Abstract
This study evaluated the potential of Aspergillus niger as an inoculant for growth promotion of vegetable seedlings. Seven vegetable species were evaluated in independent experiments carried out in 22 + 1 factorial schemes, with two doses of conidia (102 and 106 per plant) applied in two inoculation methods (seed treatment and in-furrow granular application), plus an uninoculated control. Experiments were carried out in a greenhouse. Growth parameters evaluated were shoot length, stem diameter, root volume, total root length, shoot and root fresh mass, shoot and root dry mass, and total dry mass. Regardless of the dose and inoculation method, seedlings inoculated with A. niger showed higher growth than uninoculated ones for all crops. The highest relative increase promoted by the fungus was observed for aboveground parts, increasing the production of shoot fresh mass of lettuce (61%), kale (40%), scarlet eggplant (101%), watermelon (38%), melon (16%), pepper (92%), and tomato (42%). Aspergillus niger inoculation also increased seedling root growth of lettuce, pepper, scarlet eggplant, watermelon, and tomato. This research shows that A. niger boosts the growth of all analyzed vegetables, appearing as a promising bio-input for vegetable seedling production.
1. Introduction
The production of seedlings is a critical step in vegetable crops since during the germination and initial development plants are overly sensitive to biotic and abiotic stresses [1,2]. Moreover, the use of high-quality, healthy, and vigorous seedlings is crucial to achieve the crop yield potential. Seedlings represent 14.8% of the production costs of tomato in soilless cultivation [3]. Therefore, commercial seedling production in highly specialized nurseries has become a trend [2].
Vegetable crops require large amounts of phytosanitary products, fertilizers, and water [4]. These practices have caused a progressive decrease in diversity and amount of soil microorganisms [2,5]. Rhizosphere microbiome engineering has been proposed as a way to reinstate beneficial plant-microorganism associations, and thus harness microbial functions in agroecosystems [5,6]. Therefore, inoculation of beneficial microorganisms during seedling production can be a strategy to introduce traits that may improve plant tolerance to biotic and abiotic stresses after transplanting [5,6,7,8]. Furthermore, this approach might allow decreasing the use of external inputs as well as be an option for organic production [9,10,11].
Plant beneficial microorganisms (PBM) can promote plant growth by various mechanisms, including phytohormone production, increasing nutrient availability, enhancing tolerance to salinity and drought, and disease suppression [8,11,12,13,14]. On the other hand, plants can select for different microbial functional traits according to environmental constraints, shaping the associated microbiota through signaling molecules such as indoleacetic acid [15], abscisic acid [16], strigolactones [17], and flavonoids [18]. For example, nutrient availability modulated functional traits recruited by plants so that PBMs showing phytohormone production were favored in rich nutrient soils while phosphate solubilizers predominated in poor nutrient soils [19]. Therefore, PBMs showing multiple mechanisms of plant growth promotion, called multifunctional microorganisms, can help plants to cope with different environmental constraints, representing a promising option for the development of inoculants [8,20]. Fungi are particularly attractive in this regard due to their capacity to produce high amounts of long-lived spores [21], allowing long shelf life of inoculants. Some species of the genera Aspergillus, Penicillium, Pythium, and Trichoderma show multiple plant-growth promotion mechanisms [22,23,24,25], but inoculants with fungi are still limited compared to bacteria [26].
Aspergillus niger v. Tiegh is a multifunctional fungus capable of phosphate solubilization [27,28,29], potassium solubilization [30], and phytohormone production [23,31,32]. Plants fertilized with phosphate solubilized by A. niger showed enhanced growth and P uptake [27,29,33]. Moreover, A. niger promoted the growth of coffee (Coffea arabica L.) and maize (Zea mays L.) seedlings [23,34]. Therefore, we hypothesized that A. niger could promote the growth of vegetable seedlings, enabling the production of seedlings with enhanced root system and aboveground parts. This research aimed at evaluating the growth of seedlings of seven vegetable species inoculated with A. niger at different doses and inoculation methods.
2. Materials and Methods
2.1. Experimental Site
The study was carried out at the Horticultural Experimental Station of the Universidade Federal de Uberlândia (UFU), located in the city of Monte Carmelo, Minas Gerais state, Brazil (18°42’43.19″ S, 47°29’55.8″ W, 873 m altitude). The experiments were performed in a greenhouse covered with 150 µm clear plastic film between March and June 2019. Average temperature and relative humidity in the period were 21.5 °C and 79.4%, respectively [35].
2.2. Experimental Design
The experiments were carried out in 22 + 1 factorial schemes. Treatments consisted in combinations of two inoculation methods (in-furrow granular application and seed treatment) and two doses of conidia (102 and 106 conidia per plant) of A. niger FS1. An additional uninoculated treatment was used as control (Table 1). Seven vegetable crops were evaluated in independent experiments: melon (Cucumis melo L., Cucurbitaceae), watermelon (Citrullus lanatus Thumb. Mansf., Cucurbitaceae), tomato (Solanum lycopersicum L., Solanaceae), pepper (Capsicum annuum L., Solanaceae), scarlet eggplant (Solanum gilo L., Solanaceae), lettuce (Lactuca sativa L., Asteraceae), and kale (Brassica oleracea L. var. acephala, Brassicaceae). Each experiment was set up in a randomized complete block design with eight repetitions, adding up 40 plots. Each plot contained eight plants, adding up 64 plants per treatment.

Table 1.
Combinations of inoculation methods and doses of Aspergillus niger in treatments.
Plants were grown in 128-cell polystyrene trays (27.7 cm3 cell−1) filled with a commercial coconut fiber substrate (Technes, São Paulo, SP, Brazil). Aspergillus niger inoculation was performed at sowing by in-furrow application of one granule of the formulation or by seed treatment (see Section 2.3). Two seeds were sown in each cell, except for Cucurbitaceae species which had just one seed per cell due to their high germinating power. After emergence, seedlings were thinned to one per cell. Seedlings were irrigated daily, and nutrients were supplied weekly by fertigation with 13.4 mL per cell of a solution containing (total amount added per cell): 5.02 µg N, 2.19 µg P, 5.55 µg K, 0.83 µg Mg, 0.083 µg Zn, 0.025 µg B, 0.0083 µg Fe, 0.083 µg Mn, and 0.92 µg S.
Experiments were evaluated after the seedling production period for each vegetable, i.e., 17, 18, 32, 33, 35, 37, and 44 days for watermelon, melon, tomato, lettuce, pepper, kale, and scarlet eggplant, respectively. The variables measured were shoot height, stem diameter, shoot fresh and dry mass, root dry mass, root volume (obtained by water displacement in a graduated test tube), and total root length, measured with the software RootReader2D (v4.3, Robert W. Holley Center for Agriculture & Health, Ithaca, NY, USA) [36]. For lettuce, it was determined the number of leaves instead of stem diameter since this species does not present a well-developed stem.
2.3. Aspergillus niger Inoculum Preparation
The fungus A. niger FS1 was obtained previously from soil under native forest in Viçosa, Minas Gerais state, Brazil (20°46′4.2″ S, 42°52′40.9″ W) [28]. The fungus was maintained on Petri dishes containing potato dextrose agar (PDA, Sigma-Aldrich, Saint Louis, MO, USA) at 30 °C in the dark. Fungal conidia were collected from 10-day old cultures using a Tween 80 0.01% (v/v) solution. The conidial suspension obtained was vacuum filtered through membranes with 0.45 µm pores and the conidia retained on the membranes were dried in a desiccator with silica gel at room temperature (25 °C) for 24 h [34]. The mass of dry conidia contained 4.5 × 107 conidia mg−1 as determined by counting in a Neubauer chamber. Dry conidia were used for producing a granular formulation and seed treatment.
The granular formulation was produced by mixing dry conidia to 26.5 g wheat flour, 3.8 g corn starch, 2.25 g granulated sugar, and 15 mL deionized water to form a homogeneous bulk [34]. The amount of conidia added to the mixture was 11.9 and 119 mg to produce concentrations of 102 and 106 conidia per granule, respectively. These amounts were calculated with respect to the average mass of each granule, which was 22.6 mg granule−1. The bulk was extruded through a noodle-maker fitted with a template of 2 mm diameter holes. The noodles were cut into 2 mm long granules, and then dried in an oven with forced air circulation at 50 °C for 48 h [34].
The conidial suspension for seed treatment was prepared by mixing the dry conidia with sterile water 2 h before sowing. The conidial suspension was pipetted over the seeds in a volume enough to cover them and gently mixed to allow homogeneous distribution of conidia on the seeds. Due to the differences in seed size among the vegetable species, the amount of conidia used to prepare the suspension was adjusted according to the volume of water necessary to cover the seeds, so that the final concentration was 10² or 106 conidia per plant.
2.4. Statistical Analyses
Data were subjected to ANOVA and post hoc comparisons of most interest were performed using the value of the least significant difference between two means at p = 0.05 (LSD 5%), calculated from the standard error of the difference between two means (SED). Multivariate analyses based on all growth parameters were carried out to cluster treatments by a hierarchical method and the Tocher optimization method. Dendrograms were constructed based on the dissimilarity between treatments calculated by Euclidean distance using the package Nbclust for R (RStudio v1.2.5001-3, Boston, MA, USA) [37]. The relative importance of measured variables on the dissimilarity between treatments was calculated as proposed by Singh [38]. Validation of clustering was conducted based on the cophenetic correlation coefficient [39] calculated in the software Genes (v2021.146, Federal University of Viçosa, Viçosa, MG, Brazil) [40].
3. Results
Significant differences (p < 0.05) between inoculated and uninoculated treatments were observed for all vegetable species (Table 2). In inoculated treatments, generally, there was no difference between the inoculation methods and doses of A. niger (Figure 1).

Table 2.
Effect of inoculation method and dose of A. niger FS1 on plant growth parameters of vegetable seedlings.

Figure 1.
Seedlings of (A) lettuce, (B) melon, (C) pepper, (D) scarlet eggplant, (E) watermelon, (F) tomato, and (G) kale inoculated or not with Aspergillus niger FS1. GR02 and GR06: in-furrow granular application at 102 and 106 conidia plant−1, respectively; TS02 and TS06: seed treatment at 102 and 106 conidia plant−1, respectively; UNI: uninoculated control. Scale bars: 2 cm.
Aspergillus niger inoculation increased root fresh mass of pepper, scarlet eggplant, watermelon, and tomato (Table 2). Root dry mass of inoculated lettuce, pepper, and scarlet eggplant was higher than the uninoculated control. Aspergillus niger inoculation also increased total root length of all species, except for melon and watermelon. Total root length was not measured for kale since the software RootReader2D was not able to read the root system due to the high density of fine roots.
Inoculated seedlings showed higher aboveground growth compared to uninoculated ones. Shoot fresh mass and shoot height of all species were higher in inoculated treatments (Table 2). Likewise, all species showed higher shoot dry mass and total dry mass when inoculated with A. niger, except for melon. Stem diameter of all species was higher in inoculated treatments, except for tomato and kale. Inoculation also increased the number of leaves of lettuce.
Cluster analysis distinguished two groups of treatments, one containing the uninoculated control and the other containing the treatments inoculated with A. niger (Figure 2). The only exception was kale, for which the inoculated treatments were further divided into two groups, one with the seed treatment at the dose of 106 conidia plant−1 (TS06) and another with the other inoculated treatments (TS02, GR02 e GR06) (Figure 2G). Cophenetic correlation coefficients confirmed the accuracy of the clustering, showing values higher than 0.8 [41] for all species: lettuce (0.97), melon (0.94), pepper (0.99), scarlet eggplant (0.99), watermelon (0.98), tomato (0.97), and kale (0.98), all significant by the t test (p < 0.01). Likewise, Tocher optimization method showed high similarity between inoculated treatments (GR02, GR06, TS02 e TS06) and high dissimilarity of these with the uninoculated control for all vegetable crops (Figure 3).
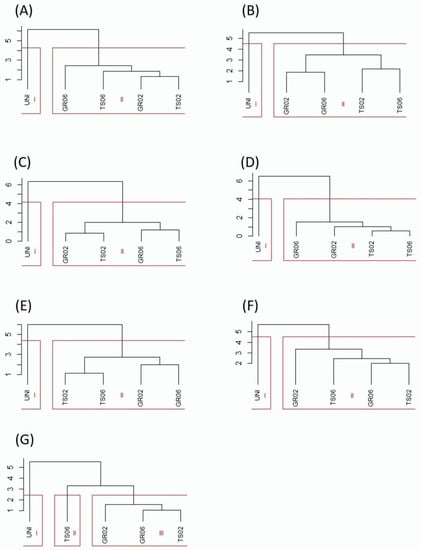
Figure 2.
Clustering of treatments with different inoculation methods and doses of Aspergillus niger FS1 in vegetables: (A) lettuce, (B) melon, (C) pepper, (D) scarlet eggplant, (E) watermelon, (F) tomato, and (G) kale. The vertical axis represents the dissimilarity (%) between groups. GR02 and GR06: in-furrow granular application at 102 and 106 conidia plant−1, respectively; TS02 and TS06: seed treatment at 102 and 106 conidia plant−1, respectively; UNI: uninoculated control.
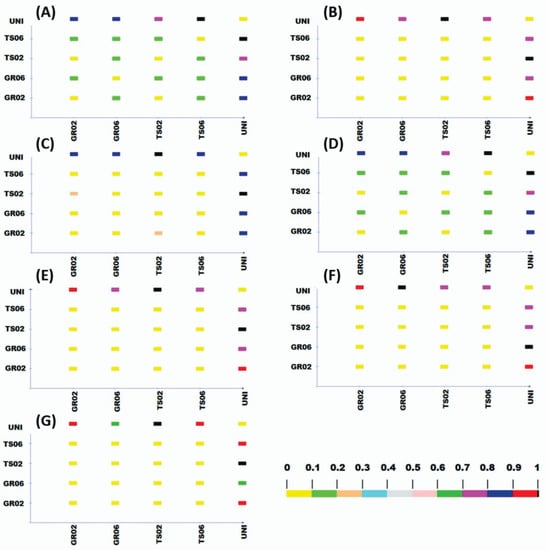
Figure 3.
Dissimilarity between treatments with different inoculation methods and doses of Aspergillus niger FS1 in vegetables: (A) lettuce, (B) melon, (C) pepper, (D) scarlet eggplant, (E) watermelon, (F) tomato, and (G) kale. Color scale determined by Tocher optimization method varies from no dissimilarity (yellow) to complete dissimilarity (black) between each pair of treatments. GR02 and GR06: in-furrow granular application at 102 and 106 conidia plant−1, respectively; TS02 and TS06: seed treatment at 102 and 106 conidia plant−1, respectively; UNI: uninoculated control.
The relative importance analysis showed that variables associated with the aboveground growth of seedlings were the main responsible for the dissimilarity observed between treatments (Table 3). Aboveground parameters (shoot fresh mass, shoot height, stem diameter, and shoot dry mass) summed up to 70.3, 78.3, 93.4, 72.2, 90.1, 72.1, and 82.3% of the dissimilarity observed between treatments in the experiments with lettuce, tomato, kale, scarlet eggplant, watermelon, melon, and pepper, respectively (Table 3).

Table 3.
Relative importance (%) of measured variables on the dissimilarity between treatments for each vegetable crop.
4. Discussion
Aspergillus niger inoculation promoted the growth of seedlings of all vegetable crops tested, increasing the shoot fresh mass of lettuce (61%), kale (40%), scarlet eggplant (101%), watermelon (38%), melon (16%), pepper (92%), and tomato (42%). Inoculation also enhanced root growth of lettuce, pepper, scarlet eggplant, watermelon, and tomato. The capacity of A. niger to produce indoleacetic acid (IAA) and gibberellic acid (GA) has been demonstrated [23,31,32]. These phytohormones regulate cell growth and elongation in plants [42] and thus may be related to the greater growth observed in inoculated seedlings. Microbial production of phytohormones has been repeatedly reported as an important mechanism of plant growth promotion [12,43,44,45,46]. Plant growth-promoting bacteria modified the architecture and functioning of tomato roots due to the production of phytohormones and other metabolites [47,48]. Volatile organic compounds (VOCs) have also been proposed as a mechanism of microbial plant growth promotion. Exposure to VOCs produced by Trichoderma asperellum increased the number of leaves and roots, plant biomass and chlorophyll content in lettuce [25]. Some VOCs were detected in A. niger cultures [49,50,51], however the potential role of these compounds on plant growth was not studied yet.
Aspergillus niger was effective at the different doses of conidia (102 and 106 conidia plant−1). Conidial germination in A. niger is very efficient, with more than 90% of conidia germinating at suitable conditions [52]. This allows efficient colonization even at low numbers of conidia, which might explain the similarity between the doses of conidia evaluated. Likewise, the inoculation methods (in-furrow granular application and seed treatment) were equally efficient to deliver the fungus to the plant root. While the granular formulation contained organic substrates that could be used by the fungus during its establishment [53], in the seed treatment there was nothing other than water. Thus, our data suggest that the fungus derived organic C from the seedling root exudates or from the substrate. Taken together, these fungal traits can make the inoculation step simpler and more economic, enabling the use of water dispersible formulations, such as a wettable powder, at low doses of conidia.
The effect of A. niger inoculation was more pronounced on aboveground parts of seedlings. Shoot mass and height of inoculated seedlings were higher than uninoculated ones for all vegetable crops tested. Aboveground growth parameters showed relative importance varying from 70.3 to 82.3% of the dissimilarity observed between treatments. GA promotes leaf expansion and stem growth and elongation [42,54], and could be involved in the enhanced growth of aboveground parts promoted by A. niger. Furthermore, since inoculated seedlings grew more and hence faster, inoculation might be used to reduce seedling production time and thus reducing costs. Moreover, inoculation in nursery can be an easy, economic, and efficient way to introduce the fungus into the field [6]. Once established in the seedling rhizosphere, A. niger would have a competitive advantage over native microbiota in the field and thus the growth promotion effect could be extended throughout the crop cycle [34].
Roots are highly responsive to fluctuations in IAA levels, which control the growth of primary and secondary roots as well as the development of adventitious roots [46]. IAA production is a trait frequently showed by plant growth-promoting microorganisms [12,19,44,46], including A. niger [23,32]. Therefore, enhanced root growth is a common effect of plant growth-promoting microorganisms [34,55,56,57]. Indeed, A. niger inoculation increased seedling root growth of lettuce, pepper, scarlet eggplant, watermelon, and tomato. Seedlings with well-developed root system can explore a higher volume of soil and hence be more efficient in reach nutrients and water in the field [2]. It should be mentioned that seedlings were exposed to root air pruning in the tray [58] and, therefore, root growth was probably underestimated. This could be the reason for the lower effects observed in roots compared to aboveground parts.
This research provides evidence of the potential of A. niger as a biofertilizer for vegetable crops. Aspergillus niger enhanced the growth of seedlings of seven species of vegetables belonging to four different botanical families—Cucurbitaceae, Solanaceae, Asteraceae, and Brassicaceae. These results suggest that A. niger has no specificity towards the host plant, in line with other reports showing growth promotion of coffee (Coffea arabica L.) [34] and maize (Zea mays L.) [23] seedlings by A. niger. This represents an advantage for the development of products with this fungus. Moreover, plants inoculated with A. niger would benefit from multiple mechanisms of plant growth promotion, such as phytohormone production [23,31,32] and solubilization of phosphate [27,28,29] and potassium [30]. Microorganisms showing these multifunctional traits may be able to help plants to cope with different environmental stresses [6,8,19,20]. Finally, the use of bio-inputs, such as the one described herein, can contribute to reducing costs in seedling production as well as increase crop productivity sustainably.
Author Contributions
Conceptualization, G.d.S.M.M., G.M.M. and G.d.O.M.; methodology, G.d.S.M.M., G.M.M. and G.d.O.M.; investigation, G.d.S.M.M.; resources, G.M.M. and G.d.O.M.; writing—original draft preparation, G.d.S.M.M.; writing—review and editing, G.d.S.M.M., G.M.M. and G.d.O.M.; supervision, G.M.M. and G.d.O.M.; project administration, G.d.O.M.; funding acquisition, G.d.O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), grant number APQ-01842-17, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant number 001, and NOOA Ciência e Tecnologia Agrícola Ltda, grant number 012/2017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset generated during the current study is available on the Mendeley Data Repository, DOI: 10.17632/nh6pzmb4tf.1.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Carmona, E.; Moreno, M.T.; Avilés, M.; Ordovás, J. Use of grape marc compost as substrate for vegetable seedlings. Sci. Hortic. 2012, 137, 69–74. [Google Scholar] [CrossRef]
- Balliu, A.; MarŠić, N.K.; Gruda, N. Seedling production. In Good Agricultural Practices for Greenhouse Vegetable Production in the South East European Countries—Principles for Sustainable Intensification of Smallholder Farms; Baudoin, W., Nersisyan, A., Shamilov, A., Hodder, A., Gutierrez, D., De Pascale, S., Nicola, S., Gruda, N., Urban, L., Tany, J., Eds.; FAO: Rome, Italy, 2017; pp. 189–206. ISBN 9789251096222. [Google Scholar]
- Popsimonova, G.; Benko, B.; Karicc, L.; Gruda, N. Production systems: Integrated and organic production, and soilless culture. In Good Agricultural Practices for Greenhouse Vegetable Production in the South East European Countries—Principles for Sustainable Intensification of Smallholder Farms; Baudoin, W., Nersisyan, A., Shamilov, A., Hodder, A., Gutierrez, D., De Pascale, S., Nicola, S., Gruda, N., Urban, L., Tany, J., Eds.; FAO: Rome, Italy, 2017; pp. 207–226. ISBN 9789251096222. [Google Scholar]
- Martin-Gorriz, B.; Gallego-Elvira, B.; Martínez-Alvarez, V.; Maestre-Valero, J.F. Life cycle assessment of fruit and vegetable production in the Region of Murcia (south-east Spain) and evaluation of impact mitigation practices. J. Clean. Prod. 2020, 265, 121656. [Google Scholar] [CrossRef]
- Pérez-Jaramillo, J.E.; Mendes, R.; Raaijmakers, J.M. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant. Mol. Biol. 2016, 90, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Toju, H.; Peay, K.G.; Yamamichi, M.; Narisawa, K.; Hiruma, K.; Naito, K.; Fukuda, S.; Ushio, M.; Nakaoka, S.; Onoda, Y.; et al. Core microbiomes for sustainable agroecosystems. Nat. Plants 2018, 4, 247–257. [Google Scholar] [CrossRef]
- Hussain, S.S.; Mehnaz, S.; Siddique, K.H.M. Harnessing the plant microbiome for improved abiotic stress tolerance. In Plant Microbiome: Stress Response; Microorganisms for Sustainability; Egamberdieva, D., Ahmad, P., Eds.; Springer: Singapore, 2018; Volume 5, pp. 21–43. ISBN 978-981-10-5513-3. [Google Scholar]
- Vassileva, M.; Flor-Peregrin, E.; Malusá, E.; Vassilev, N. Towards better understanding of the interactions and efficient application of plant beneficial prebiotics, probiotics, postbiotics and synbiotics. Front. Plant. Sci. 2020, 11, 1068. [Google Scholar] [CrossRef]
- Meemken, E.M.; Qaim, M. Organic agriculture, food security, and the environment. Annu. Rev. Resour. Econ. 2018, 10, 39–63. [Google Scholar] [CrossRef] [Green Version]
- Seufert, V.; Ramankutty, N.; Mayerhofer, T. What is this thing called organic?—How organic farming is codified in regulations. Food Policy 2017, 68, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, S.M.; Naveed, M.; Zahir, Z.A.; Asghar, H.N. Plant-microbe interactions for sustainable agriculture: Fundamentals and recent advances. In Plant Microbe Symbiosis: Fundamentals and Advances; Arora, N.K., Ed.; Springer: New Delhi, India, 2013; pp. 51–103. ISBN 978-81-322-1287-4. [Google Scholar]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant. Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, A.; Srivastava, P.; Choudhary, K.K.; Dikshit, A. Plant growth-promoting microorganisms in sustainable agriculture. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Kumar, A., Singh, A.K., Choudhary, K.K., Eds.; Elsevier: Duxford, UK, 2019; pp. 1–19. ISBN 9780128170045. [Google Scholar]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Yang, S.; Meng, L.; Wang, B.-G. The plant hormone abscisic acid regulates the growth and metabolism of endophytic fungus Aspergillus nidulans. Sci. Rep. 2018, 8, 6504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochange, S.; Goormachtig, S.; Lopez-Raez, J.A.; Gutjahr, C. The role of strigolactones in plant–microbe interactions. In Strigolactones—Biology and Applications; Koltai, H., Prandi, C., Eds.; Springer: Cham, Switzerland, 2019; pp. 121–142. [Google Scholar]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, P.B.; van Elsas, J.D.; Mallon, C.; Borges, L.G.d.A.; Passaglia, L.M.P. Efficiency of probiotic traits in plant inoculation is determined by environmental constrains. Soil Biol. Biochem. 2020, 148, 107893. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Lopez, A.; Martos, V.; Reyes, A.; Maksimovic, I.; Eichler-Löbermann, B.; Malusà, E. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 2015, 99, 4983–4996. [Google Scholar] [CrossRef] [PubMed]
- Sussman, A.S. Longevity and survivability of fungi. In The Fungi—An Advanced Treatise; Ainsworth, G.C., Sussman, A.S., Eds.; Academic Press: New York, NY, USA, 1968; pp. 447–486. [Google Scholar]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant growth-promoting fungi (PGPF): Phytostimulation and induced systemic resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Singapore, 2017; Volume 2, pp. 135–191. ISBN 9789811065934. [Google Scholar]
- Lubna; Asaf, S.; Hamayun, M.; Gul, H.; Lee, I.-J.; Hussain, A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J. Plant. Interact. 2018, 13, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, N.; le Floch, G.; Vallance, J.; Gerbore, J.; Grizard, D.; Rey, P. Pythium oligandrum: An example of opportunistic success. Microbiology 2012, 158, 2679–2694. [Google Scholar] [CrossRef]
- Wonglom, P.; Ito, S.-i.; Sunpapao, A. Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum T1 mediate antifungal activity, defense response and promote plant growth in lettuce (Lactuca sativa). Fungal Ecol. 2020, 43, 100867. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.O.; da Silva, N.M.R.M.; Anastácio, T.C.; Vassilev, N.B.; Ribeiro, J.I.; da Silva, I.R.; Costa, M.D. Optimization of Aspergillus niger rock phosphate solubilization in solid-state fermentation and use of the resulting product as a P fertilizer. Microb. Biotechnol. 2015, 8, 930–939. [Google Scholar] [CrossRef]
- Mendes, G.O.; Freitas, A.L.M.; Pereira, O.L.; Silva, I.R.; Vassilev, N.B.; Costa, M.D. Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources. Ann. Microbiol. 2014, 64, 239–249. [Google Scholar] [CrossRef]
- Mendes, G.O.; Galvez, A.; Vassileva, M.; Vassilev, N. Fermentation liquid containing microbially solubilized P significantly improved plant growth and P uptake in both soil and soilless experiments. Appl. Soil Ecol. 2017, 117–118, 208–211. [Google Scholar] [CrossRef]
- Lopes-Assad, M.L.; Avansini, S.H.; Rosa, M.M.; de Carvalho, J.R.P.; Ceccato-Antonini, S.R.; Carvalho, J.R.P.; Antonini, S.R.C.; de Carvalho, J.R.P.; Ceccato-Antonini, S.R. The solubilization of potassium-bearing rock powder by Aspergillus niger in small-scale batch fermentations. Can. J. Microbiol. 2010, 56, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Cihangir, N. Stimulation of the gibberellic acid synthesis by Aspergillus niger in submerged culture using a precursor. World J. Microbiol. Biotechnol. 2002, 18, 727–739. [Google Scholar] [CrossRef]
- Seyis Bilkay, I.; Karakoç, Ş.; Aksöz, N. Indole-3-acetic acid and gibberellic acid production in Aspergillus niger. Turk. J. Biol. 2010, 34, 313–318. [Google Scholar] [CrossRef]
- Vassilev, N.; Franco, I.; Vassileva, M.; Azcon, R. Improved plant growth with rock phosphate solubilized by Aspergillus niger grown on sugar-beet waste. Bioresour. Technol. 1996, 55, 237–241. [Google Scholar] [CrossRef]
- Araújo, V.C.; Rossati, K.F.; Xavier, L.V.; de Oliveira, V.A.; Carmo, G.J.d.S.; de Assis, G.A.; Mendes, G.d.O. Enhanced growth in nursery of coffee seedlings inoculated with the rhizosphere fungus Aspergillus niger for field transplantation. Rhizosphere 2020, 15, 100236. [Google Scholar] [CrossRef]
- SISMET Sistema de Monitoramento Meteorológico—Cooxupé (Monte Carmelo). Available online: http://sismet.cooxupe.com.br:9000/ (accessed on 20 February 2022).
- Clark, R.T.; Famoso, A.N.; Zhao, K.; Shaff, J.E.; Craft, E.J.; Bustamante, C.D.; Mccouch, S.R.; Aneshansley, D.J.; Kochian, L.V. High-throughput two-dimensional root system phenotyping platform facilitates genetic analysis of root growth and development. Plant. Cell Environ. 2013, 36, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. Nbclust: An R package for determining the relevant number of clusters in a data set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Singh, D. The relative importance of characters affecting genetic divergence. Indian J. Genet. Plant. Breed. 1981, 41, 237–245. [Google Scholar]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Cruz, C.D. Genes: A software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 2013, 35, 271–276. [Google Scholar] [CrossRef]
- Cruz, C.D.; Regazzi, A.J.; Carneiro, P.C.S. Modelos Biométrico Aplicados ao Melhoramento Genético; UFV: Viçosa, Brazil, 2012. [Google Scholar]
- Cleland, R.E. Introduction: Nature, occurrence and functioning of plant hormones. In Biochemistry and Molecular Biology of Plant Hormones; Hooykaas, P.J.J., Hall, M.A., Libbenga, K.R., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1999; Volume 33, pp. 3–22. ISBN 0 444 89825 5. [Google Scholar]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Spaepen, S. Plant hormones produced by microbes. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 247–256. ISBN 978-3-319-08574-6. [Google Scholar]
- Afzal Khan, S.; Hamayun, M.; Kim, H.Y.; Yoon, H.J.; Lee, I.J.; Kim, J.G. Gibberellin production and plant growth promotion by a newly isolated strain of Gliomastix murorum. World J. Microbiol. Biotechnol. 2009, 25, 829–833. [Google Scholar] [CrossRef]
- Fu, S.-F.; Wei, J.-Y.; Chen, H.-W.; Liu, Y.-Y.; Lu, H.-Y.; Chou, J.-Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant. Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef] [Green Version]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Miras-Moreno, B.; Lucini, L.; Valentinuzzi, F.; Mimmo, T.; Pii, Y.; Cesco, S.; Rodrigues, E.P.; Oliveira, A.L.M. de Inoculation with plant growth-promoting bacteria alters the rhizosphere functioning of tomato plants. Appl. Soil Ecol. 2021, 158, 103784. [Google Scholar] [CrossRef]
- Takishita, Y.; Charron, J.B.; Smith, D.L. Biocontrol rhizobacterium Pseudomonas sp. 23S induces systemic resistance in Tomato (Solanum lycopersicum L.) against bacterial canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Gao, P.; Korley, F.; Martin, J.; Chen, B.T. Determination of unique microbial volatile organic compounds produced by five Aspergillus species commonly found in problem buildings. Am. Ind. Hyg. Assoc. J. 2002, 63, 135–140. [Google Scholar] [CrossRef]
- Fiedler, K.; Schütz, E.; Geh, S. Detection of microbial volatile organic compounds (MVOCs) produced by moulds on various materials. Int. J. Hyg. Environ. Health 2001, 204, 111–121. [Google Scholar] [CrossRef]
- Lima, M.A.S.; De Oliveira, M.D.C.F.; Pimenta, A.T.Á.; Uchôa, P.K.S. Aspergillus niger: A hundred years of contribution to the natural products chemistry. J. Braz. Chem. Soc. 2019, 30, 2029–2059. [Google Scholar] [CrossRef]
- Abdel-Rahim, A.M.; Arbab, H.A. Factors affecting spore germination in Aspergillus niger. Mycopathologia 1985, 89, 75–79. [Google Scholar] [CrossRef]
- Burges, H.D. Formulation of Microbial Biopesticides—Beneficial Microorganisms, Nematodes and Seed Treatments; Burges, H.D., Ed.; Springer Science+Business Media: Dordrecht, The Netherlands, 1998; ISBN 978-94-010-6066-0. [Google Scholar]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant. Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amprayn, K.O.; Rose, M.T.; Kecskés, M.; Pereg, L.; Nguyen, H.T.; Kennedy, I.R. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl. Soil Ecol. 2012, 61, 295–299. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in arabidopsis. Plant. Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils 2005, 42, 97–108. [Google Scholar] [CrossRef]
- Miranda, S.; Ribeiro, R.; Ricci, M.; Almeida, D. Avaliação de substratos alternativos para produção de mudas de alface em bandejas. Embrapa 1998, 24, 1–6. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).