Effects of Growth Stage on the Characterization of Enterotoxin A-Producing Staphylococcus aureus-Derived Membrane Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Measurement of Gene Expression in Enterotoxin A-Producing Staphylococcus aureus
2.3. Isolation of Membrane Vesicles (MVs)
2.4. Measurement of Particle Size Distribution
2.5. Transmission Electron Microscopy (TEM) Analysis
2.6. Evaluation of the Storage Stability of MVs
2.7. Detection of SEA Using Western Blot Analysis
2.8. SDS-PAGE and Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS)
2.9. MV-Induced Inflammation-Related Mediator Release in Rat Basophilic Leukemia (RBL-2H3) Cells
2.10. Expression Level of MV-Induced Inflammation-Related Genes in HaCaT Cells
2.11. Statistical Analysis
3. Results
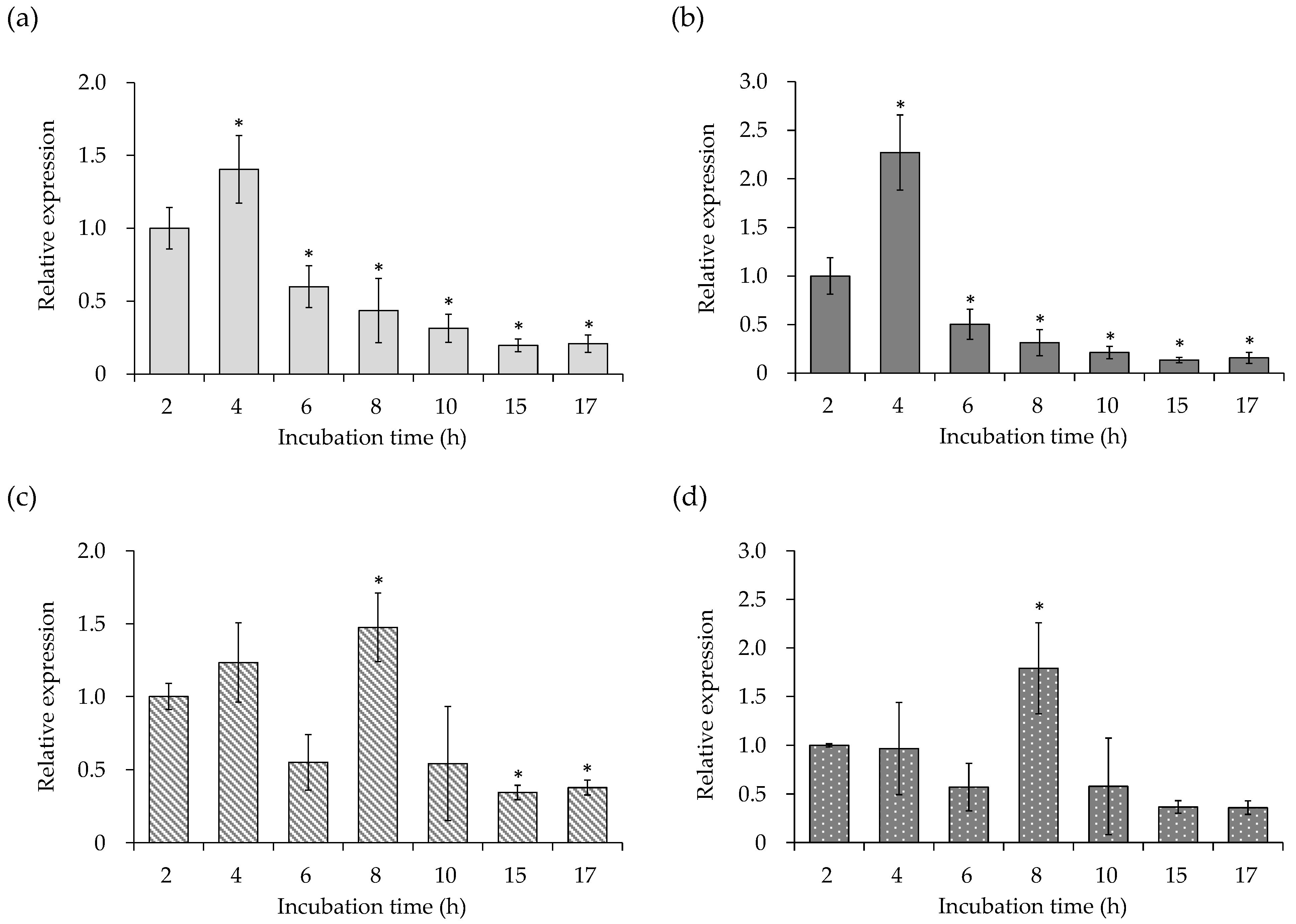
3.1. Gene Expression of Virulence Factors of the SEA-Producing Strain
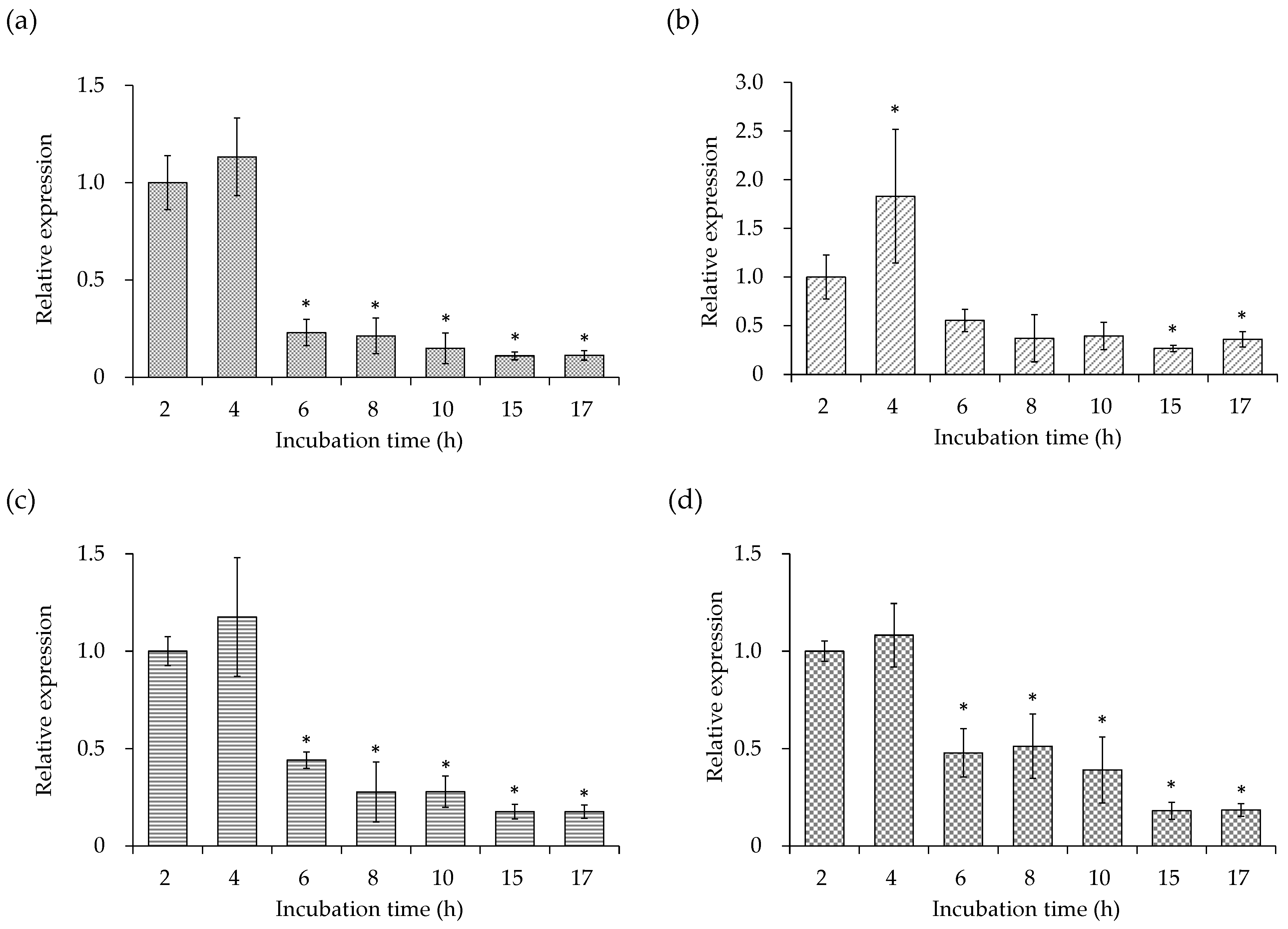
3.2. Cell Membrane-Related Gene Expression of the SEA-Producing Strain
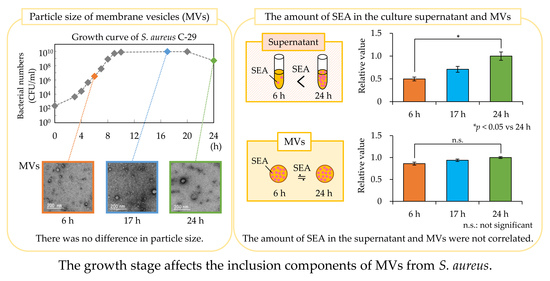
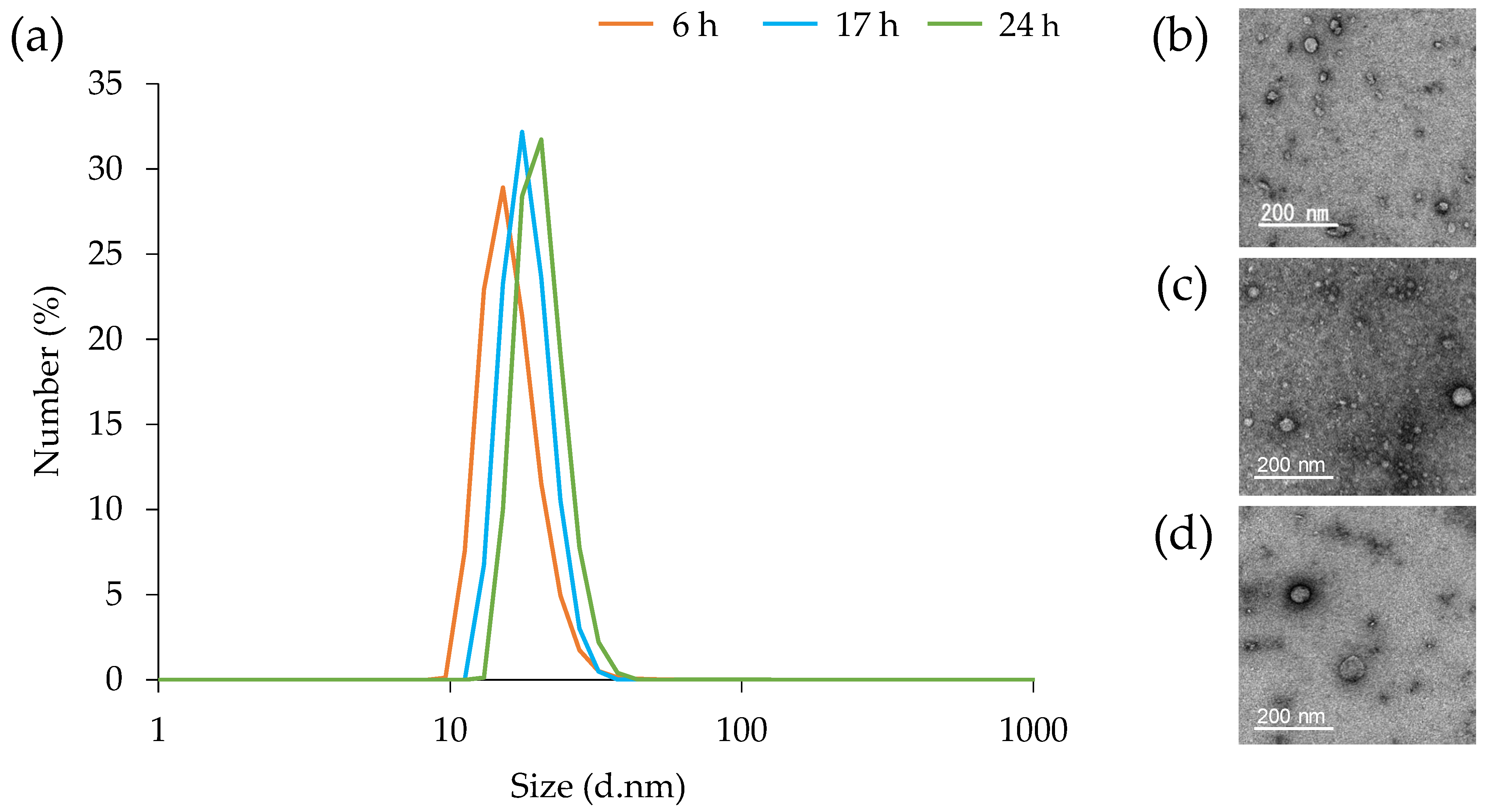
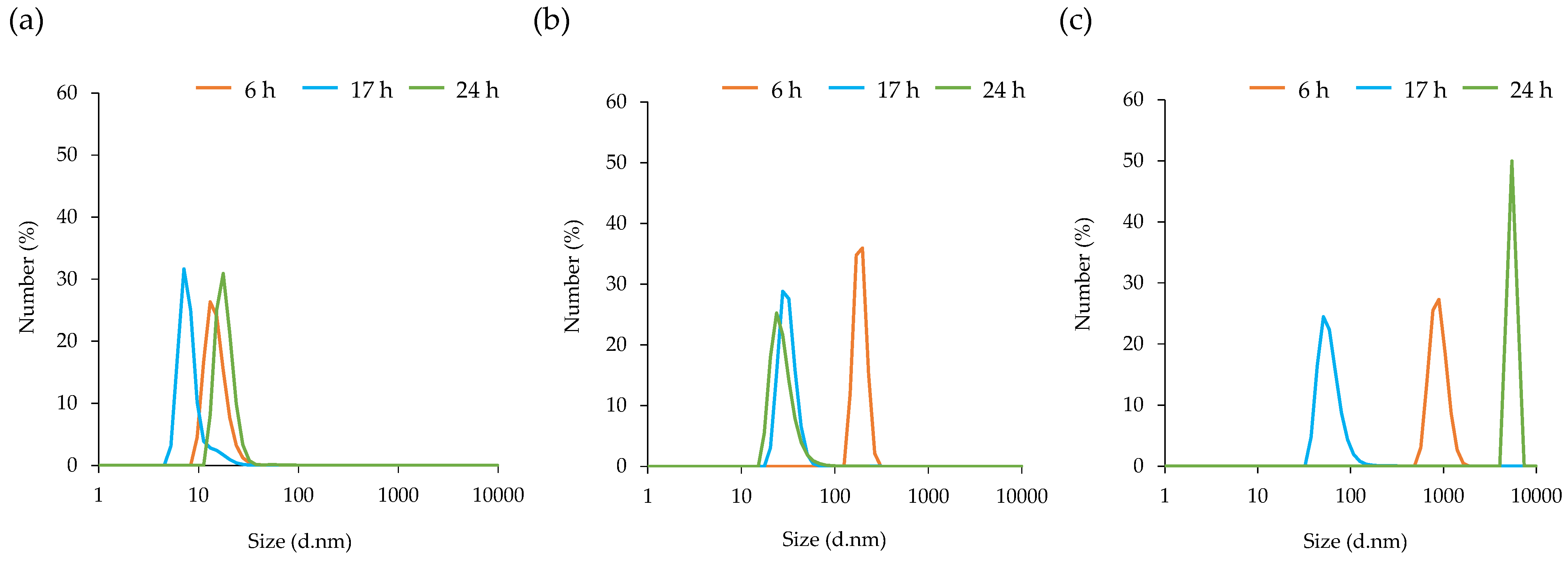
3.3. Particle Size Distribution of MVs
3.4. Storage Stability of MVs
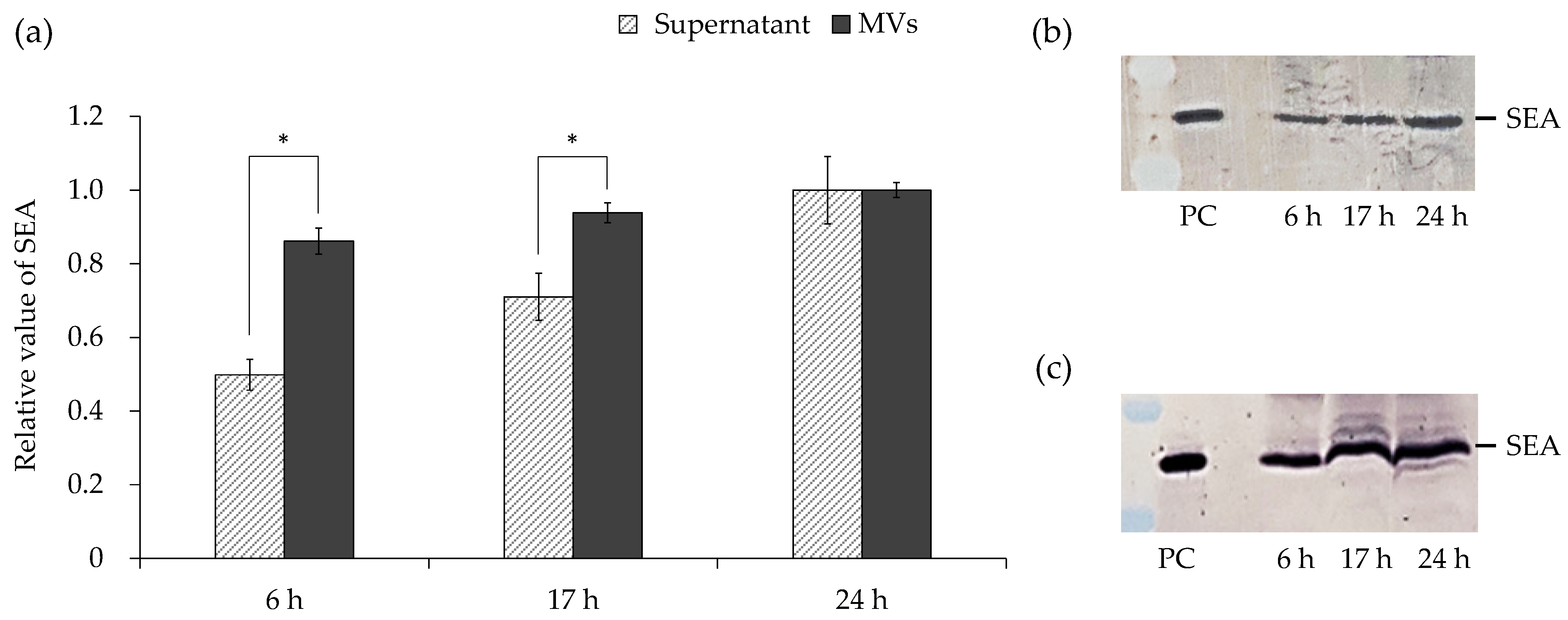
3.5. Amount of SEA in Culture Supernatant and MVs
3.6. Cargo Protein Composition in MVs
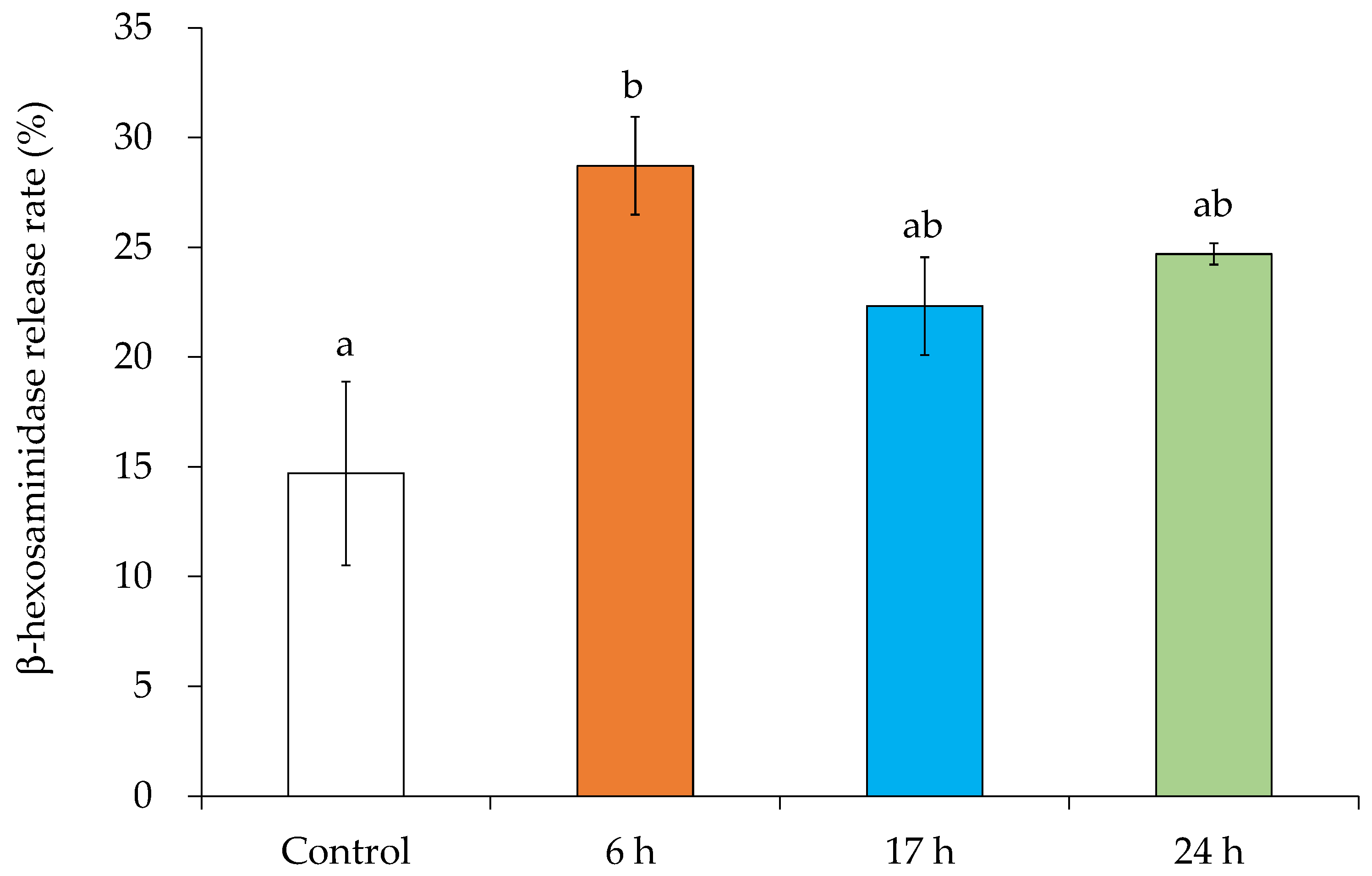
3.7. MV-Induced Inflammatory Mediator Release
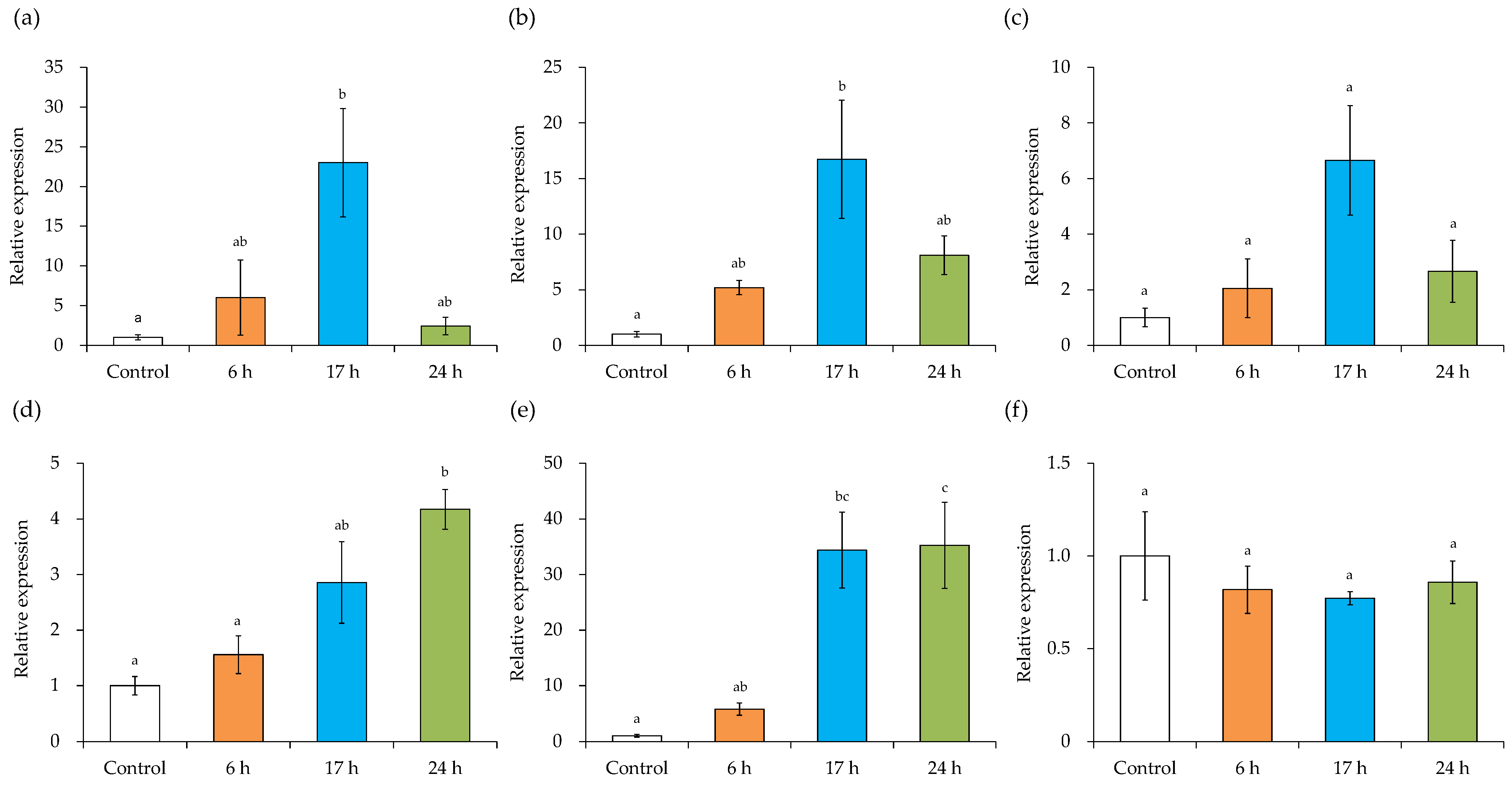
3.8. Expression Level of MV-Induced Inflammation-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powers, M.E.; Wardenburg, J.B. Igniting the fire: Staphylococcus aureus virulence factors in the pathogenesis of sepsis. PLoS Pathog. 2014, 10, e1003871. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Piémont, Y.; Godail, G.F.; Bes, M.; Peter, M.O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine leukocidin—Producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Loir, Y.L.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2003, 1, 63–76. [Google Scholar] [PubMed]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef]
- Betley, M.J.; Mekalanos, J.J. Staphylococcal enterotoxin A is encoded by phage. Science 1985, 229, 185–187. [Google Scholar] [CrossRef]
- Sumby, P.; Waldor, M.K. Transcription of the toxin genes present within the staphylococcal phage phiSa3ms is intimately linked with the phage’s life cycle. J. Bacteriol. 2003, 185, 6841–6851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremaine, M.T.; Brockman, D.K.; Betley, M.J. Staphylococcal enterotoxin A gene (sea) expression is not affected by the accessory gene regulator (agr). Infect. Immun. 1993, 61, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Deghorain, M.; Melderen, L.V. The staphylococci phages family: An overview. Viruses 2012, 4, 3316–3335. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.; Oh, M.H.; Jun, S.H.; Kim, S.I.; Choi, C.W.; Kwon, H.I.; Na, S.H.; Kim, Y.J.; Nicholas, A.; Selasi, G.N.; et al. Variation among Staphylococcus aureus membrane vesicle proteomes affects cytotoxicity of host cells. Microb. Pathog. 2016, 93, 185–193. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Gho, Y.S. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef] [PubMed]
- Nagakubo, T.; Nomura, N.; Toyofuku, M. Cracking open bacterial membrane vesicles. Front. Microbiol. 2020, 10, 3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlatterer, K.; Beck, C.; Hanzelmann, D.; Lebtig, M.; Fehrenbacher, B.; Schaller, M.; Ebner, P.; Nega, M.; Otto, M.; Lretschmer, D.; et al. The mechanism behind bacterial lipoprotein release: Phenol-soluble modulins mediate Toll-like receptor 2 activation via extracellular vesicle release from Staphylococcus aureus. MBio 2018, 9, e01851-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.H.L.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Björnsdottir, H.; Rudin, A.D.; Klose, F.P.; Elmwall, J.; Welin, A.; Stylianou, M.; Christenson, K.; Urban, C.F.; Forsman, H.; Dahlgren, C.; et al. Phenol-soluble modulin α peptide toxins from aggressive Staphylococcus aureus induce rapid formation of neutrophil extracellular traps through a reactive oxygen species-independent pathway. Front. Immunol. 2017, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Hanzelmann, D.; Joo, H.S.; Franz-Wachtel, M.; Hertlein, T.; Stevanovic, S.; Macek, B.; Wolz, C.; Götz, F.; Otto, M.; Kretschmer, D.; et al. Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat. Commun. 2016, 7, 12304. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: A novel mechanism of enzyme secretion. J. Bacteriol. 1995, 177, 3998–4008. [Google Scholar] [CrossRef] [Green Version]
- Hyyryläinen, H.L.; Pietiäinen, M.; Lunden, T.; Ekman, A.; Gardemeister, M.; Murtomäki-Repo, S.; Antelmann, H.; Hecker, M.; Valmu, L.; Sarvas, M.; et al. The density of negative charge in the cell wall influences two-component signal transduction in Bacillus subtilis. Microbiology 2007, 153, 2126–2136. [Google Scholar] [CrossRef] [Green Version]
- Ernst, C.M.; Peschel, A. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol. Microbiol. 2011, 80, 290–299. [Google Scholar] [CrossRef]
- Dowhan, W. A retrospective: Use of Escherichia coli as a vehicle to study phospholipid synthesis and function. Biochim. Biophys. Acta 2013, 1831, 471–494. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Muraih, J.K.; Tishbi, N.; Herskowitz, J.; Victor, R.L.; Silverman, J.; Uwumarenogie, S.; Taylor, S.D.; Palmer, M.; Mintzer, E. Cardiolipin prevents membrane translocation and permeabilization by daptomycin. J. Biol. Chem. 2014, 289, 11584–11591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, N.N.; Bayer, A.S. Correlation of cell membrane lipid profiles with daptomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 1082–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peleg, A.Y.; Miyakis, S.; Ward, D.V.; Earl, A.M.; Rubio, A.; Cameron, D.R.; Pillai, S.; Moellering, R.C., Jr.; Eliopoulos, G.M. Whole genome characterization of the mechanisms of daptomycin resistance in clinical and laboratory derived isolates of Staphylococcus aureus. PLoS ONE 2012, 7, e28316. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Mishra, N.N.; Sakoulas, G.; Nonejuie, P.; Nast, C.C.; Pogliano, J.; Chen, K.T.; Ellison, S.N.; Yeaman, M.R.; Yang, S.J. Heterogeneity of mprF sequences in methicillin-resistant Staphylococcus aureus clinical isolates: Role in cross-resistance between daptomycin and host defense antimicrobial peptides. Antimicrob. Agents Chemother. 2014, 58, 7462–7467. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.H.; Lee, J.H.; Kim, S.I.; Choi, C.W.; Park, T.I.; Jung, H.R.; Cho, J.W.; Kim, S.H.; Lee, J.C. Staphylococcus aureus-derived membrane vesicles exacerbate skin inflammation in atopic dermatitis. Clin. Exp. Allergy 2017, 47, 85–96. [Google Scholar] [CrossRef]
- Kwon, H.I.; Jeong, N.H.; Kim, S.Y.; Kim, M.H.; Son, J.H.; Jun, S.H.; Kim, S.; Jeon, H.; Kang, S.C.; Kim, S.H.; et al. Inhibitory effects of thymol on the cytotoxicity and inflammatory responses induced by Staphylococcus aureus extracellular vesicles in cultured keratinocytes. Microb. Pathog. 2019, 134, 103603. [Google Scholar] [CrossRef] [PubMed]
- Tokuhira, N.; Kitagishi, Y.; Suzuki, M.; Minami, A.; Nakanishi, A.; Ono, Y.; Kobayashi, K.; Matsuda, S.; Ogura, Y. PI3K/AKT/PTEN pathway as a target for Crohn’s disease therapy. Int. J. Mol. Med. 2015, 35, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, N.; Yang, Y.; Li, X.; Zhang, M.; Huang, J.; Wang, X.; Long, X. MiR-26a inhibits proliferation and migration of HaCaT keratinocytes through regulating PTEN expression. Gene 2016, 594, 117–124. [Google Scholar] [CrossRef]
- Xu, L.; Leng, H.; Shi, X.; Ji, J.; Fu, J. MiR-155 promotes cell proliferation and inhibits apoptosis by PTEN signaling pathway in the psoriasis. Biomed. Pharmacother. 2017, 90, 524–530. [Google Scholar] [CrossRef]
- Shimamura, Y.; Kidokoro, S.; Murata, M. Survey and properties of Staphylococcus aureus isolated from Japanese-style desserts. Biosci. Biotechnol. Biochem. 2006, 70, 1571–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, E.H.; Park, J.H.; Kim, J.Y.; Chung, Y.C.; Jeong, H.G. Inhibitory mechanism of saponins derived from roots of Platycodon grandiflorum on anaphylactic reaction and IgE-mediated allergic response in mast cells. Food Chem. Toxicol. 2009, 47, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Ebner, P.; Luqman, A.; Reichert, S.; Hauf, K.; Popella, P.; Forchhammer, K.; Otto, M.; Götz, F. Non-classical protein excretion is boosted by PSMα-induced cell leakage. Cell Rep. 2017, 20, 1278–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.S.; Joo, H.S.; Duong, A.C.; Dieringer, T.D.; Tan, V.Y.; Song, Y.; Fischer, E.R.; Cheung, G.Y.C.; Li, M.; Otto, M. Essential Staphylococcus aureus toxin export system. Nat. Med. 2013, 19, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, Y.; Dong, M. Distribution, polymorphism and temporal expression of egc in Staphylococcus aureus isolates from various foods in China. Food Control 2013, 29, 279–285. [Google Scholar] [CrossRef]
- Carnes, E.C.; Lopez, D.M.; Donegan, N.P.; Cheung, A.; Gresham, H.; Timmins, G.S.; Brinker, C.J. Confinement-inducedquorumsensing of individual Staphylococcus aureus bacteria. Nat. Chem. Biol. 2010, 6, 41–45. [Google Scholar] [CrossRef]
- Koprivnjak, T.; Mlakar, V.; Swanson, L.; Fournier, B.; Peschel, A.; Weiss, J.P. Cation-induced transcriptional regulation of the dlt operon of Staphylococcus aureus. J. Bacteriol. 2006, 188, 3622–3630. [Google Scholar] [CrossRef] [Green Version]
- Peschel, A.; Vuong, C.; Otto, M.; Götz, F. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob. Agents Chemother. 2000, 44, 2845–2847. [Google Scholar] [CrossRef] [Green Version]
- Wecke, J.; Perego, M.; Fischer, W. D-alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb. Drug Resist. 1996, 2, 123–129. [Google Scholar] [CrossRef]
- Heptinstall, S.; Archibald, A.R.; Baddiley, J. Teichoic acids and membrane function in bacteria. Nature 1970, 225, 519–521. [Google Scholar] [CrossRef]
- Lambert, P.A.; Hancock, I.C.; Baddiley, J. Influence of alanyl ester residues on the binding of magnesium ions to teichoic acids. Biochem. J. 1975, 151, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Rose, R.K.; Hogg, S.D. Competitive binding of calcium and magnesium to streptococcal lipoteichoic acid. Biochim. Biophys. Acta 1995, 1245, 94–98. [Google Scholar] [CrossRef]
- Koprivnjak, T.; Peschel, A.; Gelb, M.H.; Liang, N.S.; Weiss, J.P. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 2002, 277, 47636–47644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Gotz, F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, L.V.; Kristian, S.A.; Weidenmaier, C.; Faigle, M.; Kessel, K.P.M.V.; Strijp, J.A.G.V.; Götz, F.; Neumeister, B.; Peschel, A. Staphylococcus aureus strains lacking d-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 2002, 186, 214–219. [Google Scholar] [CrossRef] [Green Version]
- White, D.C.; Frerman, F.E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J. Bacteriol. 1967, 94, 1854–1867. [Google Scholar] [CrossRef] [Green Version]
- Lennarz, W.J.; Nesbitt, J.A., III; Reiss, J. The participation of sRNA in the enzymatic synthesis of O-l-lysyl phosphatidylglycerol in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 1966, 55, 934–941. [Google Scholar] [CrossRef] [Green Version]
- Lennarz, W.J.; Bonsen, P.P.M.; Deenen, L.L.M.V. Substrate specificity of O-l-lysylphosphatidylglycerol synthetase. Enzymatic studies on the structure of O-l-lysylphosphatidylglycerol. Biochemistry 1967, 6, 2307–2312. [Google Scholar] [CrossRef]
- Nesbitt, J.A., III; Lennarz, W.J. Participation of aminoacyl transfer ribonucleic acid in aminoacyl phosphatidylglycerol synthesis. I. Specificity of lysyl phosphatidylglycerol synthetase. J. Biol. Chem. 1968, 243, 3088–3095. [Google Scholar] [CrossRef]
- Oku, Y.; Kurokawa, K.; Ichihashi, N.; Sekimizu, K. Characterization of the Staphylococcus aureus mprF gene, involved in lysinylation of phosphatidylglycerol. Microbiology 2004, 150, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Short, S.A.; White, D.C. Biosynthesis of cardiolipin from phosphatidylglycerol in Staphylococcus aureus. J. Bacteriol. 1972, 109, 820–826. [Google Scholar] [CrossRef] [Green Version]
- Tropp, B.E. Cardiolipin synthase from Escherichia coli. Biochem. Biophys. Acta 1997, 1348, 192–200. [Google Scholar] [CrossRef]
- Koprivnjak, T.; Zhang, D.; Ernst, C.M.; Peschel, A.; Nauseef, W.M.; Weiss, J.P. Characterization of Staphylococcus aureus cardiolipin synthases 1 and 2 and their contribution to accumulation of cardiolipin in stationary phase and within phagocytes. J. Bacteriol. 2011, 193, 4134–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreoni, F.; Toyofuku, M.; Menzi, C.; Kalawong, R.; Shambat, S.M.; François, P.; Zinkernagel, A.S.; Eberl, L. Antibiotics stimulate formation of vesicles in Staphylococcus aureus in both phage-dependent and-independent fashions and via different routes. Antimicrob. Agents Chemother. 2019, 63, e01439-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagakubo, T.; Tahara, Y.O.; Miyata, M.; Nomura, N.; Toyofuku, M. Mycolic acid-containing bacteria trigger distinct types of membrane vesicles through different routes. Iscience 2021, 24, 102015. [Google Scholar] [CrossRef] [PubMed]
- Haurat, M.F.; Aduse-Opoku, J.; Rangarajan, M.; Dorobantu, L.; Gray, M.R.; Curtis, M.A.; Feldman, M.F. Selective sorting of cargo proteins into bacterial membrane vesicles. J. Biol. Chem. 2011, 286, 1269–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Defourny, K.A.Y.; Smid, E.J.; Abee, T. Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaban, N.; Rasooly, A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000, 61, 1–10. [Google Scholar] [CrossRef]
- Biswas, R.; Voggu, L.; Simon, U.K.; Hentschel, P.; Thumm, G.; Gotz, F. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 2006, 259, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Wang, X.; Thompson, C.D.; Weidenmaier, C.; Lee, J.C. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 2018, 9, 1379. [Google Scholar] [CrossRef] [Green Version]
- Turner, H.; Kinet, J.P. Signalling through the high-affinity IgE receptor FcεRI. Nature 1999, 402, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B.; Austen, K.F.; Wasserman, S.I. Immunologic release of β-hexosaminidase and β-glucuronidase from purified rat serosal mast cells. J. Immunol. 1979, 123, 1445–1450. [Google Scholar] [PubMed]
- Soto, E.O.; Pecht, I. A monoclonal antibody that inhibits secretion from rat basophilic leukemia cells and binds to a novel membrane component. J. Immunol. 1988, 141, 4324–4332. [Google Scholar] [PubMed]
- Komisar, J.; Rivera, J.; Vega, A.; Tseng, J. Effects of staphylococcal enterotoxin B on rodent mast cells. Infect. Immun. 1992, 60, 2969–2975. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.; Harbeck, R.; Bina, P.; Reiser, R.F.; Yang, E.; Norris, D.A.; Hanifin, J.M.; Sampson, H.A. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J. Clin. Investig. 1993, 92, 1374–1380. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Kim, S.M.; Kim, J.M.; Oh, B.M.; Kim, J.Y.; Jung, H.J.; Lim, H.J.; Kim, B.S.; Lee, W.J.; Lee, S.J.; et al. Potential immunoinflammatory role of staphylococcal enterotoxin A in atopic dermatitis: Immunohistopathological analysis and in vitro assay. Ann. Dermatol. 2013, 25, 173–180. [Google Scholar] [CrossRef]
- Gurung, M.; Moon, D.C.; Choi, C.W.; Lee, J.H.; Bae, Y.C.; Kim, J.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, S.I.; et al. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE 2011, 6, e27958. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wang, F.F.; Cao, H.W.; Yang, J.Y. MiR-223 regulates proliferation and apoptosis of IL-22-stimulated HaCat human keratinocyte cell lines via the PTEN/Akt pathway. Life Sci. 2019, 230, 28–34. [Google Scholar] [CrossRef]
- Boniface, K.; Bernard, F.X.; Garcia, M.; Gurney, A.L.; Lecron, J.C.; Morel, F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005, 174, 3695–3702. [Google Scholar] [CrossRef] [Green Version]
- Wolk, K.; Witte, E.; Wallace, E.; Döcke, W.D.; Kunz, S.; Asadullah, K.; Volk, H.D.; Sterry, W.; Sabat, R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur. J. Immunol. 2006, 36, 1309–1323. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Sun, M.; Zhang, S.; Huang, J.; Man, M.; Hu, L. Phospholipase C epsilon mediates cytokine cascade induced by acute disruption of epidermal permeability barrier in mice. Biochem. Biophys. Rep. 2020, 24, 100869. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| 16S rRNA | CGTGCTACAATGGACAATACAAA | ATCTACGATTACTAGCGATTCCA |
| sea | GATCAATTTATGGCTAGACG | CGAAGGTTCTGTAGAAGTATGA |
| RNAIII | CGATGTTGTTTACGATAGCTT | CCATCCCAACTTAATAACCA |
| psmα1 | TATCAAAAGCTTAATCGAACAATTC | CCCCTTCAAATAAGATGTTCATATC |
| psmα3 | CATTCACATGGAATTCGTAGCA | TCGTTTTGTCCTCCTGTATGTTG |
| dltD | TGACCCATTTAATCCTGCAATTG | TCTGTAGAACCACCAGCACCTAATAA |
| mprF | TTGTAGGTTTCGGTGGCTTT | GATGCATCGAAAACATGGAA |
| pgsA | TGGCTTCCCTTAGCGATTTTGT | CAGTTACGGCAAATTCTCTGGC |
| cls2 | ATTAGAGTTAATCGTTGATGAGCAAT | TTACGGATGTCTTGTATTAGGTCAT |
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| GAPDH | GGACCTGACCTGCCGTCTAG | GAGGAGTGGGTGTCGCTGTT |
| IL-1β | CCTGTCCTGCGTGTTGAAAGA | GGGAACTGGGCAGACTCAAA |
| IL-8 | TTGGCAGCCTTCCTGATTTC | TGGTCCACTCTCAATCACTCTCA |
| TNF-α | CCCAGGGACCTCTCTCTAATC | ATGGGCTACAGGCTTGTCACT |
| IL-6 | TGGCTGAAAAAGATGGATGCT | TCTGCACAGCTCTGGCTTGT |
| MCP-1 | TCGCTCAGCCAGATGCAAT | TGGCCACAATGGTCTTGAAG |
| PTEN | GTTTACCGGCAGCATCAAAT | CCCCCACTTTAGTGCACAGT |
| Biological Process | Protein | MW | Score | Coverage | Accession |
|---|---|---|---|---|---|
| Glycolysis | Dihydrolipoyllysine-residue acetyltransferase-component of pyruvate dehydrogenase complex | 46,395 | 598 | 36 | Q6GHZ0 |
| Carbohydrate metabolism | Formate acetyltransferase | 84,862 | 500 | 21 | Q2G1D8 |
| Glycolysis and virulence | Enolase | 47,117 | 427 | 21 | O69174 |
| Pyrimidine biosynthesis | CTP synthase | 59,992 | 164 | 7 | P65924 |
| Glycolysis | Dihydrolipoyl dehydrogenase | 49,451 | 148 | 11 | Q6GAB8 |
| Cell wall biogenesis/ degradation | Bifunctional autolysin | 137,528 | 134 | 3 | Q6GI31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanashi, Y.; Shimamura, Y.; Sasahara, H.; Komuro, M.; Sasaki, K.; Morimitsu, Y.; Masuda, S. Effects of Growth Stage on the Characterization of Enterotoxin A-Producing Staphylococcus aureus-Derived Membrane Vesicles. Microorganisms 2022, 10, 574. https://doi.org/10.3390/microorganisms10030574
Yamanashi Y, Shimamura Y, Sasahara H, Komuro M, Sasaki K, Morimitsu Y, Masuda S. Effects of Growth Stage on the Characterization of Enterotoxin A-Producing Staphylococcus aureus-Derived Membrane Vesicles. Microorganisms. 2022; 10(3):574. https://doi.org/10.3390/microorganisms10030574
Chicago/Turabian StyleYamanashi, Yuka, Yuko Shimamura, Haruka Sasahara, Misaki Komuro, Kuniaki Sasaki, Yasujiro Morimitsu, and Shuichi Masuda. 2022. "Effects of Growth Stage on the Characterization of Enterotoxin A-Producing Staphylococcus aureus-Derived Membrane Vesicles" Microorganisms 10, no. 3: 574. https://doi.org/10.3390/microorganisms10030574
APA StyleYamanashi, Y., Shimamura, Y., Sasahara, H., Komuro, M., Sasaki, K., Morimitsu, Y., & Masuda, S. (2022). Effects of Growth Stage on the Characterization of Enterotoxin A-Producing Staphylococcus aureus-Derived Membrane Vesicles. Microorganisms, 10(3), 574. https://doi.org/10.3390/microorganisms10030574