Hospital-Onset Bloodstream Infections Caused by Eight Sentinel Bacteria: A Nationwide Study in Israel, 2018–2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Laboratory Methods
2.4. Definitions
2.5. Incidence
2.6. Mortality
2.7. Statistical Analysis
2.8. Ethical Considerations
3. Results
3.1. Study Participants
3.2. Incidence
3.3. Mortality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaye, K.S.; Marchaim, D.; Chen, T.Y.; Baures, T.; Anderson, D.J.; Choi, Y.; Sloane, R.; Schmader, K.E. Effect of nosocomial bloodstream infections on mortality, length of stay, and hospital costs in older adults. J. Am. Geriatr. Soc. 2014, 62, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, F.; Hulstaert, F.; Van de Sande, S.; Devriese, S.; Morales, I.; Parmentier, Y. Hospital-acquired, laboratory-confirmed bloodstream infections: Linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J. Hosp. Infect. 2010, 75, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two european point prevalence surveys, 2016 to 2017. Eurosurveillance 2018, 23, 1800516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, M.; Al-Hasan, M.N. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin. Microbiol. Infect. 2013, 19, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Søgaard, M.; Lyytikäinen, O.; Laupland, K.B.; Schønheyder, H.C. Monitoring the epidemiology of bloodstream infections: Aims, methods and importance. Expert Rev. Anti-Infect. Ther. 2013, 11, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- ECDC European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2015. In Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2017. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net); European Centre for Disease Prevention and Control: Solna, Sweden, 2020. [Google Scholar]
- Kadri, S.S.; Lai, Y.L.; Warner, S.; Strich, J.R.; Babiker, A.; Ricotta, E.E.; Demirkale, C.Y.; Dekker, J.P.; Palmore, T.N.; Rhee, C.; et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: A retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect. Dis. 2021, 21, 241–251. [Google Scholar] [CrossRef]
- Ministry of Health Israel Inpatient Institutions and Day Hospital Units in Israel 2018, Part I: Hospitalization Trends; Ministry of Health: Jerusalem, Israel, 2020.
- Centers for Disease Control and Prevention Multidrug-Resistant Organism & Clostridioides Difficile (MDRO/CDI) Infection Surveillance and LabID Event Reporting Module 2021; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022.
- Tacconelli, E.; Magrini, N.; Carmeli, Y.; Harbarth, S.; Kahlmeter, G.; Kluytmans, J.; Mendelson, M.; Pulcini, C.; Singh, N.; Theuretzbacher, U. WHO Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; pp. 1–7. [Google Scholar]
- United States Centers for Disease Control and Prevention NHSN Organisms List. Available online: https://www.cdc.gov/nhsn/xls/2018-NHSN-Organisms-List-Validation.xlsx (accessed on 1 May 2020).
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibovici, L.; Schønheyder, H.; Pitlik, S.D.; Samra, Z.; Møller, J.K. Bacteraemia caused by hospital-type micro-organisms during hospital stay. J. Hosp. Infect. 2000, 44, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Leibovici, L.; Shraga, I.; Drucker, M.; Konigsberger, H.; Samra, Z.; Pitlik, S.D. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 1998, 244, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, E.H.; Sherman, G.; Ward, S.; Fraser, V.J.; Kollef, M.H. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000, 118, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.L.; Lassen, A.T.; Gradel, K.O.; Jensen, T.G.; Kolmos, H.J.; Hallas, J.; Pedersen, C. Bacteremia is associated with excess long-term mortality: A 12-year population-based cohort study. J. Infect. 2015, 70, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Osterholzer, J.J.; Langa, K.M.; Angus, D.C.; Iwashyna, T.J. Late mortality after sepsis: Propensity matched cohort study. BMJ 2016, 353, i2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibovici, L. Long-term consequences of severe infections. Clin. Microbiol. Infect. 2013, 19, 510–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laupland, K.B. Incidence of bloodstream infection: A review of population-based studies. Clin. Microbiol. Infect. 2013, 19, 492–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Duerden, B.; Fry, C.; Johnson, A.P.; Wilcox, M.H. The control of methicillin-resistant Staphylococcus aureus blood stream infections in England. Open Forum Infect. Dis. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Furuya, E.Y.; Dick, A.W.; Herzig, C.T.A.; Pogorzelska-Maziarz, M.; Larson, E.L.; Stone, P.W. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect. Control Hosp. Epidemiol. 2016, 37, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health Israel Summary of Intensive Care Unit-Acquired Bacteremia, 2019; Ministry of Health: Jerusalem, Israel, 2020.
- Timbrook, T.T.; Morton, J.B.; Mcconeghy, K.W.; Caffrey, A.R.; Mylonakis, E.; LaPlante, K.L. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: A systematic review and meta-analysis. Clin. Infect. Dis. 2017, 64, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghdadi, J.D.; Brook, R.H.; Uslan, D.Z.; Needleman, J.; Bell, D.S.; Cunningham, W.E.; Wong, M.D. Association of a Care Bundle for Early Sepsis Management with Mortality among Patients with Hospital-Onset or Community-Onset Sepsis. JAMA Intern. Med. 2020, 180, 707–716. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | N = 6752 | |
|---|---|---|
| Female sex, n (%) | 2874 | (42.6) |
| Age, mean (±SD) | 68.5 | (±16.3) |
| Days from admission to bacteremia, median (IQR) | 15 | (8–29) |
| Hospital size (beds), n (%) | ||
| <300 | 673 | (10) |
| 300–700 | 2393 | (35.4) |
| >700 | 3686 | (54.6) |
| Department at onset, n (%) | ||
| Medical | 4533 | (67.1) |
| ICU | 943 | (14) |
| Surgical | 1184 | (17.5) |
| Not reported | 92 | (1.4) |
| Organism, n (%) | ||
| Escherichia coli | 1491 | (19.8) |
| Klebsiella pneumoniae | 1659 | (22.1) |
| Staphylococcus aureus | 1315 | (17.5) |
| Pseudomonas aeruginosa | 1175 | (15.6) |
| Enterococcus faecalis | 778 | (10.4) |
| Enterococcus faecium | 405 | (5.4) |
| Acinetobacter baumannii | 654 | (8.7) |
| Streptococcus pneumoniae | 43 | (0.6) |
| Events with ≥2 sentinel organisms isolated within 5 days, n (%) | 675 | (10) |
| Events with antimicrobial resistant organisms a, n (%) | 2984 | (44.2) |
| Category | n | BSI per 1000 Admissions (95% CI) | BSI per 1000 Admissions at Risk a (95% CI) | BSI per 10,000 Patient-Days (95% CI) | BSI per 10,000 Patient-Days at Risk b (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| All events | 6752 | 2.84 | (2.77–2.91) | 8.66 | (8.45–8.86) | 6.88 | (6.72–7.05) | 9.63 | (6.72–7.05) | |
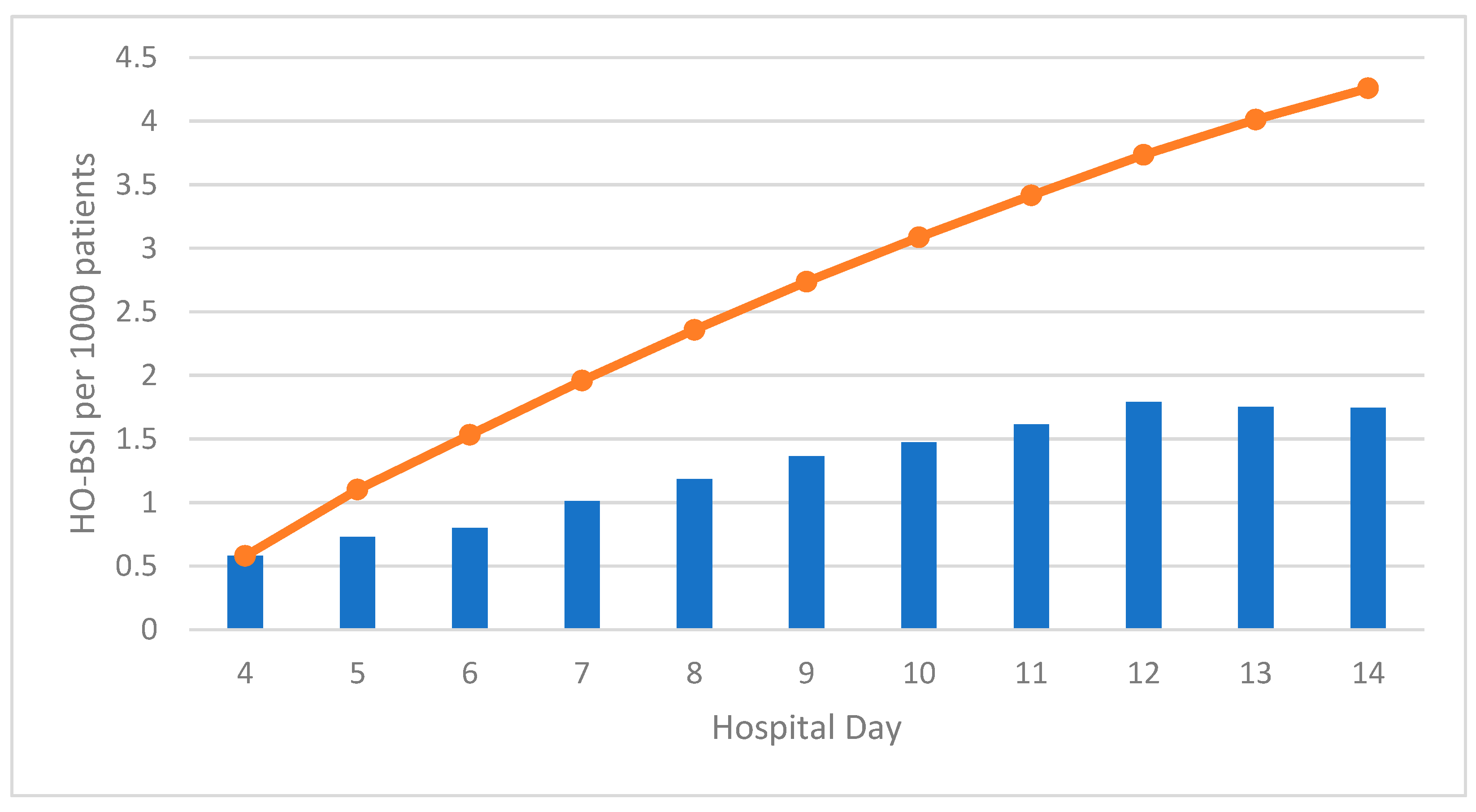
| Hospital day of onset | 4–7 | 1528 | 1.96 | (1.86–2.06) | Not calculated (denominator data not available) | 2.18 | (2.07–2.29) | Not calculated (denominator data not available) | ||
| 8–14 | 1793 | 6.84 | (6.53–7.16) | 4.06 | (3.87–4.25) | |||||
| ≥15 | 3431 | 35.04 | (33.89–36.19) | 12.43 | (12.01–12.84) | |||||
| Hospital size (beds) | <300 | 673 | 1.61 | (1.49–1.73) | 5.66 | (5.23-6.08) | 4.64 | (4.29–4.99) | 7.31 | (4.29–4.99) |
| 300–700 | 2393 | 2.71 | (2.6–2.81) | 7.96 | (7.64-8.28) | 6.61 | (6.34–6.87) | 9.25 | (6.34–6.87) | |
| >700 | 3686 | 3.43 | (3.32–3.55) | 10.23 | (9.9–10.56) | 7.78 | (7.53–8.03) | 10.53 | (7.53–8.03) | |
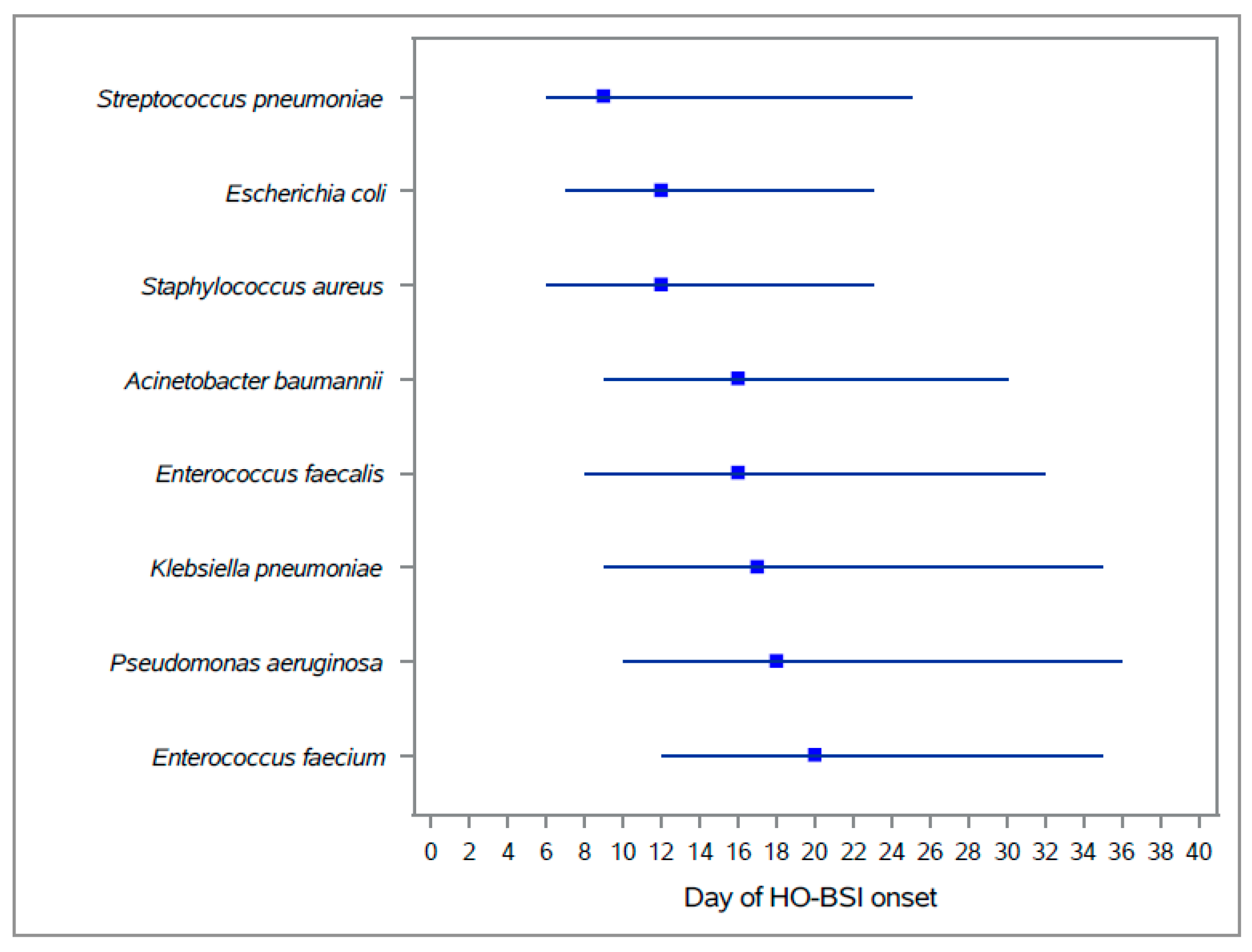
| Organism | Escherichia coli | 1491 | 0.63 | (0.6–0.66) | 1.91 | (1.81–2.01) | 1.52 | (1.44–1.6) | 2.13 | (1.44–1.6) |
| Klebsiella pneumoniae | 1659 | 0.70 | (0.66–0.73) | 2.13 | (2.03–2.23) | 1.69 | (1.61–1.77) | 2.37 | (1.61–1.77) | |
| Staphylococcus aureus | 1315 | 0.55 | (0.52–0.58) | 1.69 | (1.6–1.78) | 1.34 | (1.27–1.41) | 1.88 | (1.27–1.41) | |
| Pseudomonas aeruginosa | 1175 | 0.49 | (0.47–0.52) | 1.51 | (1.42–1.59) | 1.20 | (1.13–1.27) | 1.68 | (1.13–1.27) | |
| Enterococcus faecalis | 778 | 0.33 | (0.3–0.35) | 1.00 | (0.93–1.07) | 0.79 | (0.74–0.85) | 1.11 | (0.74–0.85) | |
| Enterococcus faecium | 405 | 0.17 | (0.15–0.19) | 0.52 | (0.47–0.57) | 0.41 | (0.37–0.45) | 0.58 | (0.37–0.45) | |
| Acinetobacter baumannii | 654 | 0.28 | (0.25–0.3) | 0.84 | (0.77–0.9) | 0.67 | (0.62–0.72) | 0.93 | (0.62–0.72) | |
| Streptococcus pneumoniae | 43 | 0.02 | (0.01–0.02) | 0.06 | (0.04–0.07) | 0.04 | (0.03–0.06) | 0.06 | (0.03–0.06) | |
| Events with antimicrobial resistant organisms c | 2984 | 1.26 | (1.21–1.3) | 3.83 | (3.69–3.96) | 3.04 | (2.93–3.15) | 4.26 | (2.93–3.15) | |
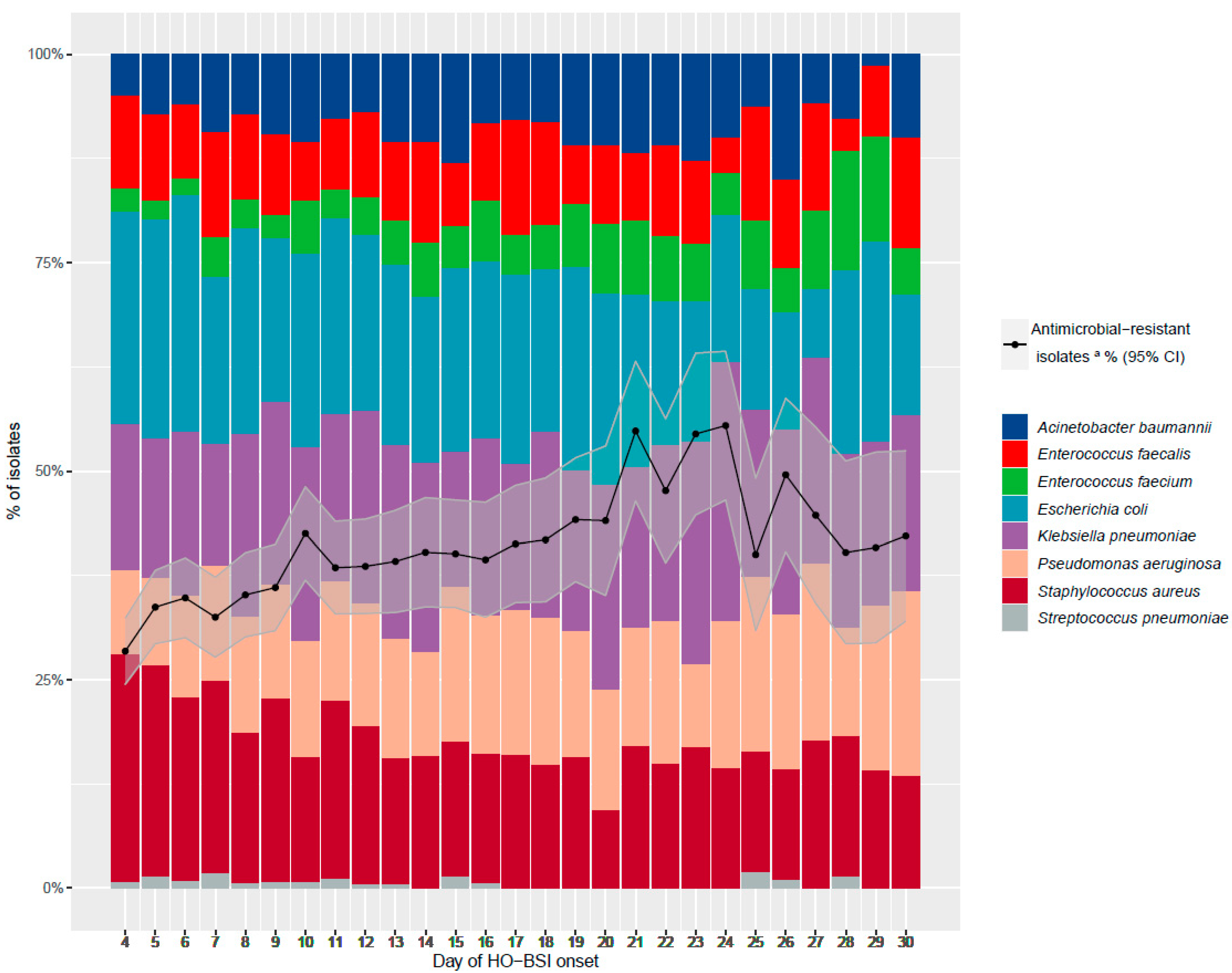
| Organism | Week 1 | Week 2 | Week 3 | Week 4+ | ||||
|---|---|---|---|---|---|---|---|---|
| % of Total | % AMR | % of Total | % AMR | % of Total | % AMR | % of Total | % AMR | |
| Escherichia coli | 25.09 | 41.39 | 21.68 | 39.39 | 21.17 | 47.77 | 14.66 | 50.63 |
| Klebsiella pneumoniae | 17.17 | 56.29 | 22.49 | 58.88 | 19.79 | 67.97 | 25.74 | 72.02 |
| Staphylococcus aureus | 24.79 | 21.31 | 18.24 | 34.35 | 15.42 | 51.11 | 13.33 | 60.66 |
| Pseudomonas aeruginosa | 11.46 | 12.04 | 13.85 | 12.41 | 16.80 | 14.29 | 18.98 | 26.46 |
| Enterococcus faecalis | 10.62 | 0 | 9.55 | 0.53 | 9.51 | 0 | 11.12 | 0 |
| Enterococcus faecium | 2.94 | 14.29 | 4.50 | 20.22 | 6.68 | 11.54 | 6.98 | 21.69 |
| Acinetobacter baumannii | 6.84 | 71.93 | 9.15 | 85.08 | 10.37 | 86.78 | 8.79 | 89.92 |
| Streptococcus pneumoniae | 1.08 | 0 | 0.56 | 0 | 0.26 | 0 | 0.41 | 0 |
| Category | N a | 14-Day Mortality % (95% CI) | 30-Day Mortality % (95% CI) | N a | 1-Year Mortality % (95% CI) | Adjusted b OR for Death within 30 Days (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| All events | 5907 | 30.6 | (28.5–32.75) | 40.2 | (38.18–42.13) | 3839 | 66.5 | (64.67–68.33) | ||
| Sex | Male | 3337 | 31.2 | (28.35–33.98) | 41.2 | (38.57–43.78) | 2164 | 66.9 | (64.49–69.34) | 1.1 (0.99–1.22) |
| Female | 2543 | 29.8 | (26.55–33.06) | 38.7 | (35.65–41.74) | 1648 | 65.9 | (63.08–68.72) | Reference | |
| Age (years) | 18–44 | 528 | 14.2 | (6.3–22.11) | 19.9 | (12.25–27.52) | 347 | 39.5 | (31.3–47.67) | |
| 45–64 | 1385 | 24.8 | (20.27–29.4) | 33.3 | (28.98–37.59) | 879 | 58.6 | (54.34–62.84) | ||
| 65–79 | 2285 | 30.7 | (27.26–34.09) | 39.9 | (36.69–43.05) | 1489 | 66.8 | (63.83–69.68) | ||
| ≥80 | 1709 | 40.3 | (36.65–43.98) | 52.4 | (49.1–55.64) | 1124 | 80.7 | (78.13–83.26) | ||
| Age, as continuous variable | 1.03 (1.02–1.03) | |||||||||
| Hospital day of onset | 4–7 | 1362 | 27.4 | (22.86–31.91) | 35.9 | (31.65–40.16) | 898 | 56.7 | (52.38–60.99) | Reference |
| 8–14 | 1577 | 31.1 | (27.04–35.23) | 39.7 | (35.86–43.53) | 998 | 65.5 | (61.89–69.17) | ||
| ≥15 | 2968 | 31.8 | (28.87–34.81) | 42.4 | (39.62–45.08) | 1943 | 71.5 | (69.17–73.91) | 1.1 (0.99–1.23) | |
| Hospital size (beds) | <300 | 588 | 32.3 | (25.66–38.96) | 42.0 | (35.85–48.16) | 375 | 72.3 | (66.94–77.6) | Non-significant b |
| 300–700 | 1780 | 30.4 | (26.58–34.32) | 39.8 | (36.17–43.38) | 1162 | 66.7 | (63.38–70.01) | ||
| >700 | 3539 | 30.4 | (27.68–33.18) | 40.0 | (37.49–42.59) | 2302 | 65.5 | (63.06–67.87) | ||
| Department | Medical | 4028 | 33.8 | (31.25–36.28) | 44.4 | (42.06–46.67) | 2672 | 72.8 | (70.85–74.81) | Reference |
| ICU | 816 | 36.2 | (30.67–41.63) | 45.5 | (40.4–50.53) | 526 | 64.1 | (58.95–69.19) | 1.24 (1.07–1.45) | |
| Surgical | 1056 | 14.6 | (9.01–20.16) | 20.3 | (14.88–25.65) | 634 | 42.6 | (36.69–48.48) | 0.34 (0.29–0.4) | |
| Organism | Escherichia coli | 1280 | 23.3 | (18.48–28.08) | 32.0 | (27.51–36.55) | 794 | 56.2 | (51.57–60.78) | Reference |
| Klebsiella pneumoniae | 1403 | 28.6 | (24.16–33) | 38.6 | (34.53–42.73) | 880 | 68.2 | (64.45–71.91) | 1.14 (0.96–1.35) | |
| Staphylococcus aureus | 1171 | 30.0 | (25.18–34.77) | 40.2 | (35.79–44.65) | 774 | 65.0 | (60.82–69.16) | 1.29 (1.08–1.53) | |
| Pseudomonas aeruginosa | 1043 | 31.1 | (26.03-36.1) | 39.8 | (35.08–44.5) | 704 | 67.0 | (62.8–71.29) | 1.42 (1.18–1.71) | |
| Enterococcus faecalis | 695 | 29.4 | (23.1–35.6) | 41.0 | (35.3–46.72) | 450 | 73.3 | (68.56–78.1) | 1.42 (1.16–1.75) | |
| Enterococcus faecium | 340 | 38.2 | (29.88–46.59) | 50.6 | (43.12–58.06) | 216 | 74.1 | (67.28–80.86) | 2.16 (1.66–2.79) | |
| Acinetobacter baumannii | 605 | 55.2 | (49.87–60.54) | 64.6 | (59.89–69.37) | 425 | 83.1 | (79.15–86.97) | 2.85 (2.3–3.55) | |
| Streptococcus pneumoniae | 38 | 42.1 | (17.91–66.3) | 50.0 | (27.52–72.48) | 28 | 64.3 | (42.15–86.42) | 2.36 (1.21–4.59) | |
| Events with ≥2 sentinel organisms isolated within 5 days | No | 5318 | 30.0 | (27.74–32.24) | 39.3 | (37.19–41.38) | 3453 | 65.7 | (63.76–67.66) | Reference |
| Yes | 589 | 36.3 | (29.89–42.78) | 48.0 | (42.23–53.87) | 386 | 73.6 | (68.45-78.7) | 1.34 (1.17–1.53) | |
| Events with antimicrobial resistant organisms c | No | 3338 | 25.3 | (22.35–28.22) | 34.1 | (31.34–36.85) | 2132 | 59.3 | (56.58–62) | Reference |
| Yes | 2569 | 37.6 | (34.51–40.62) | 48.0 | (45.25–50.82) | 1707 | 75.5 | (73.17–77.86) | 1.57 (1.4–1.77) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nutman, A.; Wullfhart, L.; Temkin, E.; Feldman, S.F.; Schechner, V.; Schwaber, M.J.; Carmeli, Y. Hospital-Onset Bloodstream Infections Caused by Eight Sentinel Bacteria: A Nationwide Study in Israel, 2018–2019. Microorganisms 2022, 10, 1009. https://doi.org/10.3390/microorganisms10051009
Nutman A, Wullfhart L, Temkin E, Feldman SF, Schechner V, Schwaber MJ, Carmeli Y. Hospital-Onset Bloodstream Infections Caused by Eight Sentinel Bacteria: A Nationwide Study in Israel, 2018–2019. Microorganisms. 2022; 10(5):1009. https://doi.org/10.3390/microorganisms10051009
Chicago/Turabian StyleNutman, Amir, Liat Wullfhart, Elizabeth Temkin, Sarah F. Feldman, Vered Schechner, Mitchell J. Schwaber, and Yehuda Carmeli. 2022. "Hospital-Onset Bloodstream Infections Caused by Eight Sentinel Bacteria: A Nationwide Study in Israel, 2018–2019" Microorganisms 10, no. 5: 1009. https://doi.org/10.3390/microorganisms10051009
APA StyleNutman, A., Wullfhart, L., Temkin, E., Feldman, S. F., Schechner, V., Schwaber, M. J., & Carmeli, Y. (2022). Hospital-Onset Bloodstream Infections Caused by Eight Sentinel Bacteria: A Nationwide Study in Israel, 2018–2019. Microorganisms, 10(5), 1009. https://doi.org/10.3390/microorganisms10051009






