Setting a Plausible Route for Saline Soil-Based Crop Cultivations by Application of Beneficial Halophyte-Associated Bacteria: A Review
Abstract
:1. Introduction
2. Salinization Effects on Plants
3. Salinity Alleviation Attempts
4. Halophytes and Halotolerant Plant Growth-Promoting Bacteria (HT-PGPB)
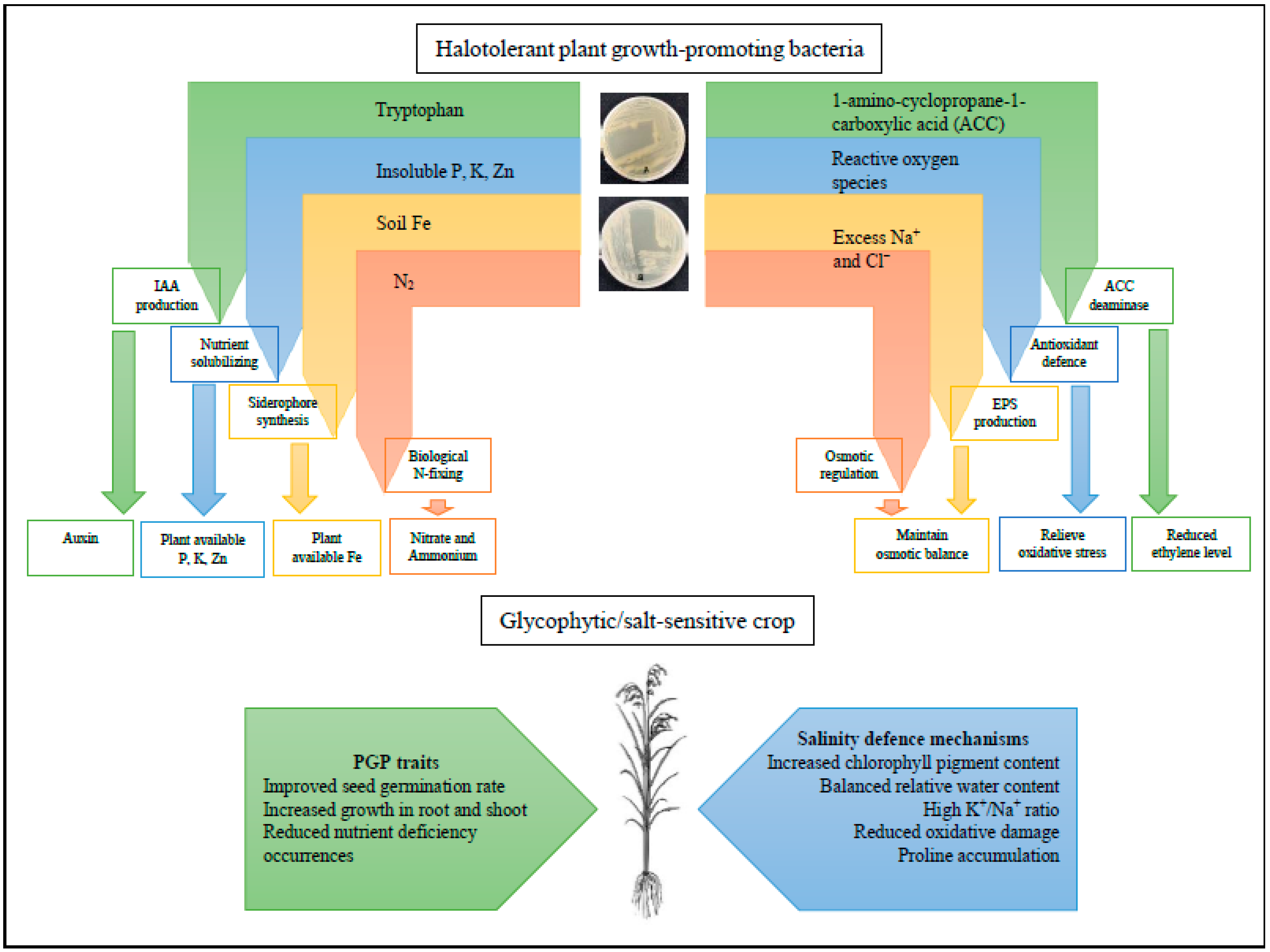
5. Plant Growth-Promoting Mechanisms by HT-PGPB
5.1. HT-PGPB Mediated Soil Nutrient Bio-Availabilities
5.2. HT-PGPB Mediated Indole-3-Acetic Acid (IAA) Production
6. Salinity Mitigating Mechanisms by HT-PGPB
6.1. HT-PGPB Modulations of Stress Ethylene
6.2. HT-PGPB Modulations of Exopolysaccharides
6.3. HT-PGPB Modulation of Antioxidant Defences
6.4. HT-PGPB Modulations of Osmotic Balance Regulation
7. Future Developments and Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schofield, R.V.; Kirkby, M. Application of salinization indicators and initial development of potential global soil salinization scenario under climatic change. Glob. Biogeochem. Cycles 2013, 17, 1–13. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations: Global Soil Partnership. Available online: https://www.fao.org/global-soil-partnership/gsasmap/en (accessed on 7 February 2022).
- Hayat, K.; Bundschuh, J.; Jan, F.; Menhas, S.; Hayat, S.; Haq, F.; Shah, M.A.; Chaudhary, H.J.; Ullah, A.; Zhang, D.; et al. Combating soil salinity with combining saline agriculture and phytomanagement with salt-accumulating plants. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1085–1115. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–53. [Google Scholar] [CrossRef] [Green Version]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Yasin, N.A.; Akram, W.; Khan, W.U.; Ahmad, S.R.; Ahmad, A.; Ali, A. Halotolerant plant growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ. Sci. Pollut. Res. 2018, 25, 236–250. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Can interaction between silicon and non–rhizobial bacteria benefit in improving nodulation and nitrogen fixation in salinity-stressed legumes? A review. Rhizosphere 2020, 15, 100229. [Google Scholar] [CrossRef]
- Golan, Y.; Shirron, N.; Avni, A.; Shmoish, M.; Gepstein, S. Cytokinin induce transcriptional reprograming and improve Arabidopsis plant performance under drought and salt stress conditions. Front. Environ. Sci. 2016, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Druzhinina, I.S.; Labbé, J.; Redman, R.; Qin, Y.; Rodriguez, R.; Zhang, C.; Tuskan, G.A.; Lin, F. Specialized microbiome of a halophyte and its role in helping non-host plants to withstand salinity. Sci. Rep. 2016, 6, 32467. [Google Scholar] [CrossRef]
- Paul, D.; Nair, S. Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J. Basic Microbiol. 2018, 48, 378–384. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; Agriculture Handbook No. 60; Government Printing Office: Washington, DC, USA, 1954; pp. 1–2. [Google Scholar]
- Sha Valli Khan, P.S.; Nagamallaiah, G.V.; Dhanunjay Rao, M.; Sergeant, K.; Hausman, J.F. Abiotic stress tolerance in plants: Insights from proteomics. Emerg. Technol. Manag. Crop Stress Toler. 2014, 2, 23–68. [Google Scholar] [CrossRef]
- Vaishnav, A.; Varma, A.; Tuteja, N.; Choudhary, D.K. PGPR-mediated amelioration of crops under salt stress. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; Volume 10, pp. 205–226. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, A.D.; Ludeke, K.L. Soil Alkalinity. In Adaptations of Desert Organisms; Springer: Berlin/Heidelberg, Germany, 1993; pp. 35–37. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant. Sci. 2018, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Sakamoto, A.; Nishiyama, Y.; Inaba, M.; Murata, N. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 2000, 123, 1047–1056. [Google Scholar] [CrossRef] [Green Version]
- Khush, G.S. What will it take to feed 5.0 billion rice consumers in 2030? Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef]
- Yan, K.; Shao, H.; Shao, C.; Chen, P.; Zhao, S.; Brestic, M.; Chen, X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant 2013, 35, 2867–2878. [Google Scholar] [CrossRef]
- Eraslan, F.; Inal, A.; Gunes, A.; Alpaslan, M. Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci. Hortic. 2007, 113, 120–128. [Google Scholar] [CrossRef]
- Othman, Y.; Al-Karakim, G.; Al-Tawaha, A.R.; Al-Horani, A. Variation in germination and ion uptake in barley genotypes under salinity conditions. World J. Agric. Sci. 2006, 2, 11–15. [Google Scholar]
- Zeng, L.; Shannon, M.C. Effects of salinity on grain yield and yield components of rice at different seeding densities. Agron. J. 2000, 92, 418–423. [Google Scholar] [CrossRef]
- Shereen, A.; Mumtaz, S.; Raza, S.; Khan, M.A.; Solangi, S. Salinity effects on seedling growth and yield components of different inbred rice lines. Pak. J. Bot. 2005, 37, 131–139. [Google Scholar]
- Flowers, T.J.; Yeo, A.R. Variability in the resistance of sodium chloride salinity within rice (Oryza sativa L.) varieties. New Phytol. 1981, 88, 363–373. [Google Scholar] [CrossRef]
- Khatun, S.; Flowers, T.J. Effects of salinity on seed set in rice. Plant Cell Environ. 1995, 18, 61–67. [Google Scholar] [CrossRef]
- Amirjani, M.R. Effect of NaCl stress on rice physiological properties. Arch. Phytopathol. Plant Prot. 2012, 45, 228–243. [Google Scholar] [CrossRef]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China. Evid.-Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genomics 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Oster, J.D. Sodic soil reclamation. In Towards the Rational Use of High Salinity Tolerant Plants; Springer: Berlin/Heidelberg, Germany, 1993; Volume 1, pp. 485–490. [Google Scholar]
- Raychev, T.; Popandova, S.; Jozefaciuk, G.; Hajnos, M.; Sokolowska, Z. Physicochemical reclamation of saline soils using coal powder. Int. Agrophys. 2001, 15, 51–54. [Google Scholar]
- Cucci, G.; Lacolla, G.; Pallara, M.; Laviano, R. Reclamation of saline and saline-sodic soils using gypsum and leaching water. Afr. J. Agric. Res. 2012, 7, 6508–6514. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, A. Biotechnological tools and trends for production and rice quality improvement: Agrobacterium-mediated genetic transformation practices for improvement of rice quality and production. In Rice Science: Biotechnology and Molecular Advancements; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Aziz, A.; Verma, D.K.; Srivastav, P.P.; Nadaf, A.B. Role of genetic engineering and biotechnology as a molecular advance tool and trend in quality improvement of rice crop. In Rice Science: Biotechnology and Molecular Advancements; CRC Press: Boca Raton, FL, USA, 2018; p. 208. [Google Scholar]
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef] [Green Version]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egamberdieva, D.; Lugtenberg, B. Use of Plant Growth-Promoting Rhizobacteria to Alleviate Salinity Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1, pp. 73–96. [Google Scholar] [CrossRef]
- Cheeseman, J.M. The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol. 2015, 206, 557–570. [Google Scholar] [CrossRef]
- Reddy, B.R.; Sethunathan, N. Salinity and the persistence of parathion in flooded soil. Soil Biol. Biochem. 1985, 17, 235–239. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: Berlin/Heidelberg, Germany, 2014; pp. 25–55. [Google Scholar] [CrossRef]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Ball, M.C.; Pidsley, S.M. Growth responses to salinity in relation to distribution of two mangrove species, Sonneratia alba and S. lanceolata, in northern Australia. Funct. Ecol. 1995, 9, 77–85. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Numana, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Menendez, E.; Garcia-Fraile, P. Plant probiotic bacteria: Solutions to feed the world. AIMS Microbiol. 2017, 3, 502–524. [Google Scholar] [CrossRef]
- Kathiresan, K.; Selvam, M.M. Evaluation of beneficial bacteria from mangrove soil. Bot. Mar. 2006, 49, 86–88. [Google Scholar] [CrossRef]
- Kushner, D.J. Growth and nutrition of halophilic bacteria. In The Biology of Halophilic Bacteria; CRC Press: Boca Raton, FL, USA, 1993; pp. 87–89. [Google Scholar]
- Ventosa, A.; Mellado, E.; Sanchez-Porro, C.; Marquez, M.C. Halophilic and Halotolerant Micro-organisms from Soils. Microbiology of Extreme Soils; Springer: Berlin/Heidelberg, Germany, 2008; pp. 87–115. [Google Scholar] [CrossRef]
- Podell, S.; Ugalde, J.A.; Narasingarao, P.; Banfield, J.F.; Heidelberg, K.B.; Allen, E.E. Assembly-driven community genomics of a hypersaline microbial ecosystem. PLoS ONE 2013, 8, e61692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Ahemad, M.; Oves, M. Functional diversity among plant growth-promoting rhizobacteria: Current Status. In Microbial Strategies for Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2009; pp. 105–132. [Google Scholar] [CrossRef]
- Hassan, T.U.; Bano, A.; Naz, I. Halophyte root powder: An alternative biofertilizer and carrier for saline land. In Soil Science and Plant Nutrition; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–9. [Google Scholar] [CrossRef]
- Soldan, R.; Mapellia, F.; Crottia, E.; Schnell, S.; Daffonchio, D.; Marascoc, R.; Fusic, M.; Borina, S.; Cardinale, M. Bacterial endophytes of mangrove propagules elicit early establishment of the natural host and promote growth of cereal crops under salt stress. Microbiol. Res. 2019, 223-225, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Bano, A. Isolation of PGPRS from rhizospheric soil of halophytes and its impact on maize (Zea mays L.) under induced soil salinity. Can. J. Microbiol. 2015, 61, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Rodríguez, M.; Llamas, I.; Béjar, V.; Sampedro, I. Silencing of phytopathogen communication by the halotolerant PGPR Staphylococcus equorum strain EN21. Microorganisms 2020, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerbab, S.; Silini, A.; Bouket, A.C.; Cherif-Silini, H.; Eshelli, M.; Rabhi, N.E.H.; Belbahri, L. Mitigation of NaCl stress in wheat by rhizosphere engineering using salt habitat adapted PGPR halotolerant bacteria. Appl. Sci. 2021, 11, 34. [Google Scholar] [CrossRef]
- Zhou, N.; Zhao, S.; Tian, C.Y. Effect of halotolerant rhizobacteria isolated from halophytes on the growth of sugar beet (Beta vulgaris L.) under salt stress. FEMS Microbiol. Lett. 2017, 364, 1–8. [Google Scholar] [CrossRef]
- Muhammad, B.; Rehman, S.; Rasul, S.; Aslam, K.; Abbas, R.; Athar, H.R.; Manzoor, I.; Muhammad, K.H.; Naqqash, T. Mining of halo-tolerant plant growth promoting rhizobacteria and their impact on wheat (Triticum aestivum L.) under saline conditions. J. King Saud Univ. Sci. 2021, 33, 101372. [Google Scholar] [CrossRef]
- Lu, L.; Chang, M.; Han, X.; Wang, Q.; Wang, J.; Yang, H.; Guan, Q.; Dai, S. Beneficial effects of endophytic Pantoea ananatis with ability to promote rice growth under saline stress. J. Appl. Microbiol. 2021, 131, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M. Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 2020, 15, e238537. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.M.; Kim, K.M.; Lee, I.J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.A.A.; Bettiol, W. Multifaceted intervention of Bacillus spp. against salinity stress and Fusarium wilt in tomato. J. Appl. Microbiol. 2021, 131, 2387–2401. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Jha, D. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Govindasamy, V.; Senthilkumar, M.; Magheshwaran, V.; Kumar, U.; Bose, P.; Sharma, V.; Annapurna, K. Bacillus and Paenibacillus spp.: Potential PGPR for sustainable agriculture. In Plant Growth and Health Promoting Bacteria. Microbiology Monographs; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 333–364. [Google Scholar] [CrossRef]
- Aeron, A.; Kumar, S.; Pandey, P.; Maheshwari, D.K. Emerging role of plant growth promoting rhizobacteria in agrobiology. In Bacteria in Agrobiology: Crop Ecosystems; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–36. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Nawaz, S. Mitigation of salinity induced negative impact on the growth and yield of wheat by plant growth-promoting rhizobacteria in naturally saline conditions. Ann. Microbiol. 2013, 63, 225–232. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharyaa, A.; Vermaa, P.; Mishraa, P.; et al. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Cheng-Song, X.I.N.; He-Zhong, D.; Zhen, L.; Wei, T.; Zhang, D.M.; Wei-Jiang, L.I.; Kong, X.Q. Effects of N, P, and K fertilizer application on cotton growing in saline soil in yellow river delta. Acta Agron. Sin. 2010, 36, 1698–1706. [Google Scholar] [CrossRef]
- Thomine, S.; Lanquar, V. Iron transport and signalling in plants. In Transporters and Pumps in Plant Signalling; Springer: Berlin/Heidelberg, Germany, 2011; Volume 7, pp. 99–131. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Halotolerant plant growth-promoting fungi and bacteria as an alternative strategy for improving nutrient availability to salinity-stressed crop plants. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–146. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safdarian, M.; Askari, H.; Nematzadeh, G.; Sofo, A. Halophile plant growth-promoting rhizobacteria induce salt tolerance traits in wheat seedlings (Triticum aestivum L.). Pedosphere, 2020; 30, 684–693. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Wu, G.H.; Tian, C.Y. Isolation of endophytic plant growth-promoting bacteria associated with the halophyte Salicornia europaea and evaluation of their promoting activity under salt stress. Curr. Microbiol. 2016, 73, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Biswas, D.R.; Marwaha, T.S. Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.): A hydroponics study under phytotron growth chamber. J. Plant Nutr. 2010, 33, 1236–1251. [Google Scholar] [CrossRef]
- Tariq, M.; Hameed, S.; Malik, K.A.; Hafeez, F.Y. Plant root associated bacteria for zinc mobilization in rice. Pak. J. Bot. 2007, 39, 245–253. [Google Scholar]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The natural history of nitrogen fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.E.; Hocking, P.J.; Simpson, R.J.; George, T.S. Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 2009, 60, 124–143. [Google Scholar] [CrossRef]
- Parmar, P.; Sindhu, S.S. Potassium solubilization by rhizosphere bacteria: Influence of nutritional and environmental conditions. J. Microbiol. Res. 2013, 3, 25–31. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Riggs, P.J.; Chelius, M.K.; Iniguez, A.L.; Kaeppler, S.M.; Triplett, E.W. Enhanced maize productivity by inoculation with diazotrophic bacteria. Funct. Plant Biol. 2001, 28, 829–836. [Google Scholar] [CrossRef]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Tairo, E.V.; Ndakidemi, P.A. Possible benefits of rhizobial inoculation and phosphorus supplementation on nutrition, growth and economic sustainability in grain legumes. Am. J. Res. Commun. 2013, 1, 532–556. [Google Scholar]
- Oberson, A.; Frossard, E.; Bühlmann, C.; Mayer, J.; Mäder, P.; Lüscher, A. Nitrogen fixation and transfer in grass clover leys under organic and conventional cropping systems. Plant Soil 2013, 371, 237–255. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, L.A.; Zalba, P.; Gomez, M.A.; Sagardoy, M.A. Phosphate-solubilization activity of bacterial strains in soil and their effect on soybean growth under greenhouse conditions. Biol. Fertil. Soil 2007, 43, 805–809. [Google Scholar] [CrossRef]
- Penfold, C. Phosphorus management in broadacre organic farming systems. RIRDC Publ. 2001, 32, 179. [Google Scholar]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Etesami, H. Enhanced phosphorus fertilizer use efficiency with microorganisms. In Nutrient Dynamics for Sustainable Crop Production; Springer: Berlin/Heidelberg, Germany, 2020; pp. 215–245. [Google Scholar] [CrossRef]
- Goswami, D.; Pithwa, S.; Dhandhukia, P.; Thakker, J.N. Delineating Kocuria turfanensis 2M4 as a credible PGPR: A novel IAA-producing bacteria isolated from saline desert. J. Plant Interact. 2014, 9, 566–576. [Google Scholar] [CrossRef]
- Choudhary, D.K. Microbial rescue to plant under habitat-imposed abiotic and biotic stresses. Appl. Microbiol. Biotechnol. 2012, 96, 1137–1155. [Google Scholar] [CrossRef]
- Patel, K.; Goswami, D.; Dhandhukia, P.; Thakker, J. Techniques to study microbial phytohormones. In Bacterial Metabolites in Sustainable Agroecosystem; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–27. [Google Scholar] [CrossRef]
- Khan, M.S.; Ahmad, E.; Zaidi, A.; Oves, M. Functional aspect of phosphate-solubilizing bacteria: Importance in crop production. In Bacteria in Agrobiology: Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2013; Volume 10, pp. 237–263. [Google Scholar] [CrossRef]
- Ma, J.F. Plant root responses to three abundant soil minerals: Silicon, aluminium and iron. Crit. Rev. Plant Sci. 2005, 24, 267–281. [Google Scholar] [CrossRef]
- Ma, Y.; Prasad, M.N.; Rajkumar, M.; Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Defago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Latour, X.; Delorme, S.; Mirleau, P.; Lemanceau, P. Identification of traits implicated in the rhizosphere competence of fluorescent pseudomonads: Description of a strategy based on population and model strain studies. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2009; pp. 285–296. [Google Scholar] [CrossRef]
- Chaiharn, M.; Lumyong, S. Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr. Microbiol. 2011, 62, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alfocea, F.; Albacete, A.; Ghanem, M.E.; Dodd, I.C. Hormonal regulation of source-sink relations to maintain crop productivity under salinity: A case study of root-to-shoot signalling in tomato. Funct. Plant Biol. 2010, 37, 592–603. [Google Scholar] [CrossRef]
- Jha, C.K.; Saraf, M. Plant growth promoting rhizobacteria (PGPR): A review. J. Agril. Res. Dev. 2015, 5, 108–119. [Google Scholar]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole acetic acid production by the indigenous isolates of azotobacter and fluorescent pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 2005, 29, 29–34. [Google Scholar]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signalling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Solano, B.; Barriuso, J.; Gutiérrez-Mañero, F.J. Physiological and molecular mechanisms of plant growth promoting rhizobacteria (PGPR). In Plant-Bacteria Interactions: Strategies and Techniques to Promote Plant Growth; Wiley and Sons: Hoboken, NJ, USA, 2008; pp. 41–54. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Co-inoculation with endophytic and rhizosphere bacteria allows reduced application rates of N-fertilizer for rice plant. Rhizosphere 2016, 2, 5–12. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef] [PubMed]
- Hajiabadi, A.A.; Arani, A.M.; Ghasemi, S.; Mohammad, H.R.; Etesami, H.; Manshadi, S.S.; Dolati, A. Mining the rhizosphere of halophytic rangeland plants for halotolerant bacteria to improve growth and yield of salinity-stressed wheat. Plant Physiol. Biochem. 2021, 163, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhang, Y.J.; Yuan, B.; Xu, P.Y.; Xing, K.; Wang, J.; Jiang, J.H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Sorty, A.M.; Meena, K.K.; Choudhary, K.; Bitla, U.M.; Minhas, P.S.; Krishnani, K.K. Effect of plant growth promoting bacteria associated with halophytic weed (Psoralea corylifolia L) on germination and seedling growth of wheat under saline conditions. Appl. Biochem. Biotechnol. 2016, 180, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shahzad, R.; Khan, A.L.; Halo, B.A.; Al-Yahyai, R.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Endophytic bacterial diversity of Avicennia marina helps to confer resistance against salinity stress in Solanum lycopersicum. J. Plant Interact. 2017, 12, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Siddikee, M.A.; Glick, B.R.; Chauhan, P.S.; Jong-Yim, W.; Sa, T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 2011, 49, 427–434. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunkaew, T.; Kantachote, D.; Nitoda, T.; Kanzaki, H.; Ritchie, R.J. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr. Polym. 2015, 115, 334–341. [Google Scholar] [CrossRef]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef] [Green Version]
- Sandhya, V.; Ali, S.K.Z.; Grover, M.R.; Venkateswarlu, G.B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Carrasco, L.; Rolda, A. Contribution of Pseudomonas mendocina and Glomus intraradices to aggregate stabilization and promotion of biological fertility in rhizosphere soil of lettuce plants under field conditions. Soil Use Manag. 2006, 22, 298–304. [Google Scholar] [CrossRef]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef] [Green Version]
- Hichem, H.; Mounir, D.; Naceur, E.A. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crop Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
- Scandalios, J.G. The rise of ROS. Trends Biochem. Sci. 2002, 27, 483–486. [Google Scholar] [CrossRef]
- Parvaiz, A.; Khalid, U.R.H.; Ashwani, K.; Muhammad, A.; Nudrat, A.A. Salt-induced changes in photosynthetic activity and oxidative defence system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 2012, 11, 2694–2703. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and anti-oxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lim, J.H.; Park, M.R.; Kim, Y.J.; Park, T.I.; Seo, Y.W.; Choi, K.G.; Yun, S.G. Enhanced antioxidant enzymes are associated with reduced hydrogen peroxide in barley roots under saline stress. BMB Rep. 2005, 38, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Jha, Y.; Subramanian, R.B. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol. Mol. Biol. Plants 2014, 20, 201–207. [Google Scholar] [CrossRef]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef]
- Deivanai, S.; Bindusara, A.S.; Prabhakaran, G.; Bhore, S.J. Culturable bacterial endophytes isolated from Mangrove tree (Rhizophora apiculata Blume) enhance seedling growth in Rice. J. Nat. Sci. Biol. Med. 2014, 5, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, E.; Sepanlou, M.G.; Badi, H.A.N. The effect of salinity on the growth, morphology and physiology of Echium amoenum Fisch & Mey. Afr. J. Biotechnol. 2011, 10, 8765–8773. [Google Scholar] [CrossRef]
- Pinedo, I.; Ledger, T.; Greve, M.; Poupin, M.J. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front Plant Sci. 2015, 6, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Tapias, D.; Moreno-Galvan, A.; Pardo-Diaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil. Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Arora, N.K.; Tewari, S.; Singh, S.; Lal, N.; Maheshwari, D.K. PGPR for protection of plant health under saline conditions. In Bacteria in Agrobiology: Stress Management; Springer: Berlin/Heidelberg, Germany, 2012; pp. 239–258. [Google Scholar] [CrossRef]
- Wood, J.M.; Bremer, E.; Csonka, L.N.; Kraemerd, R.; Poolmane, B.; HeideSmith, T.V.D. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 437–460. [Google Scholar] [CrossRef] [Green Version]
- Creus, C.M.; Sueldo, R.J.; Barassi, C.A. Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can. J. Bot. 2004, 82, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Arora, N.K.; Singhal, V.; Maheshwari, D.K. Salinity induced accumulation of poly-b-hydroxybutyrate in rhizobia indicating its role in cell protection. World J. Microbiol. Biotechnol. 2006, 22, 603–606. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2014, 35, 425–437. [Google Scholar] [CrossRef]
- Prado, F.E.; Boero, C.; Gallardo, M.; Gonzalez, J.A. Effect of NaCl on germination, growth and soluble sugar content in Chenopodium quinoa Willd. seeds. Bot. Bull. Acad. Sin. 2000, 41, 27–34. [Google Scholar]
- Kavi Kishor, P.B.; Sangam, S.; Amrutha, R.N.; Sri Laxmi, P.; Naidu, K.R.; Rao, K.R. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Zhu, M.; Zhou, M.; Shabala, L.; Shabala, S. Linking osmotic adjustment and stomatal characteristics with salinity stress tolerance in contrasting barley accessions. Funct. Plant Biol. 2015, 42, 252–263. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Saad, A.M.; El-Saadony, M.T.; Merwad, A.R.M.; Rady, M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020, 30, 101878. [Google Scholar] [CrossRef]
- Tirry, N.; Kouchou, A.; Laghmari, G.; Lemjereb, M.; Hnadi, H.; Amrani, K.; Bahafid, W.; Ghachtouli, N.E. Improved salinity tolerance of Medicago sativa and soil enzyme activities by PGPR. Biocatal. Agric. Biotechnol. 2021, 31, 101914. [Google Scholar] [CrossRef]
- Kearl, J.; McNary, C.; Lowman, J.S.; Mei, C.; Aanderud, Z.T.; Smith, S.T.; West, J.; Colton, E.; Hamson, M.; Nielsen, B.L. Salt-tolerant halophyte rhizosphere bacteria stimulate growth of alfalfa in salty soil. Front. Microbiol. 2019, 10, 1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamilova, F.; Okon, Y.; de Weert, S.; Hora, K. Commercialization of microbes: Manufacturing, inoculation, best practice for objective field testing, and registration. In Principles of Plant-Microbe Interactions; Springer: Berlin/Heidelberg, Germany, 2015; Volume 33, pp. 319–327. [Google Scholar] [CrossRef]
- Ehsan, S.; Begum, R.A.; Nor, G.M.; Maulud, K.N.A. Current and potential impacts of sea level rise in the coastal areas of Malaysia. IOP Conf. Ser. Earth Environ. Sci. 2019, 228, 012023. [Google Scholar] [CrossRef]
- Selamat, A.; Ismail, M.R. Growth and production of rice for the increased Malaysian population as affected by global warming trends: Forecast for 2057. Trans. Malays. Soc. Plant Physiol. 2008, 17, 20–34. [Google Scholar]
- Bueno Batista, M.; Dixon, R. Manipulating nitrogen regulation in diazotrophic bacteria for agronomic benefit. Biochem. Soc. Trans. 2019, 47, 603–614. [Google Scholar] [CrossRef] [Green Version]
| HT-PGPB | Source | Crop | PGP Traits | Citations |
|---|---|---|---|---|
| Micrococcus yunnanensis | Avicennia marina | Rice (Oryza sativa) | IAA 1, Siderophore 2, Ammonia 3 | Soldan et al., 2019 [60] |
| Arthrobacter pascens | Suaeda fruticosa | Maize (Zea mays) | P-solubilization 4, Siderophore, Antioxidants 5 | Ullah and Bano, 2015 [61] |
| Bacillus sp. | Atriplex leucoclada | Maize (Zea mays) | P-solubilization, Siderophore, Antioxidants | Ullah and Bano, 2015 [61] |
| Staphylococcus equorum | Salicornia hispanica | Tomato (Solanum lycopersicum) | ACC deaminase 6, P-solubilization, BNF 7, Siderophore | Vega et al., 2019 [62] |
| Bacillus atrophaeus | Suaeda mollis | Wheat (Triticum aestivum) | P-solubilization, IAA, BNF | Kerbab et al., 2021 [63] |
| Arthrobacter agilis | Halocnemum strobilaceum | Sugar beets (Beta vulgaris) | P-solubilization, IAA, ACC deaminase | Zhou et al., 2017 [64] |
| Alcaligenes faecalis | Atriplex lentiformis | Wheat (Triticum aestivum) | P-solubilization, IAA, N-fixing, Antioxidants, Ammonia | Muhammad et al., 2021 [65] |
| Pantoea ananatis | Oryza sativa | Rice (Oryza sativa) | ACC deaminase, P-solubilization, IAA, Siderophore | Lu et al., 2021 [66] |
| Bacillus tequilensis | Oryza sativa | Rice (Oryza sativa) | IAA, N-fixing, P-solubilization, K-solubilization 8, EPS 9 | Shultana et al., 2020 [67] |
| Bacillus aryabhattai and Arthrobacter woluwensis | Coastal plants | Soybean (Glycine max) | IAA, EPS, Antioxidants | Khan et al., 2021 [68] |
| Bacillus velezensis | NA * | Tomato (Solanum lycopersicum) | IAA, P-solubilization, BNF, Antioxidants | Medeiros and Bettiol, 2021 [69] |
| HT-PGPB | Halophyte | Nutrients | Mechanisms | Citation |
|---|---|---|---|---|
| Bacillus atrophaeus | Suaeda mollis | Nitrogen | BNF | Kerbab et al., 2021 [63] |
| Alcaligenes faecalis | Atriplex lentiformis | Nitrogen | BNF | Muhammad et al., 2021 [65] |
| Agrobacierium tumefacien | Arthrocnemum indicum | Nitrogen | BNF | Sharma et al., 2016 [79] |
| Variovorax paradoxus | Suaeda physophora | Iron | Siderophore | Zhou et al., 2017 [64] |
| Micrococcus yunnanensis | Nitraria tangutorum | Iron | Siderophore | Zhou et al., 2017 [64] |
| Alcaligenes faecalis | Sesbania aculeata | Phosphorus | P-solubilization | Muhammad et al., 2021 [65] |
| Enterobacter asburiae and Arthrobacter aurescens | Echinochloa stagnina | Phosphorus | P-solubilization | Safdarian et al., 2018 [80] |
| Bacillus endophyticus and Bacillus tequilensis | Salicornia europaea | Phosphorus | P-solubilization | Zhao et al., 2016 [81] |
| Bacillus mucilaginosus and Azotobacter chroococcum, | NA | Potassium | K-solubilization | Singh et al., 2010 [82] |
| Azospirillum lipoferum | NA | Zinc | Zn-solubilization 1 | Tariq et al., 2007 [83] |
| HT-PGPB | Halophyte | Targeted Crop | Mode of Actions on Plant | Citation |
|---|---|---|---|---|
| Variovorax paradoxus | Suaeda physophora | Sugar beet (Beta vulgaris) | Enhance the growth of shoot and root | Zhou et al., 2017 [64] |
| Planococcus rifietoensis | Kalidium capsicum | Sugar beet (Beta vulgaris) | Enhance the growth of shoot and root | Zhou et al., 2017 [64] |
| Alcaligenes faecalis. | Sesbania aculeata and Atriplex lentiformis | Wheat (Triticum aestivum) | Enhance growth parameters and plant biomass | Muhammad et al., 2021 [65] |
| Bacillus atrophaeus | Salicornia spp. | Wheat (Triticum aestivum) | Enhance the growth of shoot and root | Safdarian et al., 2020 [80] |
| Bacillus sp., Pseudomonas sp., and Microbacterium sp. | Limonium sinense | L. sinense | Enhance the growth of shoot and root | Qin et al., 2014 [116] |
| Bacillus sp., Marinobacterium sp., and Sinorhizobium sp. | Psoralea corylifolia L. | Wheat (Triticum aestivum) | Enhance germination rate and root elongation | Sorty et al., 2016 [117] |
| Bacillus pumilus and Exiguobacterium sp. | Avicennia marina | Tomato (Solanum lycopersicum) | Enhance the growth of shoot and root, leaf numbers and internodes | Ali et al., 2017 [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teo, H.M.; A., A.; A., W.A.; Bhubalan, K.; S., S.N.M.; C. I., M.S.; Ng, L.C. Setting a Plausible Route for Saline Soil-Based Crop Cultivations by Application of Beneficial Halophyte-Associated Bacteria: A Review. Microorganisms 2022, 10, 657. https://doi.org/10.3390/microorganisms10030657
Teo HM, A. A, A. WA, Bhubalan K, S. SNM, C. I. MS, Ng LC. Setting a Plausible Route for Saline Soil-Based Crop Cultivations by Application of Beneficial Halophyte-Associated Bacteria: A Review. Microorganisms. 2022; 10(3):657. https://doi.org/10.3390/microorganisms10030657
Chicago/Turabian StyleTeo, Han Meng, Aziz A., Wahizatul A. A., Kesaven Bhubalan, Siti Nordahliawate M. S., Muhamad Syazlie C. I., and Lee Chuen Ng. 2022. "Setting a Plausible Route for Saline Soil-Based Crop Cultivations by Application of Beneficial Halophyte-Associated Bacteria: A Review" Microorganisms 10, no. 3: 657. https://doi.org/10.3390/microorganisms10030657
APA StyleTeo, H. M., A., A., A., W. A., Bhubalan, K., S., S. N. M., C. I., M. S., & Ng, L. C. (2022). Setting a Plausible Route for Saline Soil-Based Crop Cultivations by Application of Beneficial Halophyte-Associated Bacteria: A Review. Microorganisms, 10(3), 657. https://doi.org/10.3390/microorganisms10030657






