Abstract
Background and Aims: Cases of Clostridioides difficile infection have been rising among the pediatric and adolescent population. Fecal microbiota transplantation (FMT) has emerged as an alternative therapy for recurrent C. difficile infection. We aim to perform the first systematic review and meta-analysis investigating the safety and efficacy of fecal microbiota transplantation for C. difficile infection in children and adolescents. Methods: A literature search was performed using variations of the keywords “pediatrics”, “C. difficile infection”, and “fecal microbiota transplantation” in PubMed, EMBASE, CINAHL, Cochrane, and Google Scholar from inception to 30 June 2022. The resulting 575 articles were independently screened by three authors. Fourteen studies that satisfied the eligibility criteria were included in the meta-analysis. Results: The pooled success rate of FMT in the overall cohort was 86% (95% confidence interval: 77–95%; p < 0.001; I2 = 70%). There were 38 serious adverse events in 36 patients with a pooled rate of 2.0% (95% confidence interval: 0.0–3.0%; p = 0.1; I2 = 0.0%) and 47 adverse events in 45 patients with a pooled rate of 15% (95% confidence interval: 5.0–25.0%; p = 0.02; I2 = 54.0%). There was no death associated with FMT. Conclusions: FMT was concluded to be an effective and safe therapy in pediatric and adolescent patients with C. difficile infection. Underlying comorbidities may impede the efficacy. A rigorous screening process of the donors is recommended prior to embarking on FMT. There is no universal and cost-effective way to monitor the long-term outcomes of FMT. While promising, metagenomic sequencing may not be available in settings with limited resources. Robust data from randomized clinical trials is warranted.
1. Introduction
Clostridioides difficile (formerly Clostridium difficile) is a nosocomial infection that causes severe watery diarrhea and is associated with increased hospitalization, healthcare costs, morbidity, and mortality [1,2,3]. In the United States (U.S.) alone, C. difficile infection (CDI) was responsible for half a million infections and over USD 1.5 billion in excess healthcare expenditure in 2011 [3]. Approximately 20% of patients who were diagnosed with CDI in 2011 had a recurrence of CDI, which is defined as persistence of symptoms within 60 days of completion of previous treatment [4]. Of those with recurrent CDI (rCDI), 29,000 patients died from rCDI [4]. Originally thought to affect adults only, CDI has also been increasing in its incidence among the pediatric population in both inpatient (hospital-acquired) and outpatient (community-acquired) settings over the past 20 years [4,5,6,7]. A 12.5-fold increase in incidence of CDI was observed among the pediatric population from 1991 to 2009 [5,6]. Furthermore, up to 35% of pediatric patients may experience recurrence after treatment with first-line agents [7]. The causes of recurrence include, but are not limited to dysbiosis of the microbiome, continued exposure to C. difficile, and immunocompromised status [5]. Although there is a substantial amount of published data available for CDI in adults, CDI in the pediatric population has been increasingly studied only in recent years. There is a paucity of information on CDI in pediatric patients compared to their adult counterparts with regard to the severity of the disease, the treatments available, the outcomes, and its impact on the pediatric patients.
The traditional treatment of CDI includes antibiotics such as fidaxomicin, oral or rectal vancomycin, and parenteral metronidazole as a single agent and/or in combination [1]. As dysbiosis and reduced biodiversity of the gut microbiome has been noted among patients with CDI or rCDI [6], fecal microbiota transplantation (FMT) has emerged in recent years as an alternative therapy for rCDI [3]. The donor sample typically originates from adults and can be attained through various preparations. For instance, the donor may be related to the recipient, such as a family member, or unrelated [3]. In either case, it is paramount that both donors and recipients undergo a screening process and have their stools analyzed for microbiome composition prior to the procedure [3,7]. The sample itself may be collected as a fresh specimen within 1–2 days prior to the transplantation or may be prepared from a frozen donor stool from the stool bank [7]. FMT can be accomplished via different routes of administration including, but not limited to, colonoscopy, capsules, and nasogastric tubes.
In adult patients, FMT has been demonstrated to be an effective therapy against CDI or rCDI [1,5]. The first successful use of FMT in the pediatric population for CDI was reported in the literature in 2010 [5]. In addition, FMT has been studied as a form of treatment in a variety of pediatric conditions such as inflammatory bowel disease, autism spectrum, and obesity [1,2,3,4,5,6,7]. Since 2010, several studies have investigated the effectiveness of FMT for the treatment of CDI in adolescents and pediatric patients. In 2019, the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition, and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition published a joint recommendation that FMT may be used in pediatric patients with rCDI [8]. However, the long-term effects (positive or negative) of alterations to the gut microbiome through FMT in the children and adolescent population remain to be seen. Therefore, it was recommended that FMT be performed only in experienced centers where the long-term effects could be monitored [8].
While FMT has been observed as an effective and safe therapy against CDI in children, adolescents, and young adults, there are currently no randomized controlled trials published in the literature and the available information is derived from observational studies, case series, and case reports [1,2,3,4,5,6,7,9,10]. Nonetheless, the current data from the literature must be studied and analyzed for better elucidation of the available information. Therefore, we aim to conduct the first systematic review and meta-analysis to determine the efficacy and safety of FMT in the treatment of CDI and/or rCDI in the patients <18 years of age, adolescents, and young adults.
2. Materials and Methods
2.1. Search Strategy
We performed a comprehensive literature search across five databases (Pubmed/Medline, EMBASE, CINAHL, Cochrane, Web of Science, and Google Scholar) using variations of the keywords “fecal microbiota transplant” and “pediatric” to identify original studies published from inception through to 30 June 2022. Results were limited to human studies published in English. There were a total of 575 studies for review.
Prior to screening the studies for eligibility to be included in the systematic review and meta-analysis, our review was registered on PROSPERO (PROSPERO registration number CRD42022343342; Registered 30 June 2022). See Supplemental Materials for detailed search terms.
2.2. Eligibility Criteria
Inclusion criteria were: (1) FMT for CDI and/or rCDI; (2) pediatric patients 21 years old or younger; (3) reporting of patient data and outcomes after first fecal infusion; (4) patients of any sex; (5) minimum follow-up of 2 months; (6) sample size of at least 5 patients; and (7) at least moderate quality of evidence. In 2017, the American Academy of Pediatrics defined adolescence from 12 to 21 years of age, and identified 21 years as the upper age limit of the pediatric population [11]. Furthermore, several FMT studies on pediatrics included age up to 21 years in the sample [5,12]. Therefore, patients of age up to 21 years old were included in our systematic review and meta-analysis.
Exclusion criteria: (1) case reports with less than 5 patients; (2) published abstracts, letters to editor, and commentaries which did not require detailed patient data or an extensive review process; (3) studies without patient data; (4) non-English studies; and (5) animal studies. Case series with more than 5 patients were included in our systematic review and meta-analysis. The threshold for the number of patients that distinguished between case series (5 or more patients) and case reports (less than 5 patients) was derived from a prior concept analysis by Abu-Zidan et al. [13].
2.3. Quality Assessment
Newcastle–Ottawa Scale (NOS) is used to evaluate the methodological quality in observational studies such as case-control and cohort studies. The risk of bias regarding the selection of subjects, comparability of subjects, and assessment of the exposure and outcome is graded by using a star system corresponding to nine items. A study is categorized as low risk of bias if a total of 8 to 9 stars are allocated, medium risk of bias if 6 to 7 stars are allocated, and high risk of bias if the study is given ≤5 stars [14].
For case series, the appraisal of quality and risk of bias was performed by a series of quality assessment tools developed by the US National Heart Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH, Bethesda, MD, USA) (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) (accessed on 1 August 2022). Similar to the NOS, a set of question items with yes/no answers were used, with a “Yes” counting as a score of 1 and a “No” as a score of 0. In this tool used for case series, there were a total of 9 questions. A score of 7–9 corresponded to good quality, while scores of 4–6 and 1–3 indicated moderate and poor quality, respectively [14].
In the final selection stage, only studies with at least a moderate level of evidence were included. Quality appraisal was performed by at least two of the following authors (K.M.T., C.H.L., T.L., and T.V.). If there was any disagreement, a senior reviewer (A.H.) evaluated the article and achieved consensus through discussion. See Supplemental Materials for quality assessment scores for each study.
2.4. Study Outcomes and Effect Size
The primary endpoint was the efficacy or clinical success of FMT in the treatment of rCDI among the pediatric patient population. The term “success” was defined as the resolution of symptoms (≥3 watery bowel movements in ≤24 h) and no requirement for further interventions for CDI for at least 8 weeks after the date of FMT [1,4]. The term “failure” or “recurrence” was used to describe an initial episode of CDI followed by persistence of symptoms or return of symptoms that needed further treatment for CDI within at least 8 weeks from the date of FMT [1,4,15]. Additionally, recurrence of CDI also required positive laboratory testing, such as a nucleic acid amplification test or stool toxin test in patients who remained symptomatic after FMT [1,15]. The effect size used in this study was the event rate (success rate). The event or success rate was calculated by dividing the number of reported events by the total sample size of the individual studies.
The secondary endpoint was the safety of FMT, which can be divided into serious adverse events (SAE) and adverse events (AE) that occurred within at least 8 weeks after FMT [15]. The SAEs and AEs were specifically labeled as attributable to FMT by the respective authors from each study. If there was no specific delineation, it was assumed that all the SAEs and AEs were related to FMT. SAEs were defined as death or any event requiring hospitalization. AEs included other adverse events that did not meet the criteria for SAE.
2.5. Study Selection and Data Extraction
A total of 575 articles were retrieved in the initial search. Two authors (K.M.T. and M.H.) independently reviewed these titles and abstracts. Afterwards, 23 articles were deemed relevant with patient data. Full texts were then reviewed by at least two of the following authors (K.M.T., C.H.L., T.L. and T.V.), after which 14 studies fulfilled the eligibility criteria. In cases of disagreement, a senior reviewer (A.H.) arbitrated the final decision for inclusion. The study selection process by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement is detailed in Figure 1. A list of 20 articles (as shown in Figure 1) that were retrieved and reviewed can be found in Supplemental Table S2. IRB review was not required as all data were extracted from published literature and no patient intervention was directly performed.
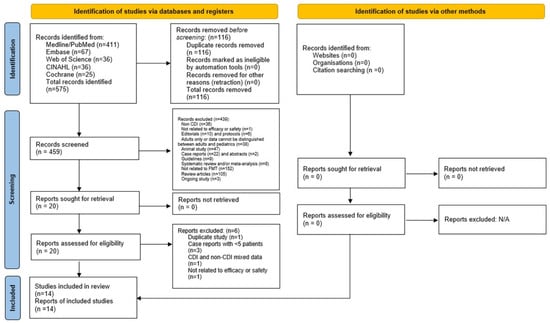
Figure 1.
Preferred reporting items for systematic review and meta-analysis.
2.6. Data Analysis
Individual estimates of each study were pooled to compute the summary estimates of the clinical success or efficacy of the FMT. A weighted summary statistic was calculated if many zero values occurred (e.g., adverse events) to prevent positive bias. The inverse variance heterogeneity (IVhet) model was fitted to account for methodological differences among the included studies for generating summary estimates [16]. The strength of evidence of heterogeneity across studies was determined by Cochran’s Q and I2 statistics [17,18,19]. The values of under 30, 30–60, 61–75, and over 75% were categorized as low, moderate, substantial, and considerable heterogeneity, respectively [20]). Subgroup analyses of the clinical success of FMT by gender were also performed. Sensitivity analysis was conducted to determine the validity of the estimated summary effect size. For the sensitivity analysis, a “leave-one-out analysis” was conducted to investigate the impact of the removal of study (one by one) on the estimates and LFK index asymmetry (Luis Furuya-Kanamori index). Publication bias was assessed by visually inspecting funnel plot and Doi plot [21,22]. In addition, Luis Furuya-Kanamori (LFK) index was used as a quantitative method to assess asymmetry of the study effects or publication bias as it has been noted in the literature that LFK index has higher sensitivity than the Egger regression statistics, particularly in meta-analysis with a small number of studies [21]. All meta-analyses were performed using MetaXL software (v. 5.3; EpiGear International, Sunrise Beach, Queensland, Australia). The 95% Clopper–Pearson exact confidence intervals and prediction intervals were calculated using R package [23,24].
3. Results
Table 1 summarizes the studies included in the systematic review and meta-analysis. There was a total of 14 studies that comprised five retrospective observational studies, five prospective observational studies, and four case series. In total, there were 904 pediatric patients who received FMT. In Nicholson, 2022 [1], the total number of patients who received FMT was 396, with success rates of 81.85% (203/248), 75.68% (112/148), and 79.55% (315/396), respectively, in the non-IBD, IBD, and overall cohorts. While efficacy was reported for the overall sample cohort, the following data was reported only for IBD cohort: gender, number of times of FMT administered, routes of FMT delivery, SAE, and AE.

Table 1.
Summary of included studies.
There were 326 male patients and 303 female patients from the studies that reported the genders of those who received FMT. Three studies (Aldrich, 2019 [4], Hourigan, 2019 [7], and Hourigan, 2015 [18]) did not distinguish the gender among the FMT patients. Nicholson, 2022 [1] reported gender data only for the IBD cohort as discussed above. The mean age of patients was 9.38 ± 2.80 years. There were five multi-center studies and nine single-center studies. All studies were performed within the U.S.A (North America), except for Li, 2022, [2] which was conducted in China (Asia). Almost all studies performed FMT for rCDI except for Nicholson, 2020 [5] and Barfield, 2018 [25], where FMT was used for both CDI and rCDI patients.
There were a total of 725 times FMT was administered since there were patients who received FMT more than once. Table 2 demonstrates the various routes via which FMT was administered. Delivery through the upper gastrointestinal tract included esophagogastroduodenoscopy, capsule, nasogastric, nasoduodenal, nasojejunal, gastric, duodenal, or jejunostomy tubes. Delivery through the lower gastrointestinal tract included colonoscopy, sigmoidoscopy, or enema. Colonoscopy was the most frequently used technique.

Table 2.
Routes of FMT administration.
Inflammatory bowel disease (IBD) was the most common concomitant disease, and was found in 337 patients (Table 3). Other concurrent gastrointestinal diseases included gastroesophageal reflux disease (n = 38), short bowel syndrome (n = 10), and celiac disease (n = 1). Neuromuscular disorders included epilepsy, Lennox–Gastaut syndrome, Emmanuel syndrome, and muscular dystrophy, while genetic disorders such as mitochondrial disease, and cystic fibrosis were also observed.

Table 3.
Comorbidities found among the patients included in the systematic review and meta-analysis.
3.1. Primary Outcome
The primary outcome was the efficacy of FMT in treating CDI or rCDI. Success was defined as the resolution of diarrhea symptoms (<3 watery bowel movements in ≤24 h) without requiring further treatment or interventions for CDI for at least 8 weeks after receiving FMT. Table 4 shows the number of patients who had success and those who had failure with the treatment in the FMT cohort. The rate of success ranged between 66 and 100%, the latter of which was found in seven studies. The gross success rate was 81.86% (740/904) while the overall failure rate was 18.14% (164/904).

Table 4.
Efficacy outcomes in the FMT cohort.
Table 5 further differentiates the FMT success cohort by gender. Some studies did not report data on gender and are thus labeled as not reported (NR). The gross success rate was 78.98% (233/295) in males and 82.51% (217/263) in females. The failure rates were 21.02% (62/295) and 17.49% (46/263) among male and female patients, respectively.

Table 5.
Efficacy outcomes by gender.
The calculated pooled rate of clinical success of FMT in the overall cohort was 86% (95% confidence interval [CI]: 77%, 95%; p < 0.001; I2 = 70%). Figure 2 shows the forest plot for clinical success or the efficacy of FMT. By gender, the calculated pooled rate of clinical success of FMT was 81% among males (95% confidence interval [CI]: 71%, 91%; p = 0.1; I2 = 40%) as opposed to an 84% success rate among their female counterparts (95% confidence interval [CI]: 78%, 90%; p = 0.4; I2 = 10%) as shown in Figure 3.
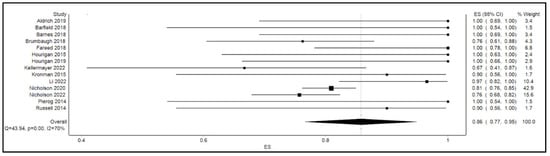
Figure 2.
Forest plot displaying clinical success or efficacy of FMT.
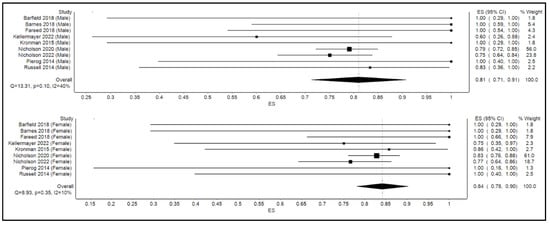
Figure 3.
Forest plot displaying clinical efficacy of FMT by gender.
Efficacy outcomes in the patients with IBD who received FMT are tabulated in Table 6. Li, 2022 [2] and Aldrich, 2019 [4] did not differentiate the results for IBD patients. In Aldrich, 2019 [4], it was noted that 14% of the 175 subjects had known or were later diagnosed with IBD, the number of which was approximated to 25. In Nicholson, 2020 [5], out of the 120 IBD patients, only 111 had data available regarding FMT. In Barfield, 2018 [25], one patient required an additional delivery of FMT after 3 months from initial FMT due to the recurrence of symptoms and achieved resolution of CDI thereafter. In Brumbaugh, 2018 [26], one IBD patient required FMT twice for success while one patient still had persistent CDI despite also receiving FMT twice. In Russell, 2014 [30], one patient continued to experience gastrointestinal symptoms but remained negative on enzyme immunoassay (EIA) for CDI. The patient’s symptoms improved after initiating treatment for Crohn’s disease. That patient was counted as a success. There was one patient who was lost to follow up after 2 months and was counted as a failure. The overall success rate of FMT for CDI in IBD patients was 75.33% (223/296) while the failure rate was 24.66% (73/296). Although the total number of patients in the IBD cohort was 337, since there were two studies that did not report results for IBD patients, the numbers of patients from those studies were not included in calculating the overall success and failure rates.

Table 6.
Efficacy outcomes by IBD.
3.2. Secondary Outcome
The secondary outcomes were serious adverse events (SAE) and adverse events (AE). Among the SAE and AE reported from the studies, only those that were determined to be attributable to FMT by the respective studies were included in our systematic review and meta-analysis. Most studies documented SAE and AE within 3 months after the administration of the FMT, except for the following: the follow-up periods of Aldrich, 2019 [4] and Barnes, 2018 [9] were 2 months and 2.5 months, respectively, while one patient in Russell, 2014 [30] was lost to follow-up after 4 months.
There were 38 SAE in 36 patients and 47 AE in 45 patients as shown in Table 7. There was no death attributable to FMT. The causes of SAE were variable and there was no single predominant cause. While there were 20 IBD-related SAE in Nicholson, 2022 [1], SAE and AE were reported only for the IBD cohort and not for the non-IBD cohort. Among the AE, diarrhea and abdominal pain were commonly recorded.

Table 7.
Serious adverse events and adverse events.
The calculated pooled rate of serious adverse events was 2.0% (95% confidence interval [CI]: 0.0%, 3.0%; p = 0.1; I2 = 0.0%), and the pooled rate of adverse events was 15% (95% confidence interval [CI]: 5.0%, 25.0%; p = 0.02; I2 = 54.0% as shown in Figure 4. Based on the validation analysis (described below), Nicholson, 2022 [1] and Nicholson, 2020 [5] were discovered to have dominant effects on the analysis. Therefore, Nicholson, 2022 [1] was removed from analyses for both SAE and AE, while Nicholson, 2020 [5] was removed from the forest plot for AE (Figure 4).
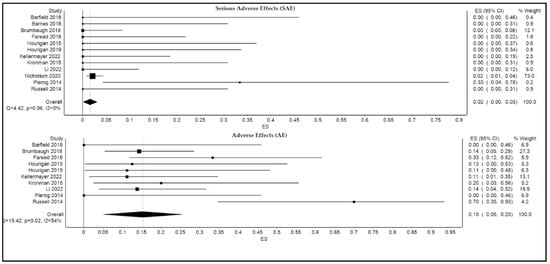
Figure 4.
Forest plot displaying pooled rates of serious adverse events and adverse events of FMT.
3.3. Validation Analysis (Leave-One-Out Analysis)
To assess if any study involved in the main analysis had a dominant effect, a leave-one-out analysis was conducted. As shown in Table 8, upon removal of each study one by one, no significant impact on the summary statistics of the primary outcome or heterogeneity was found. However, in the analysis of the secondary outcomes, one study in both SAE and AE (Nicholson, 2022) and one in AE (Nicholson, 2020) had dominant effects on the summary statistics, which were excluded from the analysis. These studies were removed from forest plot analysis for SAE and AE.

Table 8.
Outputs of sensitivity analysis (n = 14).
4. Discussion
To our best knowledge, this is the first systematic review and meta-analysis of the safety and efficacy of FMT via all routes of administration in treating CDI and rCDI amongst pediatric patients. A prior systematic review by Iqbal et al., in 2018 demonstrated the effectiveness of encapsulated FMT to treat rCDI in patients of all ages [31]. The overall success rate from that systematic review was 92.6% (316/341); the overall success rate from our meta-analysis was 81.86% (740/904). The discrepancy in the efficacy can be explained by several factors. Firstly, Iqbal et al., included patients from all ages and there was only one study that consisted of data solely from pediatric patients [31,32]. Moreover, the review focused on FMT delivered via capsules only. On the other hand, our review focused solely on pediatric and adolescent patients up to and including the age of 21 and FMT administered via any route. These variables could have accounted for the difference in efficacy. Indeed, studies have concluded that encapsulated FMT may be considered in adult patients who previously failed to achieve resolution of CDI by other methods of FMT delivery such as colonoscopy [31,33,34]. A prior systematic review by Iqbal et al., concluded that encapsulated FMT was similar in efficacy and was associated with fewer adverse events when compared to colonoscopy [31]. In the same study, 8 out of 10 patients who previously failed FMT with colonoscopy achieved resolution of rCDI with encapsulated FMT [31]. Furthermore, routes such as colonoscopy, enema, nasogastric, or nasoduodenal tubes have been demonstrated to be inconvenient for patients [33], while oral preparation has been associated with greater ease of administration [34].
Our study confirmed a pooled success rate of 86% (95% CI: 77–95%, p < 0.001). Hence, FMT may still be used as an effective treatment for rCDI in pediatric patients. In fact, based on the success rate reported in the literature for the pediatric patient population, Infectious Diseases Society of America (IDSA) recommended, in 2017, that FMT may be considered in those who continued to experience multiple recurrences of CDI despite treatment with standard antibiotics [35,36].
However, despite its efficacy, FMT still remains relatively poorly regulated and standardized [36]. As discussed above, the routes of FMT administration may impact its effectiveness. Comorbidities and the gut microbiome composition of the recipient or the donor may also interfere with its therapeutic potential and may also be associated with adverse outcomes. In addition to the variabilities in success rate, since the delivery of FMT often requires a procedure such as colonoscopy, esophagogastroduodenoscopy, or use of enteral tubes, it also carries procedure-related risks [35]. The most common adverse outcomes from our review were diarrhea and abdominal pain or discomfort. The findings were consistent with the results previously reported in the literature for both adults and pediatrics [31,36]. Concerns have also been raised regarding SAE, such as transmission of multi-drug resistant organisms (MDRO), and blood-borne infections [35]. In 2019, the United States Food and Drug Administration released a safety alert and recommendations for more comprehensive screening and testing of the donors after two immunocompromised adult patients received FMT from a donor with positive extended-spectrum beta-lactamase (ESBL) producing Escherichia coli [36]. Both patients developed the infection and one subsequently expired.
In our review, the rate of SAE and AE was low overall. The pooled rate for SAE was 2.0% (95% CI: 0.0–3.0%, p = 0.1) and the pooled rate for AE was 15% (95% CI: 5.0–25.0%, p = 0.02). The pooled rate for AE was slightly higher but comparable to the 12.5% that was derived from a systematic review of adult FMT patients by Iqbal et al. [31]. We also did not identify any deaths attributable to FMT, similar to the results by Iqbal et al., [31]. Furthermore, there was no infection caused by an MDRO after receiving FMT. Nonetheless, the causative pathogens from the aspiration pneumonia, appendicitis, or the infection unrelated to the gastrointestinal tract, which were part of SAE in our meta-analysis, were not known. It is also unclear whether the IBD-related hospitalizations or exacerbations were related to an infection episode. Moreover, viral RNA of SARS-CoV-2 has been found in the feces of infected patients [37]. Although there has not yet been documented transmission of COVID-19 via feces, the FDA recommended screening for the virus in potential donors [36]. Taking everything into consideration, we concur that donors should undergo a comprehensive screening process for infectious agents, including COVID-19, prior to the transplantation. While FMT has been successfully used to decolonize adult patients with MDROs, the available data is limited for similar use in pediatric patients and further research is warranted [38].
It is worth noting that both the recommendation from the FDA and the onset of the COVID-19 pandemic have led to changes not only in the practice of FMT administration but also the availability and access of FMT for pediatric patients [39]. For instance, the FDA also limited use of FMT to only emergent situations in 2020 and early 2021 during the peak of the COVID-19 pandemic [39]. A survey of pediatric gastroenterologists also showed that some practitioners took additional precautions such as increased screening of the donors or avoidance of FMT in immunocompromised patients. Some physicians increased the utilization of antibiotics such as fidaxomicin and oral vancomycin, while others entirely paused the program [39].
Dysbiosis has been established among IBD patients [40]. The success rate for FMT for CDI in pediatric patients with IBD from our review was 75.00% and was effective but was lower than the pooled success rate of 86.00%. The results were similar to prior observational studies such as Nicholson, 2022 [1], where the efficacy rates for overall, IBD, and non-IBD cohorts were 79.55, 75.68, and 81.85%, respectively. Nevertheless, Nicholson et al., concluded that there was no difference in FMT success between children with IBD and without IBD [1]. However, the authors discovered that a high proportion of children with FMT failure was found among those with clinically active IBD [1]. There are several confounding variables that can potentially influence the outcomes of FMT for CDI among IBD patients. Some examples include the type of stool received (e.g., fresh versus frozen stools), time from diagnosis of CDI until FMT, severity of CDI or IBD symptoms, mode of delivery, and concomitant medications, among others [1,40]. A systematic review and meta-analysis by Tariq et al. concluded that FMT is an effective therapy for rCDI in adult patients with IBD [41]. A similar analysis for pediatric patients has not been completed to the best of our knowledge.
Lastly, FMT has been associated with the restoration of the gut microbiome in CDI patients to the levels of healthy children [3]. Since the patients are at an age where the gut microbiome is still undergoing development at the time of receiving FMT, manipulation of the microbiome may influence metabolic or immune dysregulations [36]. In particular, restoration was noted in the levels of Lachnospiraceae, Ruminococcaceae, Bifidobacteriaceae, Erysipelotrichaceae, and Bacteroidaceae, the latter of which has been believed to be a key protective member of the gut microbiota [3]. Decolonization of Enterobacteriaceae, which can remain abundant in treatment-naive CDI patients prior to FMT, was also observed [3]. In other words, metagenomics may be a potential modality to predict long-term outcome of FMT in pediatric patients with rCDI. However, the presence of comorbidities such as IBD, immunocompromised status, or neurologic conditions may impede the process of reconstituting the gut microbiome and can result in the failure of FMT or requiring multiple administration of FMT [3]. The cost of metagenomic sequencing also presents a practical barrier to be performed in every patient receiving FMT. Thus, metagenomic sequencing of the gut microbiome may not be used as a predictor of long-term outcome in pediatric patients with complicated background comorbidities and/or in settings with limited resources [3].
Limitations
We acknowledge several limitations. First, there were no randomized controlled trials in the previously published literature to include in our meta-analysis. Second, two studies needed to be excluded from the secondary outcome analysis to avoid a predominant effect on the analysis. Third, given that this is a systematic review and meta-analysis, we were not able to control for confounding variables in the patient qualities or the procedural protocols. Moreover, the determination of whether SAE or AE were attributable to FMT was made by the original authors. Since we were unable to review the original data of the studies, it is possible that the number of SAE or AE related to FMT may not accurately reflect the true number of adverse events. Fourth, there was evidence of publication bias present as shown by the Doi plot in Figure 5, Funnel plot in Figure 6, and an LFK index of greater than 4.
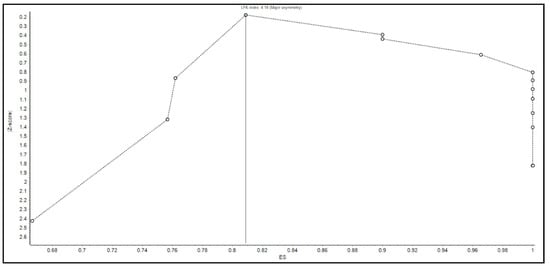
Figure 5.
Doi plot for assessing the evidence of publication bias.
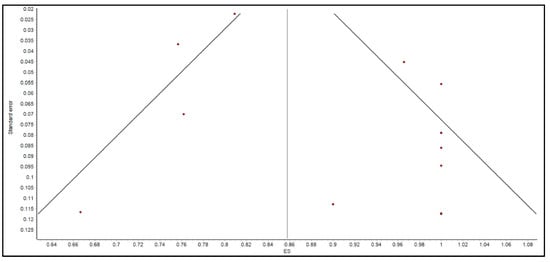
Figure 6.
Funnel plot for assessing the evidence of publication bias.
Lastly, we did not include Cho, 2019 [42] in our meta-analysis. The efficacy rate reported by Cho et al., was 75% from eight patients [42], which was comparable to that of the overall cohort of 904 patients (86%) and the IBD cohort of 296 patients (75.33%) from our meta-analysis. In addition, there was one SAE from Cho, 2019 [42] with abdominal pain and fever that was ultimately concluded to be related to influenza and not to FMT by the original authors. There were no other SAE or AE attributable to FMT from Cho, 2019 [42]. Therefore, while the study was not included in the meta-analysis, the impact was determined to be negligible in the current meta-analysis.
5. Conclusions and Future Directions
Despite its efficacy and safety profile, FMT remains a poorly regulated and standardized therapeutic modality. While there have been established data on FMT for adult patients with rCDI, similar information for the pediatric population has only emerged recently. Our systematic review and meta-analysis demonstrated that FMT can be an effective and safe therapy for pediatric and adolescent patients with rCDI. However, the effectiveness may be hindered by the presence of underlying conditions such as IBD or immunodeficiency. The availability and accessibility of FMT were also further deterred by the COVID-19 pandemic and the risk of transferring virulent pathogens during FMT. Although metagenomics provided promising data, there has yet to be an established tool to predict the long-term outcomes for the children who received FMT. There may also only be a few medical centers where such long-term monitoring can be provided. There is much to be explored regarding the use of FMT in the pediatric patient population, and robust data from randomized clinical trials is warranted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10122450/s1, Table S1: Search terms used to identify and filter studies from literature, Table S2: List of studies for which full articles were retrieved and reviewed, Table S3: Risk of bias assessment for observational cohort studies with Newcastle–Ottawa Scale, and Table S4: Risk of bias assessment for case series with NIH quality assessment tool. References [1,2,3,4,5,7,9,10,25,26,27,28,29,30,43,44,45,46,47,48] are cited in the supplementary materials.
Author Contributions
Conceptualization: K.M.T., M.H. and A.S.H.; methodology: K.M.T. and A.S.H. data retrieval: K.M.T., M.H., C.-H.L., T.L. and T.V.; formal analysis: K.B.; writing—original draft preparation: K.M.T. and K.B.; writing—review and editing: K.M.T., M.H. and K.B., C.-H.L., S.M. and A.S.H.; supervision—A.S.H., T.L., T.V. and S.M. share equal co-authorship. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study since this is a systematic review and meta-analysis based on the data that have already been published in the literature.
Informed Consent Statement
Not applicable. This is a systematic review and meta-analysis based on the data that have already been published in the literature.
Data Availability Statement
Data supporting the statements can be found on PubMed, EMBASE, CINAHL, Web of Science, Cochrane and Google Scholar.
Acknowledgments
We acknowledge Aidybert Weeks, librarian from Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, for her assistance with literature search. T.L., T.V., and S.M. share equal co-authorship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicholson, M.R.; Alexander, E.; Ballal, S.; Davidovics, Z.; Docktor, M.; Dole, M.; Gisser, J.M.; Goyal, A.; Hourigan, S.K.; Jensen, M.K.; et al. Efficacy and Outcomes of Faecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection in Children with Inflammatory Bowel Disease. J. Crohns Colitis 2022, 16, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, F.; Li, Y.; Hu, H.; Xiao, Y.; Xu, Q.; Li, D.; Yu, G.; Wang, Y.; Zhang, T. Characteristics and management of children with Clostridioides difficile infection at a tertiary pediatric hospital in China. Braz. J. Infect. Dis. 2022, 26, 102380. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, R.; Wu, Q.; Nagy-Szakal, D.; Queliza, K.; Ihekweazu, F.D.; Bocchini, C.E.; Magee, A.R.; Oezguen, N.; Spinler, J.K.; Hollister, E.B.; et al. Fecal Microbiota Transplantation Commonly Failed in Children with Co-Morbidities. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, A.M.; Argo, T.; Koehler, T.J.; Olivero, R. Analysis of Treatment Outcomes for Recurrent Clostridium difficile Infections and Fecal Microbiota Transplantation in a Pediatric Hospital. Pediatr. Infect. Dis. J. 2019, 38, 32–36. [Google Scholar] [CrossRef]
- Nicholson, M.R.; Mitchell, P.D.; Alexander, E.; Ballal, S.; Bartlett, M.; Becker, P.; Davidovics, Z.; Docktor, M.; Dole, M.; Felix, G.; et al. Efficacy of Fecal Microbiota Transplantation for Clostridium difficile Infection in Children. Clin. Gastroenterol. Hepatol. 2020, 18, 612–619.e1. [Google Scholar] [CrossRef]
- Nicholson, M.R.; Thomsen, I.P.; Slaughter, J.C.; Creech, C.B.; Edwards, K.M. Novel risk factors for recurrent Clostridium difficile infection in children. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 18–22. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Ahn, M.; Gibson, K.M.; Pérez-Losada, M.; Felix, G.; Weidner, M.; Leibowitz, I.; E Niederhuber, J.; Sears, C.L.; Crandall, K.A.; et al. Fecal Transplant in Children with Clostridioides difficile Gives Sustained Reduction in Antimicrobial Resistance and Potential Pathogen Burden. Open Forum Infect. Dis. 2019, 6, ofz379. [Google Scholar] [CrossRef]
- Davidovics, Z.H.; Michail, S.; Nicholson, M.R.; Kociolek, L.; Pai, N.; Hansen, R.; Schwerd, T.; Maspons, A.; Shamir, R.; Szajewska, H.; et al. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection and Other Conditions in Children: A Joint Position Paper from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 130–143. [Google Scholar] [CrossRef]
- Barnes, D.; Ng, K.; Smits, S.; Sonnenburg, J.; Kassam, Z.; Park, K.T. Competitively Selected Donor Fecal Microbiota Transplantation: Butyrate Concentration and Diversity as Measures of Donor Quality. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 185–187. [Google Scholar] [CrossRef]
- Fareed, S.; Sarode, N.; Stewart, F.J.; Malik, A.; Laghaie, E.; Khizer, S.; Yan, F.; Pratte, Z.; Lewis, J.; Immergluck, L.C. Applying fecal microbiota transplantation (FMT) to treat recurrent Clostridium difficile infections (rCDI) in children. PeerJ 2018, 6, e4663. [Google Scholar] [CrossRef]
- Hardin, A.P.; Hackell, J.M.; Simon, G.R.; Boudreau, A.D.A.; Baker, C.N.; Barden, G.A. Committee on Practice And Ambulatory Medicine. Age limit of pediatrics. Pediatrics 2017, 140, e20172151. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Popov, J.; Pai, N. Fecal microbial transplant for the treatment of pediatric inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 10304–10315. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zidan, F.M.; Abbas, A.K.; Hefny, A.F. Clinical “case series”: A concept analysis. Afr. Health Sci. 2012, 12, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel Members; Jensen, M.D.; Ryan, D.H.; Donato, K.A.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; et al. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity 2014, 22, S5–S39. [Google Scholar] [CrossRef]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.J.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018, 67, 1920–1941. [Google Scholar] [CrossRef]
- Doi, S.A.R.; Furuya-Kanamori, L. Selecting the best meta-analytic estimator for evidence-based practice: A simulation study. Int. J. Evid. Based Healthc. 2020, 18, 86–94. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kanwal, F.; White, D. “Systematic Reviews and Meta-analyses” in Clinical Gastroenterology and Hepatology. Clin. Gastroenterol. Hepatol. 2012, 10, 1184–1186. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Fagan, T. Exact 95% confidence intervals for differences in binomial proportions. Comput. Biol. Med. 1999, 29, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Lin, L. Use of Prediction Intervals in Network Meta-analysis. JAMA Netw. Open 2019, 2, e199735. [Google Scholar] [CrossRef] [PubMed]
- Barfield, E.; Small, L.; Navallo, L.; Solomon, A. Going to the Bank: Fecal Microbiota Transplantation in Pediatrics. Clin. Pediatr. 2018, 57, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, D.E.; De Zoeten, E.F.; Pyo-Twist, A.; Fidanza, S.; Hughes, S.; Dolan, S.A.; Child, J.; Dominguez, S.R. An Intragastric Fecal Microbiota Transplantation Program for Treatment of Recurrent Clostridium difficile in Children is Efficacious, Safe, and Inexpensive. J. Pediatr. 2018, 194, 123–127.e1. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, S.K.; Chen, L.A.; Grigoryan, Z.; Laroche, G.; Weidner, M.; Sears, C.L.; Oliva-Hemker, M. Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 42, 741–752. [Google Scholar] [CrossRef]
- Pierog, A.; Mencin, A.; Reilly, N.R. Fecal microbiota transplantation in children with recurrent Clostridium difficile infection. Pediatr. Infect. Dis. J. 2014, 33, 1198–1200. [Google Scholar] [CrossRef]
- Kronman, M.P.; Nielson, H.J.; Adler, A.L.; Giefer, M.J.; Wahbeh, G.; Singh, N.; Zerr, D.M.; Suskind, D.L. Fecal microbiota transplantation via nasogastric tube for recurrent Clostridioides difficile infection in pediatric patients. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 23–26. [Google Scholar] [CrossRef]
- Russell, G.H.; Kaplan, J.L.; Youngster, I.; Baril-Dore, M.; Schindelar, L.; Hohmann, E.; Winter, H.S. Fecal transplant for recurrent Clostridium difficile infection in children with and without inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 588–592. [Google Scholar] [CrossRef]
- Iqbal, U.; Anwar, H.; Karim, M.A. Safety and efficacy of encapsulated fecal microbiota transplantation for recurrent Clostridium difficile infection: A systematic review. Eur. J. Gastroenterol. Hepatol. 2018, 30, 730–734. [Google Scholar] [CrossRef]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014, 312, 1772–1778, Erratum in JAMA 2015, 313, 729. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.T.; Obrenovich, M.E.; Cadnum, J.L.; Jencson, A.L.; Jain, A.K.; Ho, E.; Donskey, C.J. Fecal Microbiota Transplantation by Freeze-Dried Oral Capsules for Recurrent Clostridium difficile Infection. Open Forum Infect. Dis. 2016, 3, ofw091. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Hamilton, M.J.; Vaughn, B.P.; Graiziger, C.T.; Newman, K.M.; Kabage, A.J.; Sadowsky, M.J.; Khoruts, A. Successful Resolution of Recurrent Clostridium difficile Infection using Freeze-Dried, Encapsulated Fecal Microbiota; Pragmatic Cohort Study. Am. J. Gastroenterol. 2017, 112, 940–947. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Nicholson, M.R.; Kahn, S.A.; Kellermayer, R. Updates and Challenges in Fecal Microbiota Transplantation for Clostridioides difficile Infection in Children. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 430–432. [Google Scholar] [CrossRef]
- Xiao, F.; Sun, J.; Xu, Y.; Li, F.; Huang, X.; Li, H.; Zhao, J.; Huang, J.; Zhao, J. Infectious SARS-CoV-2 in Feces of Patient with Severe COVID-19. Emerg. Infect. Dis. 2020, 26, 1920–1922. [Google Scholar] [CrossRef]
- Chen, C.C.; Chiu, C.H. Current and future applications of fecal microbiota transplantation for children. Biomed. J. 2022, 45, 11–18. [Google Scholar] [CrossRef]
- Nicholson, M.R.; Hourigan, S.K.; Conrad, M.; Goyal, A.; Jensen, K.; Kelsen, J.; Kennedy, M.; Weatherly, M.; Kahn, S.A. Current Challenges in Fecal Microbiota Transplantation for Clostridioides difficile Infection in Children. Am. J. Gastroenterol. 2021, 116, 1954–1956. [Google Scholar] [CrossRef]
- Kellermayer, R. Fecal microbiota transplantation: Great potential with many challenges. Transl. Gastroenterol. Hepatol. 2019, 4, 40. [Google Scholar] [CrossRef]
- Tariq, R.; Syed, T.; Yadav, D.; Prokop, L.J.; Singh, S.; Loftus, E.V., Jr.; Pardi, D.S.; Khanna, S. Outcomes of Fecal Microbiota Transplantation for, C. difficile Infection in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2021; Online ahead of print. [Google Scholar] [CrossRef]
- Cho, S.; Spencer, E.; Hirten, R.; Grinspan, A.; Dubinsky, M.C. Fecal Microbiota Transplant for Recurrent Clostridium difficile Infection in Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 343–347. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Wang, Y.-Z.; Li, X.-L.; Hu, H.; Liu, H.-F.; Li, D.; Xiao, Y.-M.; Zhang, T. Safety of fecal microbiota transplantation in Chinese children: A single-center retrospective study. World J. Clin. Cases 2018, 6, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Hu, H.; Xiao, Y.; Li, D.; Yu, G.; Yu, D.; Zhang, T.; Wang, Y. Clinical Efficacy and Microbiome Changes Following Fecal Microbiota Transplantation in Children with Recurrent Clostridium difficile Infection. Front. Microbiol. 2018, 9, 2622. [Google Scholar] [CrossRef] [PubMed]
- Drewes, J.L.; Corona, A.; Sanchez, U.; Fan, Y.; Hourigan, S.K.; Weidner, M.; Sidhu, S.D.; Simner, P.J.; Wang, H.; Timp, W.; et al. Transmission and clearance of potential procarcinogenic bacteria during fecal microbiota transplantation for recurrent Clostridioides difficile. JCI Insight 2019, 4, e130848. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, H.; Kronman, M.P.; Suskind, D.L. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infections in Pediatric Hematopoietic Stem Cell Transplant Recipients. J Pediatr. Infect. Dis. Soc. 2018, 7, e6–e8. [Google Scholar] [CrossRef]
- Walia, R.; Garg, S.; Song, Y.; Girotra, M.; Cuffari, C.; Fricke, W.F.; Dutta, S.K. Efficacy of fecal microbiota transplantation in 2 children with recurrent Clostridium difficile infection and its impact on their growth and gut microbiome. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 565–570. [Google Scholar] [CrossRef]
- Ooijevaar, R.E.; van Rossen, T.M.; Vandenbroucke-Grauls, C.M.J.E.; Budding, A.E.; Kneepkens, C.M.F.; de Meij, T.G.J. Fecestransplantatie voor kinderen met recidiverende C. difficile-infecties [Faecal transplants for children with recurrent infections]. Ned. Tijdschr. Geneeskd. 2019, 163, D3739. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).