Anti-Tumor Effects of Heat-Killed L. reuteri MG5346 and L. casei MG4584 against Human Colorectal Carcinoma through Caspase-9-Dependent Apoptosis in Xenograft Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Preparation
2.2. Cell Culture
2.3. Cell Cytotoxicity
2.4. Cell Morphology
2.5. Annexin V-FITC and Propidium Iodide Staining Assay
2.6. Human Colorectal Cancer Xenografts in BALB/c Nude Mice
2.7. Western Blot Analysis
2.8. Immunohistochemistry (IHC)
2.9. Statistical Analysis
3. Results
3.1. Cytotoxic Effect of Heat-Killed Bifidobacterium and Lactobacillus Bacterial Strains on Human Colorectal Carcinoma RKO Cells
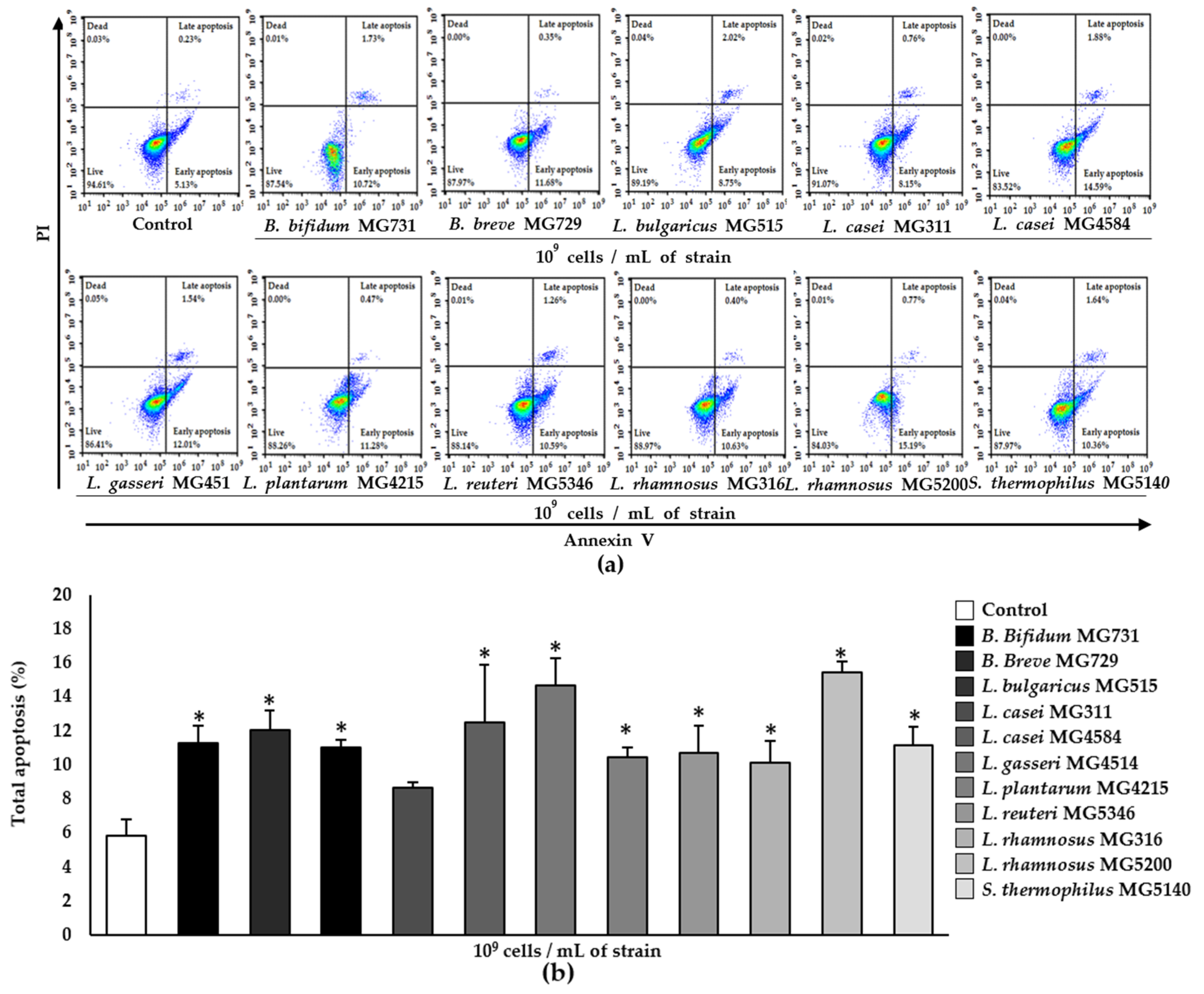
3.2. Apoptotic Effect of Heat-Killed Bifidobacterium and Lactobacillus Strains on RKO Cells
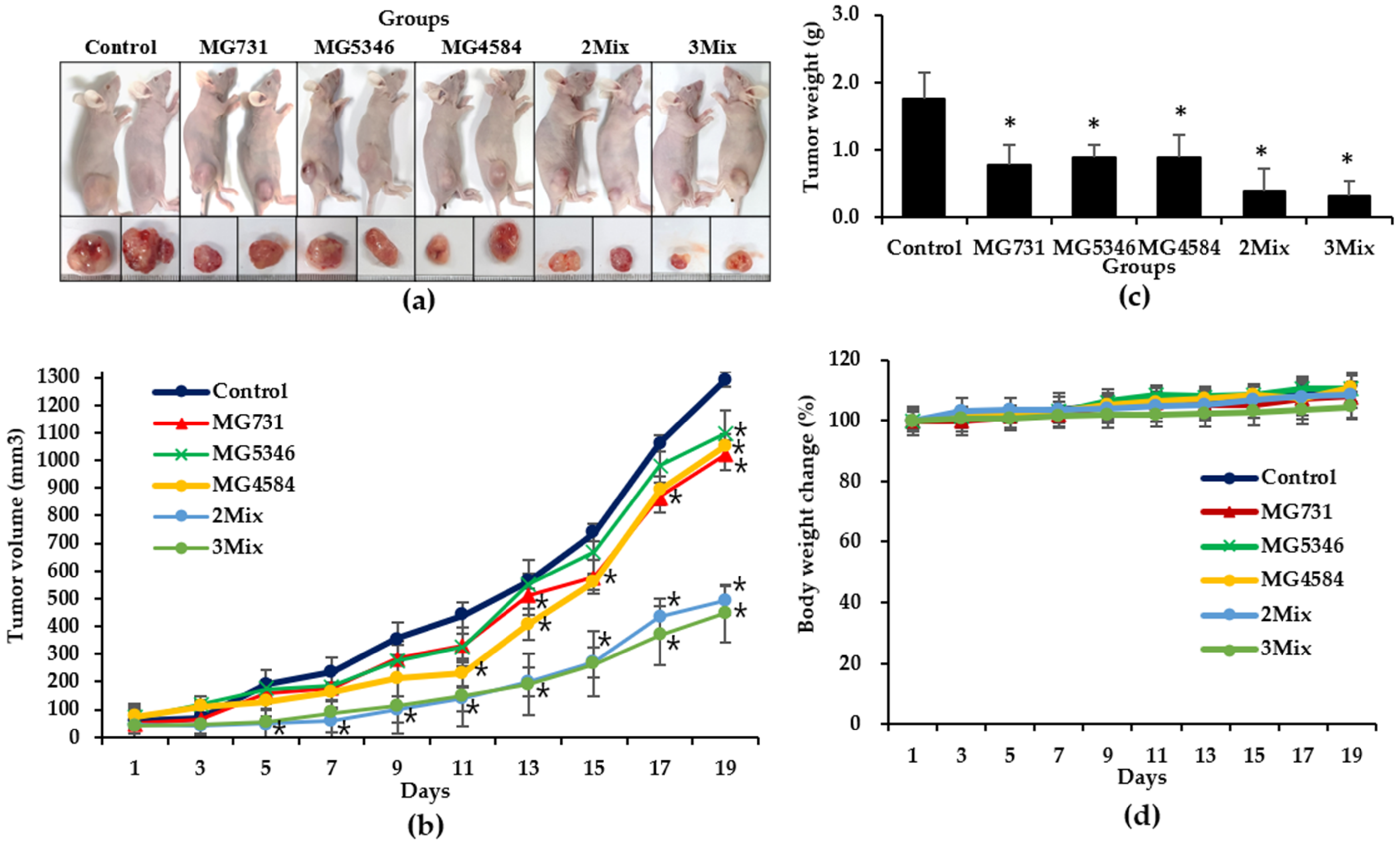
3.3. Anti-Tumor Effects of Heat-Killed Bifidobacterium and Lactobacillus Strains in Xenograft Model Bearing RKO Cells
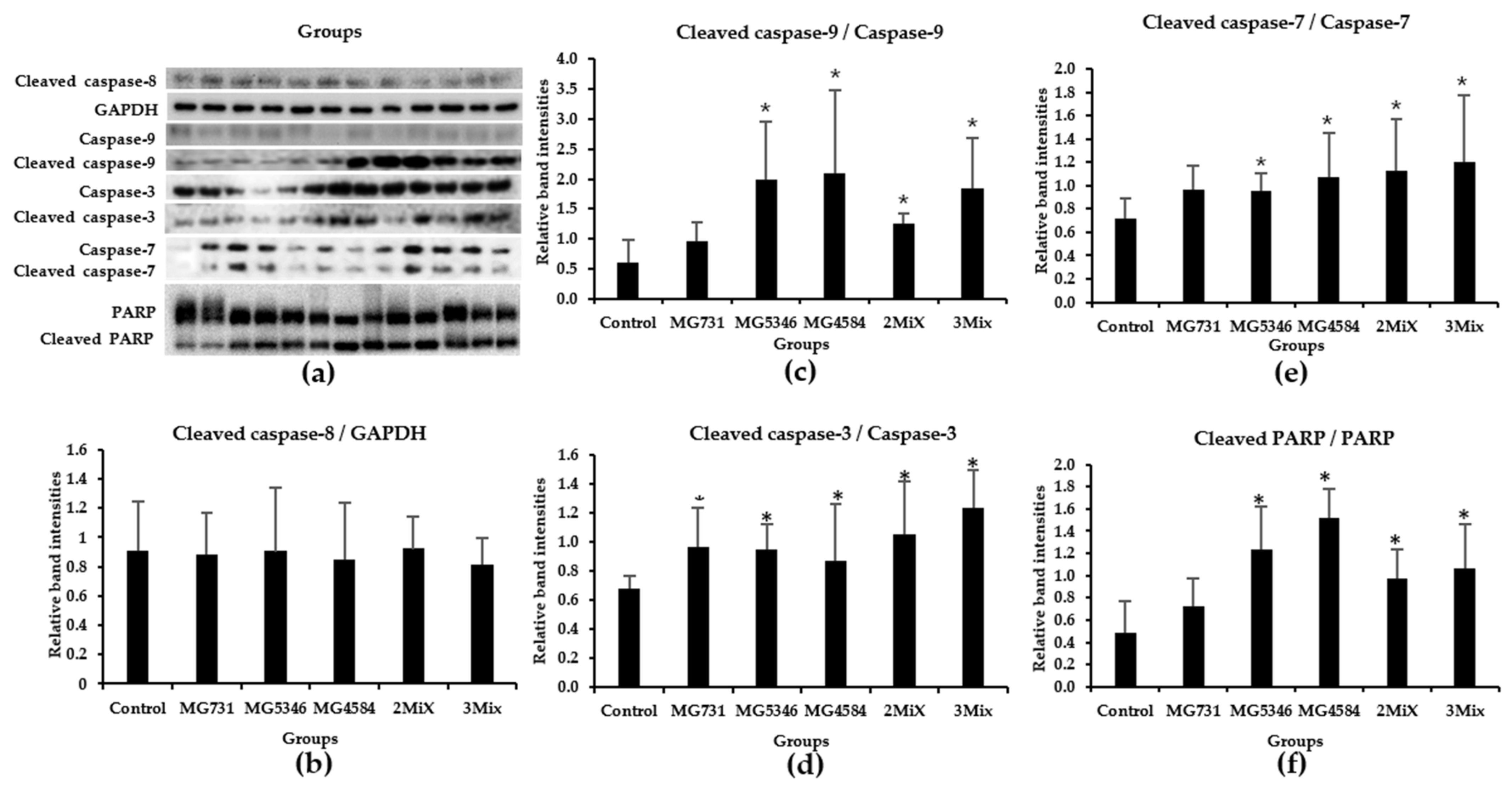
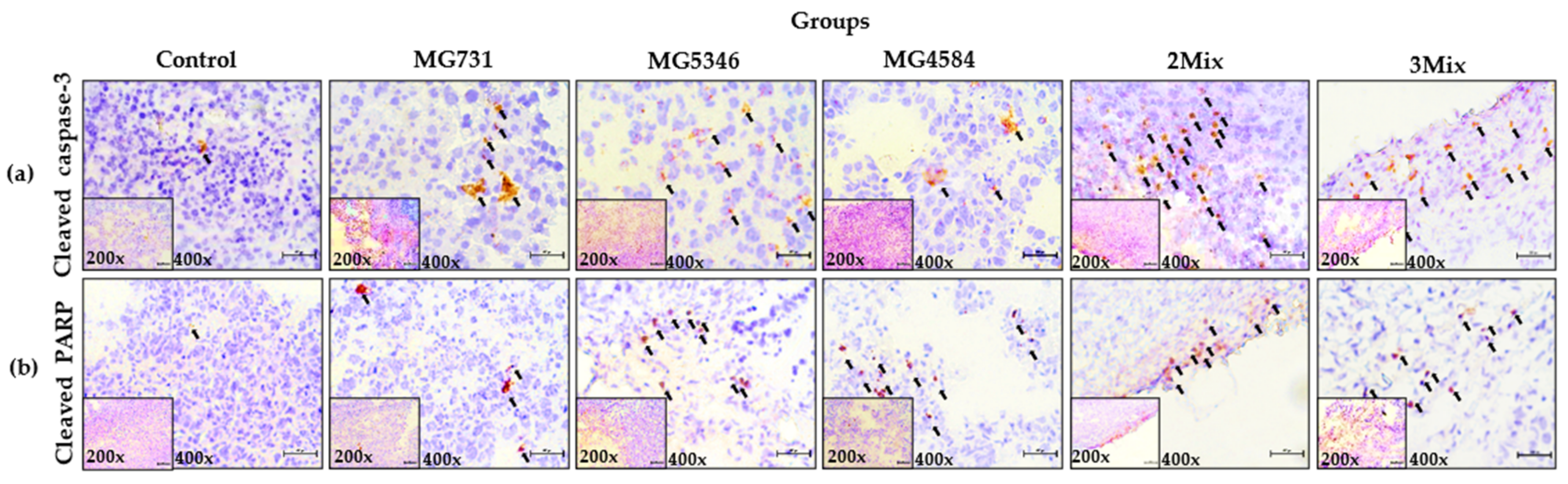
3.4. Caspase-9-Dependent Apoptosis in RKO Cell–Derived Tumors Induced by Heat-Killed Bifidobacterium and Lactobacillus Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Hsieh, P.S.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Yang, S.F.; Lin, C.W. Antibacterial activity of viable and heat-killed probiotic strains against oral pathogens. Lett. Appl. Microbiol. 2020, 70, 310–317. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, J.H.; Yang, H.; Kang, S.D.; Song, S.; Lee, D.; Lee, J.S.; Yoon Park, J.H.; Byun, S.; Lee, K.W. Heat-Killed Lactobacillus plantarum KCTC 13314BP Enhances Phagocytic Activity and Immunomodulatory Effects via Activation of MAPK and STAT3 Pathways. J. Microbiol. Biotechnol. 2019, 29, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.B.; Shih, H.Y.; Huang, C.H.; Li, L.T.; Chen, C.C.; Fang, H.W. Live and heat-killed Lactobacillus rhamnosus GG upregulate gene expression of pro-inflammatory cytokines in 5-fluorouracil-pretreated Caco-2 cells. Support Care Cancer 2014, 22, 1647–1654. [Google Scholar] [CrossRef]
- Song, M.W.; Jang, H.J.; Kim, K.T.; Paik, H.D. Probiotic and antioxidant properties of novel Lactobacillus brevis KCCM 12203P isolated from kimchi and evaluation of immune-stimulating activities of its heat-killed cells in raw 264.7 cells. J. Microbiol. Biotechnol. 2019, 29, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, G.H.; Cho, H.S. Postbiotics for cancer prevention and treatment. Korean J. Microbiol. 2021, 57, 142–153. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rad, A.H.; Aghebati-Maleki, L.; Kafil, H.S.; Abbasi, A. Molecular mechanisms of postbiotics in colorectal cancer prevention and treatment. Crit. Rev. Food Sci. Nutr. 2020, 61, 1787–1803. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.; In, L.L.; Kumar, A.; Ahmed, N.; Nagoor, N.H. Cytotoxic and apoptotic effects of heat killed Mycobacterium indicus pranii (MIP) on various human cancer cell lines. Sci. Rep. 2016, 6, 19833. [Google Scholar] [CrossRef] [Green Version]
- Chuah, L.O.; Foo, H.L.; Loh, T.C.; Mohammed Alitheen, N.B.; Yeap, S.K.; Abdul Mutalib, N.E.; Abdul Rahim, R.; Yusoff, K. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef] [Green Version]
- Karimi Ardestani, S.; Tafvizi, F.; Tajabadi Ebrahimi, M. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum. Exp. Toxicol. 2019, 38, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, S.; Azarpira, N.; Moazamian, E. Anti-colon cancer activity of Bifidobacterium metabolites on colon cancer cell line SW742. Turk. J. Gastroenterol. 2019, 30, 835–842. [Google Scholar] [CrossRef]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef] [PubMed]
- Negarandeh, R.; Salehifar, E.; Saghafi, F.; Jalali, H.; Janbabaei, G.; Abdhaghighi, M.J.; Nosrati, A. Evaluation of adverse effects of chemotherapy regimens of 5-fluoropyrimidines derivatives and their association with DPYD polymorphisms in colorectal cancer patients. BMC Cancer 2020, 20, 560. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, R.; Zhang, W.; Yao, X.; Li, W.; Xu, L.; Sun, X.; Zhao, L. Cysteine-rich intestinal protein 1 suppresses apoptosis and chemosensitivity to 5-fluorouracil in colorectal cancer through ubiquitin-mediated Fas degradation. J. Exp. Clin. Cancer Res. 2019, 38, 120. [Google Scholar] [CrossRef] [PubMed]
- Bressenot, A.; Marchal, S.; Bezdetnaya, L.; Garrier, J.; Guillemin, F.; Plenat, F. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J. Histochem. Cytochem. 2009, 57, 289–300. [Google Scholar] [CrossRef]
- Lee, H.H.; Jung, J.; Moon, A.; Kang, H.; Cho, H. Antitumor and Anti-Invasive Effect of Apigenin on Human Breast Carcinoma through Suppression of IL-6 Expression. Int. J. Mol. Sci. 2019, 20, 3143. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Lee, S.; Lee, K.; Shin, Y.S.; Kang, H.; Cho, H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. DARU J. Pharm. Sci. 2015, 23, 35. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.Y.; Kim, G.Y.; Lee, J.H.; Choi, B.T.; Yoo, Y.H.; Choi, Y.H. Apoptosis induction of human prostate carcinoma DU145 cells by diallyl disulfide via modulation of JNK and PI3K/AKT signaling pathways. Int. J. Mol. Sci. 2012, 13, 14158–14171. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, S.Y.; Yoon, Y.; Park, C.; Sohn, J.; Jeong, J.J.; Jeon, B.N.; Jang, M.; An, C.; Lee, S.; et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat. Microbiol. 2021, 6, 277–288. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chu, S.H.; Jeon, J.Y.; Lee, M.K.; Park, J.H.; Lee, D.C.; Lee, J.W.; Kim, N.K. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: A double-blind, randomized, placebo-controlled trial. Dig. Liver Dis. 2014, 46, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Said, S.; Rajandram, R.; Wang, Z.; Roslani, A.C.; Chin, K.F. Pre-surgical Administration of Microbial Cell Preparation in Colorectal Cancer Patients: A Randomized Controlled Trial. World J. Surg. 2016, 40, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Kim, H.; Yang, K.M. An Aqueous Extract of a Bifidobacterium Species Induces Apoptosis and Inhibits Invasiveness of Non-Small Cell Lung Cancer Cells. J. Microbiol. Biotechnol. 2020, 30, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, B.S.; Ghadimi-Darsajini, A.; Nekouian, R.; Iragian, G.R. In vitro activity of probiotic Lactobacillus reuteri against gastric cancer progression by downregulation of urokinase plasminogen activator/urokinase plasminogen activator receptor gene expression. J. Cancer Res. Ther. 2017, 13, 246–251. [Google Scholar] [CrossRef]
- Gong, R.H.; Yang, D.J.; Kwan, H.Y.; Lyu, A.P.; Chen, G.Q.; Bian, Z.X. Cell death mechanisms induced by synergistic effects of halofuginone and artemisinin in colorectal cancer cells. Int. J. Med. Sci. 2022, 19, 175–185. [Google Scholar] [CrossRef]
- Cai, J.; Hu, D.; Sakya, J.; Sun, T.; Wang, D.; Wang, L.; Mao, X.; Su, Z. ABIN-1 is a key regulator in RIPK1-dependent apoptosis (RDA) and necroptosis, and ABIN-1 deficiency potentiates necroptosis-based cancer therapy in colorectal cancer. Cell Death Dis. 2021, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Fujiya, M.; Tanaka, H.; Ueno, N.; Moriichi, K.; Sasajima, J.; Ikuta, K.; Akutsu, H.; Tanabe, H.; Kohgo, Y. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 2016, 7, 12365. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenoir, M.; Del Carmen, S.; Cortes-Perez, N.G.; Lozano-Ojalvo, D.; Munoz-Provencio, D.; Chain, F.; Langella, P.; de Moreno de LeBlanc, A.; LeBlanc, J.G.; Bermudez-Humaran, L.G. Lactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancer. J. Gastroenterol. 2016, 51, 862–873. [Google Scholar] [CrossRef]
- Li, M.; Song, L.H.; Yue, G.G.; Lee, J.K.; Zhao, L.M.; Li, L.; Zhou, X.; Tsui, S.K.; Ng, S.S.; Fung, K.P.; et al. Bigelovin triggered apoptosis in colorectal cancer in vitro and in vivo via upregulating death receptor 5 and reactive oxidative species. Sci. Rep. 2017, 7, 42176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, M.; Davoodi, J.; Zali, M.R.; Foroumadi, A. Concomitant activation of caspase-9 and down-regulation of IAP proteins as a mechanism of apoptotic death in HepG2, T47D and HCT-116 cells upon exposure to a derivative from 4-aryl-4H-chromenes family. Biomed. Pharmacother. 2011, 65, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Altonsy, M.O.; Andrews, S.C.; Tuohy, K.M. Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: Mediation by the mitochondrial pathway. Int. J. Food Microbiol. 2010, 137, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, X.; Zhang, L.; Zhang, Y.; Li, H.; Jiao, Y. Whole peptidoglycan extracts from the Lactobacillus paracasei subsp. paracasei M5 strain exert anticancer activity in vitro. Biomed. Res. Int. 2018, 2018, 2871710. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Xie, N.; Wang, Y. Cooperative effect of Bifidobacteria lipoteichoic acid combined with 5-fluorouracil on hepatoma-22 cells growth and apoptosis. Bull. Cancer 2015, 102, 204–212. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Kang, C.-H.; Kim, G.-H.; Cho, H. Anti-Tumor Effects of Heat-Killed L. reuteri MG5346 and L. casei MG4584 against Human Colorectal Carcinoma through Caspase-9-Dependent Apoptosis in Xenograft Model. Microorganisms 2022, 10, 533. https://doi.org/10.3390/microorganisms10030533
Kim S-J, Kang C-H, Kim G-H, Cho H. Anti-Tumor Effects of Heat-Killed L. reuteri MG5346 and L. casei MG4584 against Human Colorectal Carcinoma through Caspase-9-Dependent Apoptosis in Xenograft Model. Microorganisms. 2022; 10(3):533. https://doi.org/10.3390/microorganisms10030533
Chicago/Turabian StyleKim, Suk-Jin, Chang-Ho Kang, Gun-Hee Kim, and Hyosun Cho. 2022. "Anti-Tumor Effects of Heat-Killed L. reuteri MG5346 and L. casei MG4584 against Human Colorectal Carcinoma through Caspase-9-Dependent Apoptosis in Xenograft Model" Microorganisms 10, no. 3: 533. https://doi.org/10.3390/microorganisms10030533
APA StyleKim, S.-J., Kang, C.-H., Kim, G.-H., & Cho, H. (2022). Anti-Tumor Effects of Heat-Killed L. reuteri MG5346 and L. casei MG4584 against Human Colorectal Carcinoma through Caspase-9-Dependent Apoptosis in Xenograft Model. Microorganisms, 10(3), 533. https://doi.org/10.3390/microorganisms10030533





