID-19 Vaccine in Solid Organ Transplant Recipients: The Prospective Multicenter ORCHESTRA Cohort
Abstract
:1. Introduction
2. Materials and Methods
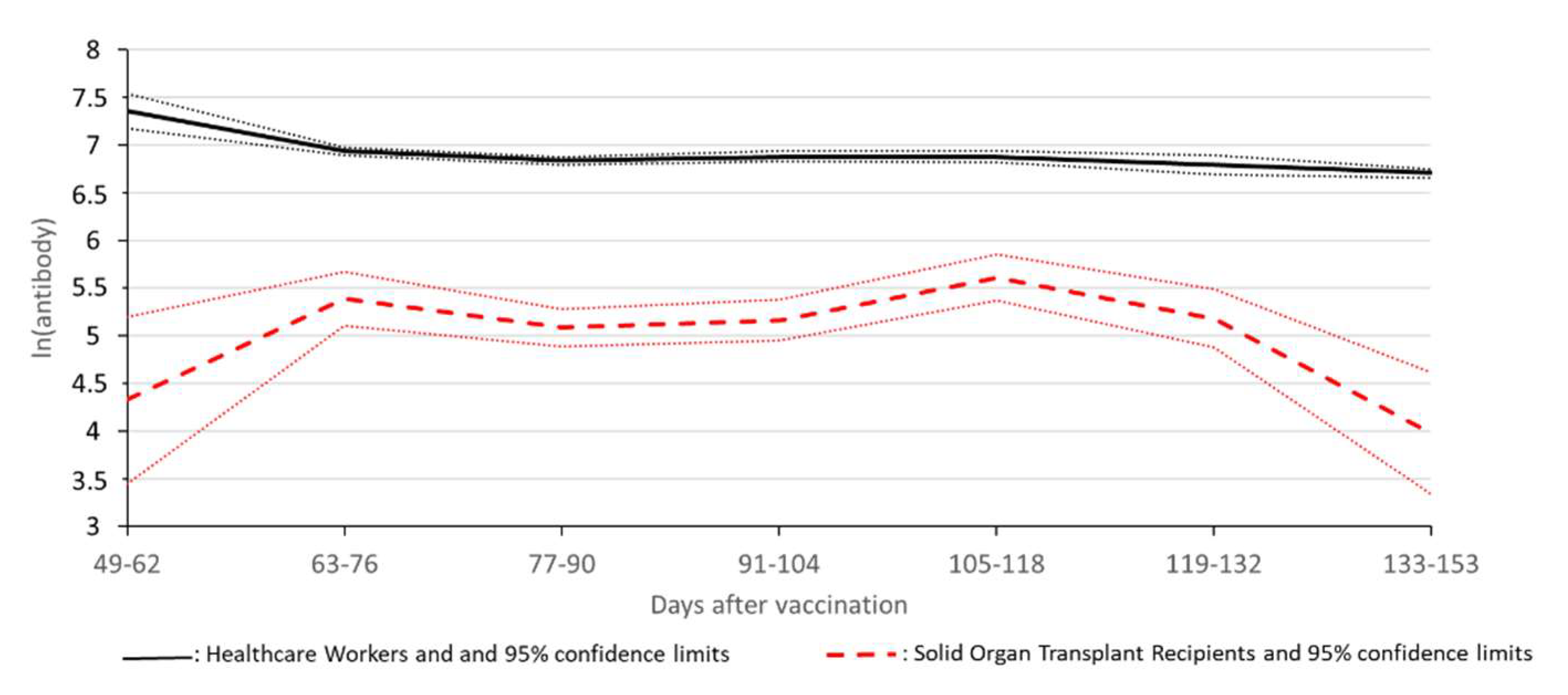
3. Results
Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Del Rio, C.; Omer, S.B.; Malani, P.N. Winter of Omicron-The Evolving COVID-19 Pandemic. JAMA 2022, 327, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Moulin, B.; Fafi-Kremer, S.; et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021, 99, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Chiang, T.P.-Y.; Ou, M.T.; Werbel, W.A.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to the Janssen COVID-19 Vaccine in Solid Organ Transplant Recipients. Transplantation 2021, 105, e82–e83. [Google Scholar] [CrossRef] [PubMed]
- Chavarot, N.; Ouedrani, A.; Marion, O.; Leruez-Ville, M.; Vilain, E.; Baaziz, M.; Del Bello, A.; Burger, C.; Sberro-Soussan, R.; Martinez, F.; et al. Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated With Belatacept. Transplantation 2021, 105, e94–e95. [Google Scholar] [CrossRef]
- Cholankeril, G.; Al-Hillan, A.; Tarlow, B.; Abrams, D.; Jacobs, J.S.; Flores, N.P.; Rana, A.; Kanwal, F.; Goss, J.A. Clinical Factors Associated With Lack of Serological Response to SARS-CoV-2 Messenger RNA Vaccine in Liver Transplantation Recipients. Liver Transplant. 2022, 28, 123–126. [Google Scholar] [CrossRef]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021, 21, 2727–2739. [Google Scholar] [CrossRef]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. JASN 2021, 32, 2153–2158. [Google Scholar] [CrossRef]
- Dębska-Ślizień, A.; Ślizień, Z.; Muchlado, M.; Kubanek, A.; Piotrowska, M.; Dąbrowska, M.; Tarasewicz, A.; Chamienia, A.; Biedunkiewicz, B.; Renke, M.; et al. Predictors of Humoral Response to mRNA COVID19 Vaccines in Kidney Transplant Recipients: A Longitudinal Study-The COViNEPH Project. Vaccines 2021, 9, 1165. [Google Scholar] [CrossRef]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Hallett, A.M.; Greenberg, R.S.; Boyarsky, B.J.; Shah, P.D.; Ou, M.T.; Teles, A.T.; Krach, M.R.; López, J.I.; Werbel, W.A.; Avery, R.K.; et al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J. Heart Lung Transplant. 2021, 40, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Havlin, J.; Svorcova, M.; Dvorackova, E.; Lastovicka, J.; Lischke, R.; Kalina, T.; Hubacek, P. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J. Heart Lung Transplant. 2021, 40, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Colmenero, J.; Pascal, M.; Escobedo, M.; Castel, M.A.; Sole-González, E.; Palou, E.; Egri, N.; Ruiz, P.; Mosquera, M.; et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am. J. Transplant. 2021, 21, 3971–3979. [Google Scholar] [CrossRef]
- Itzhaki Ben Zadok, O.; Shaul, A.A.; Ben-Avraham, B.; Yaari, V.; Ben Zvi, H.; Shostak, Y.; Pertzov, B.; Eliakim-Raz, N.; Abed, G.; Abuhazira, M.; et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients—A prospective cohort study. Eur. J. Heart Fail. 2021, 23, 1555–1559. [Google Scholar] [CrossRef]
- Korth, J.; Jahn, M.; Dorsch, O.; Anastasiou, O.E.; Sorge-Hädicke, B.; Eisenberger, U.; Gäckler, A.; Dittmer, U.; Witzke, O.; Wilde, B.; et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 2021, 13, 756. [Google Scholar] [CrossRef]
- Marinaki, S.; Adamopoulos, S.; Degiannis, D.; Roussos, S.; Pavlopoulou, I.D.; Hatzakis, A.; Boletis, I.N. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 2913–2915. [Google Scholar] [CrossRef]
- Pedersen, R.M.; Bang, L.L.; Tornby, D.S.; Kierkegaard, H.; Nilsson, A.C.; Johansen, I.S.; Bistrup, C.; Jensen, T.G.; Justesen, U.S.; Andersen, T.E. The SARS-CoV-2-neutralizing capacity of kidney transplant recipients 4 weeks after receiving a second dose of the BNT162b2 vaccine. Kidney Int. 2021, 100, 1129–1131. [Google Scholar] [CrossRef]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J. Hepatol. 2021, 75, 435–438. [Google Scholar] [CrossRef]
- Rashidi-Alavijeh, J.; Frey, A.; Passenberg, M.; Korth, J.; Zmudzinski, J.; Anastasiou, O.; Saner, F.; Jahn, M.; Lange, C.; Willuweit, K. Humoral Response to SARS-CoV-2 Vaccination in Liver Transplant Recipients-A Single-Center Experience. Vaccines 2021, 9, 738. [Google Scholar] [CrossRef]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef] [PubMed]
- Rozen-Zvi, B.; Yahav, D.; Agur, T.; Zingerman, B.; Ben-Zvi, H.; Atamna, A.; Tau, N.; Mashraki, T.; Nesher, E.; Rahamimov, R. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin. Microbiol. Infect. 2021, 27, 1173.e1–1173.e4. [Google Scholar] [CrossRef] [PubMed]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Proß, V.; Bergmann, Y.; et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021, 131, e150175. [Google Scholar] [CrossRef] [PubMed]
- Schramm, R.; Costard-Jäckle, A.; Rivinius, R.; Fischer, B.; Müller, B.; Boeken, U.; Haneya, A.; Provaznik, Z.; Knabbe, C.; Gummert, J. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin. Res. Cardiol. 2021, 110, 1142–1149. [Google Scholar] [CrossRef]
- Shostak, Y.; Shafran, N.; Heching, M.; Rosengarten, D.; Shtraichman, O.; Shitenberg, D.; Amor, S.M.; Yahav, D.; Ben Zvi, H.; Pertzov, B.; et al. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir. Med. 2021, 9, e52–e53. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Chiang, T.P.Y.; Teles, A.T.; Greenberg, R.S.; Krach, M.R.; Ou, M.T.; Massie, A.B.; Tobian, A.A.; Garonzik-Wang, J.M.; Segev, D.L.; et al. Antibody Kinetics and Durability in SARS-CoV-2 mRNA Vaccinated Solid Organ Transplant Recipients. Transplantation 2021, 105, e137–e138. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Giannella, M.; Pierrotti, L.C.; Helantera, I.; Manuel, O. SARS-CoV-2 vaccination in solid-organ transplant recipients: What the clinician needs to know. Transpl. Int. 2021, 34, 1776–1788. [Google Scholar] [CrossRef]
- Fung, M.; Babik, J.M. COVID-19 in Immunocompromised Hosts: What We Know So Far. Clin. Infect. Dis. 2021, 72, 340–350. [Google Scholar] [CrossRef]
- Kotton, C.N. Belt and Suspenders: Vaccines and Tixagevimab/Cilgavimab for Prevention of COVID-19 in Immunocompromised Patients. Ann. Intern. Med. 2022. [Google Scholar] [CrossRef]
- Yahav, D.; Rozen-Zvi, B.; Mashraki, T.; Atamna, A.; Ben-Zvi, H.; Bar-Haim, E.; Rahamimov, R. Immunosuppression reduction when administering a booster dose of the BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant recipients without adequate humoral response following two vaccine doses: Protocol for a randomised controlled trial (BECAME study). BMJ Open 2021, 11, e055611. [Google Scholar] [CrossRef] [PubMed]
- Hirzel, C.; Ferreira, V.H.; L’Huillier, A.G.; Hoschler, K.; Cordero, E.; Limaye, A.P.; Englund, J.A.; Reid, G.; Humar, A.; Kumar, D.; et al. Humoral response to natural influenza infection in solid organ transplant recipients. Am. J. Transplant. 2019, 19, 2318–2328. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, Q.; Madhira, V.; Olex, A.L.; Anzalone, A.J.; Vinson, A.; Singh, J.A.; French, E.; Abraham, A.G.; Mathew, J.; et al. Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US. JAMA Intern. Med. 2022, 182, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef]
- Werbel, W.A.; Boyarsky, B.J.; Ou, M.T.; Massie, A.B.; Tobian, A.A.; Garonzik-Wang, J.M.; Segev, D.L. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann. Intern. Med. 2021, 174, 1330–1332. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3990–4002. [Google Scholar] [CrossRef]
| Total n = 1062 (%) | Positive Antibody Response at 3 ± 1 Month N = 556 (%) | Negative Antibody Response at 3 ± 1 Month N = 506 (%) | p | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (mean ± SD) (years) | 58.28 ± 13.10 | 56.47 ± 13.61 | 60.26 ± 12.23 | <0.001 |
| Age group | <0.001 | |||
| <39 y | 93 (8.76%) | 63 (67.74%) | 30 (32.26%) | |
| 40–49 y | 156 (14.69%) | 93 (59.62%) | 63 (40.38%) | |
| 50–59 y | 268 (25.24%) | 146 (54.48%) | 122 (45.52%) | |
| 60–69 y | 309 (29.10%) | 148 (47.90%) | 161 (52.10%) | |
| ≥70 y | 236 (22.22%) | 106 (44.92%) | 130 (55.08%) | |
| Sex | 0.967 | |||
| Male | 704 (66.29%) | 368 (52.27%) | 336 (47.73%) | |
| Female | 353 (33.40%) | 185 (52.41%) | 168 (47.59%) | |
| Comorbidities | 0.005 | |||
| No | 347 (32.67%) | 203 (58.50%) | 144 (41.5%) | |
| Yes | 715 (67.33%) | 353 (49.37%) | 362 (50.63%) | |
| Type of graft | <0.001 | |||
| Kidney | 677 (63.75%) | 312 (46.09%) | 365 (53.91%) | |
| Heart | 177 (16.67%) | 86 (48.59%) | 91 (51.41%) | |
| Liver | 182 (17.14%) | 144 (79.12%) | 38 (20.88%) | |
| Lung | 26 (2.45%) | 14 (53.85%) | 12 (46.15%) | |
| Type of vaccine | <0.001 | |||
| BNT162b2 | 928 (87.38%) | 463 (49.89%) | 465 (50.11%) | |
| mRNA-1273 | 134 (12.62%) | 93 (69.40%) | 41 (30.60%) | |
| Time from transplant to vaccination | <0.001 | |||
| Less than 1 year | 58 (5.46%) | 21 (36.21%) | 37 (63.79%) | |
| 1 to 3 years | 166 (15.63%) | 68 (40.96%) | 98 (59.04%) | |
| More than 3 years | 836 (78.72%) | 465 (55.62%) | 371 (44.38%) | |
| Induction regimen in the last 6 months | 0.340 | |||
| No | 1061 (99.90%) | 555 (52.31%) | 506 (47.69%) | |
| Any | 1 (0.09%) | 1 (100%) | 0 (0.00%) | |
| Immunosuppressive drugs at the time of vaccination | ||||
| Calcineurin inhibitors | 1007 (94.82%) | 520 (51.64%) | 487 (48.36%) | 0.046 |
| Tacrolimus | 763 (71.85%) | 384 (50.33%) | 379 (49.67%) | 0.035 |
| Cyclosporine | 246 (23.16%) | 136 (55.28%) | 110 (44.72%) | 0.294 |
| Anti-metabolites | 663 (62.43%) | 284 (42.84%) | 379 (57.16%) | <0.001 |
| Mycophenolate mofetil | 626 (58.95%) | 252 (40.26%) | 374 (59.74%) | <0.001 |
| Azathioprine | 37 (3.48%) | 32 (86.49%) | 5 (13.51%) | <0.001 |
| mTOR | 144 (13.56%) | 90 (62.50%) | 54 (37.50%) | 0.009 |
| Everolimus | 128 (12.05%) | 78 (60.94%) | 50 (39.06%) | 0.038 |
| Sirolimus | 16 (1.51%) | 12 (75.00%) | 4 (25.00%) | 0.068 |
| Steroids | 709 (66.76%) | 312 (44.01%) | 397 (55.99%) | <0.001 |
| Impaired graft function | <0.001 | |||
| Good | 830 (78.15%) | 475 (57.23%) | 355 (42.77%) | |
| Impaired or Failure | 222 (20.90%) | 75 (33.78%) | 147 (66.22%) | |
| Time between first dose and assessment of antibody response | 0.038 | |||
| 40–70 d | 170 (16.01%) | 74 (43.53%) | 96 (56.47%) | |
| 70–100 d | 500 (47.08%) | 262 (52.40%) | 238 (47.60%) | |
| 100–130 d | 298 (28.06%) | 170 (57.05%) | 128 (42.95%) | |
| 130–160 d | 75 (7.06%) | 37 (49.33%) | 38 (50.67%) | |
| >160 d | 19 (1.79%) | 13 (68.42%) | 6 (31.58%) |
| Variable | OR (95% CI) | p-Value (α = 0.05) |
|---|---|---|
| Sex | - | - |
| Male | 1 (ref) | - |
| Female | 0.91 (0.67 1.24) | 0.568 |
| Age | - | - |
| Categorical increase (<39 y; 40–49 y; 50–59 y; 60–69 y; ≥70 y) | 0.67 (0.60 0.76) | <0.001 |
| Type of graft | - | - |
| Kidney | 1 (ref) | |
| Heart | 0.64 (0.39 1.07) | 0.090 |
| Liver | 2.71 (1.55 4.72) | <0.001 |
| Lung | 1.16 (0.46 2.95) | 0.750 |
| Time from transplant to vaccination | - | - |
| Less than 1 year | 1 (ref) | |
| 1 to 3 years | 1.79 (0.87 3.67) | 0.111 |
| More than 3 years | 4.92 (2.56 9.45) | 0.000 |
| Time from vaccination onset to serological assessment | ||
| Categorical increase (40–70 d; 70–100 d; 100–130 d; 130–160 d; >160 d) | 1.30 (1.10 1.53) | <0.001 |
| Comorbidities | ||
| No | 1 (ref) | |
| Yes | 0.60 (0.43 0.83) | 0.002 |
| Type of vaccine | ||
| BNT162b2 | 1 (ref) | |
| mRNA-1273 | 3.57 (2.25 5.67) | <0.001 |
| Immunosuppressive drugs at the time of vaccination | ||
| Cyclosporine | 0.71 (0.30 1.67) | 0.429 |
| Tacrolimus | 0.52 (0.23 1.16) | 0.111 |
| Azathioprine | 3.43 (1.20 9.82) | 0.022 |
| Mycophenolates | 0.29 (0.20 0.43) | <0.001 |
| Sirolimus | 0.70 (0.18 2.66) | 0.598 |
| Everolimus | 0.72 (0.43 1.20) | 0.212 |
| Steroids | 0.44 (0.30 0.65) | 0.000 |
| Impaired graft function | ||
| Good | 1 (ref) | |
| Impaired, Failure, and others | 0.38 (0.26 0.55) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannella, M.; Righi, E.; Pascale, R.; Rinaldi, M.; Caroccia, N.; Gamberini, C.; Palacios-Baena, Z.R.; Caponcello, G.; Morelli, M.C.; Tamè, M.; et al. Evaluation of the Kinetics of Antibody Response to COVID-19 Vaccine in Solid Organ Transplant Recipients: The Prospective Multicenter ORCHESTRA Cohort. Microorganisms 2022, 10, 1021. https://doi.org/10.3390/microorganisms10051021
Giannella M, Righi E, Pascale R, Rinaldi M, Caroccia N, Gamberini C, Palacios-Baena ZR, Caponcello G, Morelli MC, Tamè M, et al. Evaluation of the Kinetics of Antibody Response to COVID-19 Vaccine in Solid Organ Transplant Recipients: The Prospective Multicenter ORCHESTRA Cohort. Microorganisms. 2022; 10(5):1021. https://doi.org/10.3390/microorganisms10051021
Chicago/Turabian StyleGiannella, Maddalena, Elda Righi, Renato Pascale, Matteo Rinaldi, Natascia Caroccia, Chiara Gamberini, Zaira R. Palacios-Baena, Giulia Caponcello, Maria Cristina Morelli, Mariarosa Tamè, and et al. 2022. "Evaluation of the Kinetics of Antibody Response to COVID-19 Vaccine in Solid Organ Transplant Recipients: The Prospective Multicenter ORCHESTRA Cohort" Microorganisms 10, no. 5: 1021. https://doi.org/10.3390/microorganisms10051021
APA StyleGiannella, M., Righi, E., Pascale, R., Rinaldi, M., Caroccia, N., Gamberini, C., Palacios-Baena, Z. R., Caponcello, G., Morelli, M. C., Tamè, M., Busutti, M., Comai, G., Potena, L., Salvaterra, E., Feltrin, G., Cillo, U., Gerosa, G., Cananzi, M., Piano, S., ... on behalf of The ORCHESTRA Study Group Workpackage. (2022). Evaluation of the Kinetics of Antibody Response to COVID-19 Vaccine in Solid Organ Transplant Recipients: The Prospective Multicenter ORCHESTRA Cohort. Microorganisms, 10(5), 1021. https://doi.org/10.3390/microorganisms10051021









