An In-Silico Evaluation of Anthraquinones as Potential Inhibitors of DNA Gyrase B of Mycobacterium tuberculosis
Abstract
1. Introduction
2. Methods
2.1. Target and Ligands Preparation
2.2. Molecular Docking-Based Virtual Screening Analyses
2.3. Pharmacokinetic, Cytotoxicity and Synthetic Accessibility Predictions
2.4. Target Fishing Predictions
2.5. Molecular Dynamic Simulations
2.6. Binding Free Energy Calculation
2.7. Visualizations of Molecular Docking and Molecular Dynamics Results
3. Results and Discussion
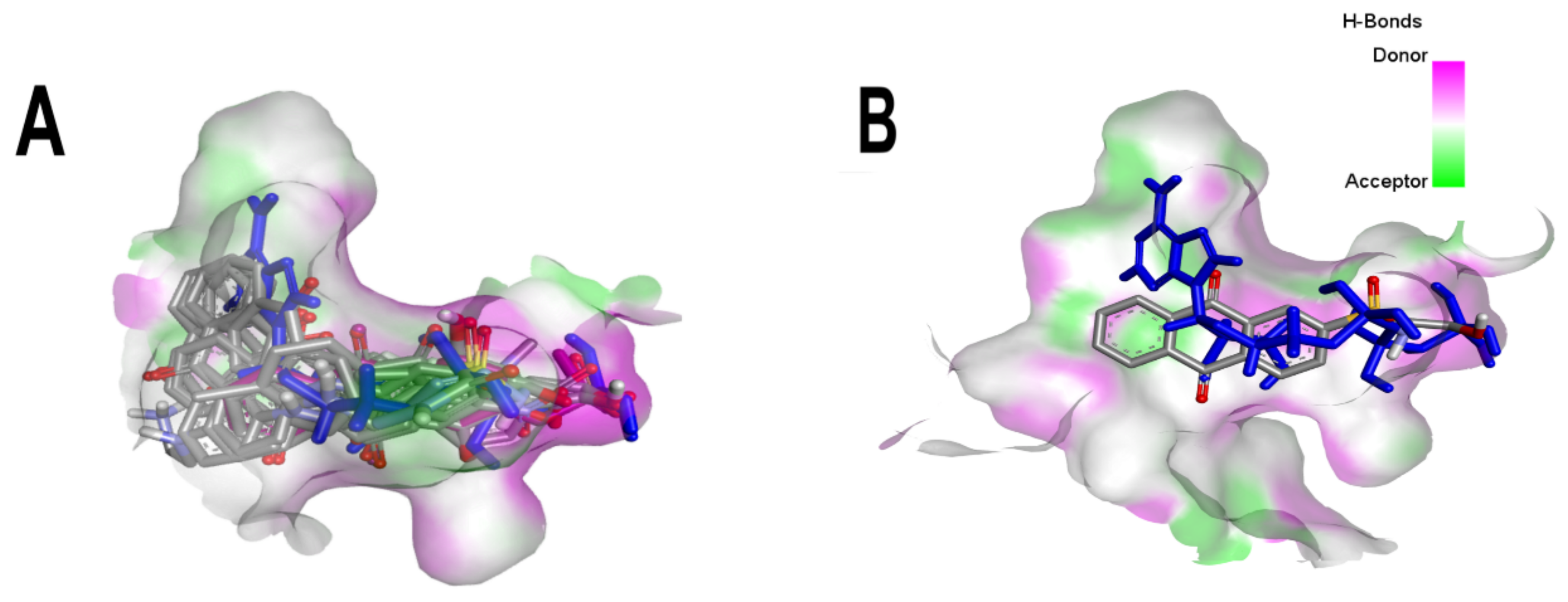
3.1. Virtual Screening of Anthraquinones
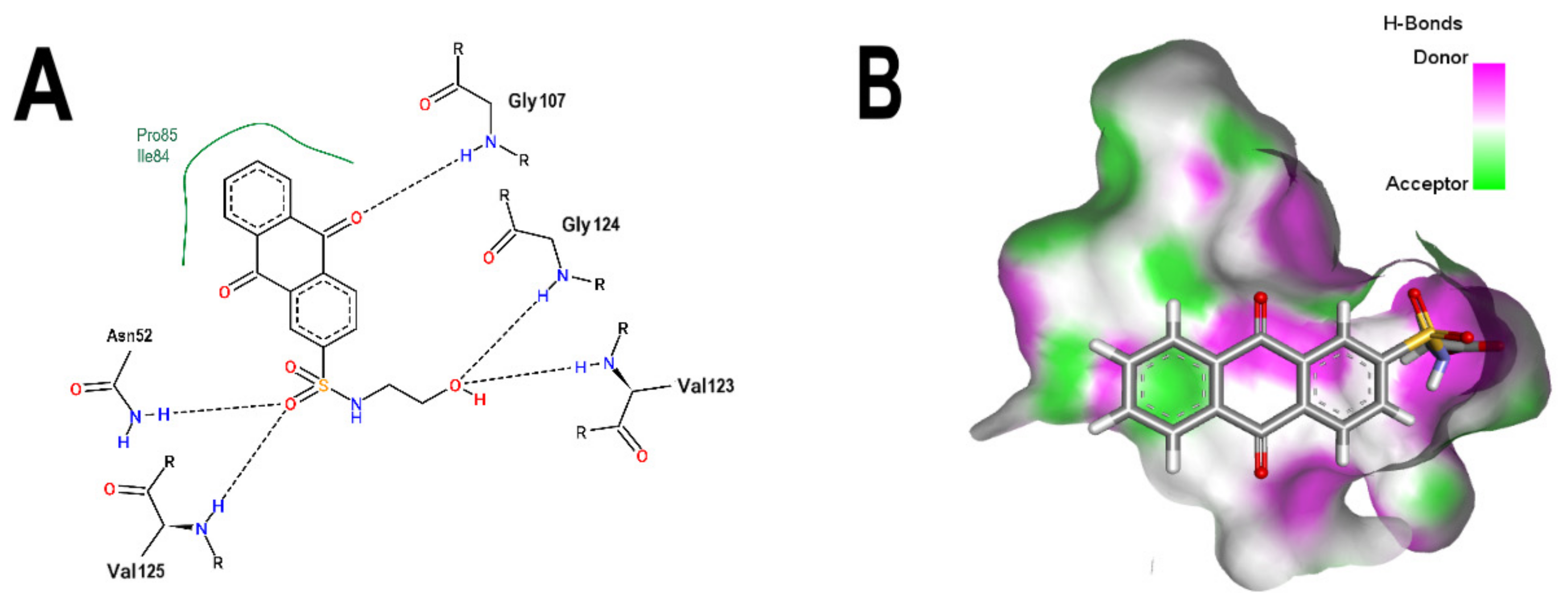
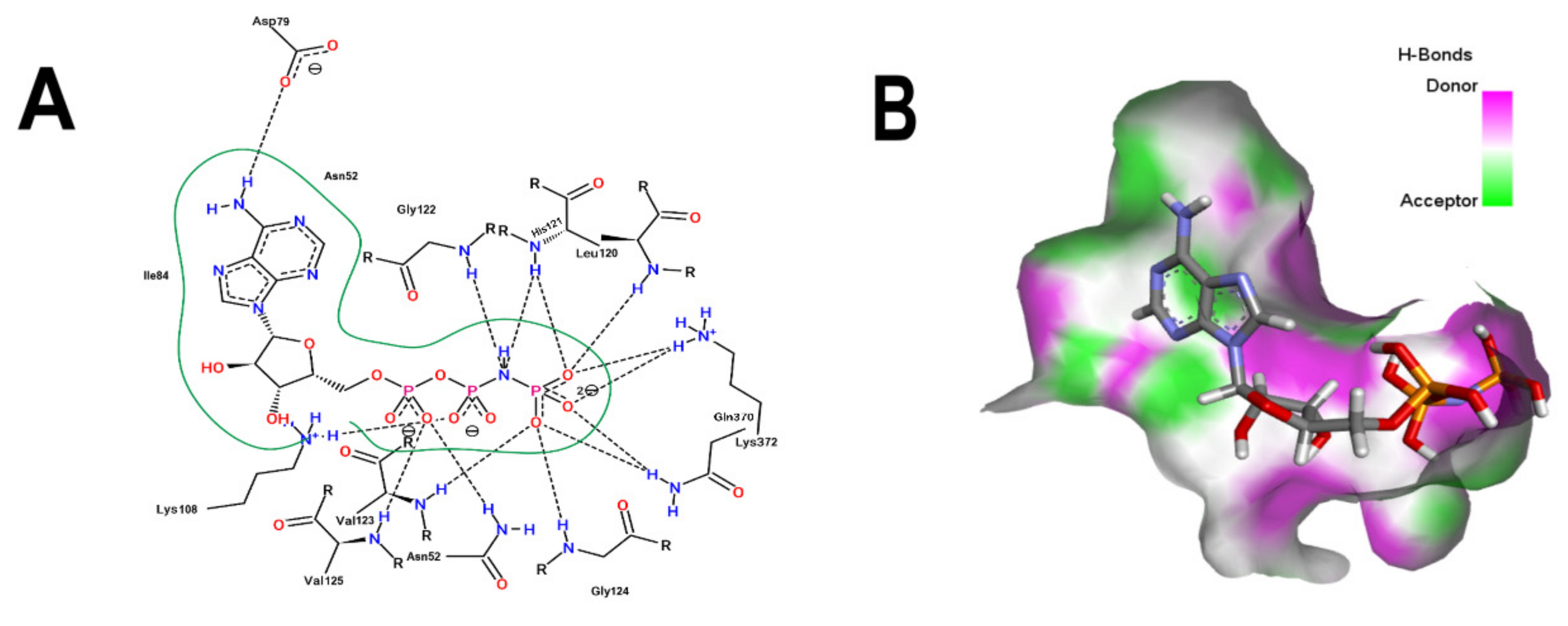
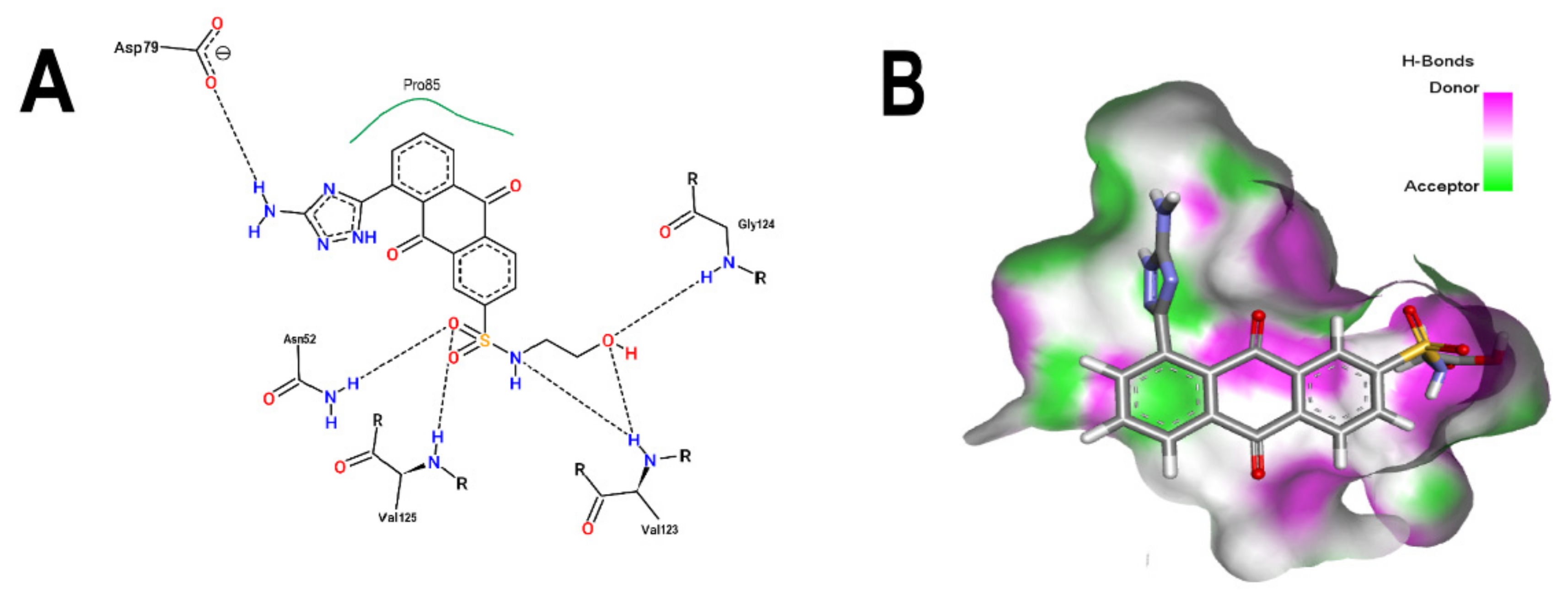
3.2. Comparative Analyses of the ANP and BS-1 Poses
3.3. Pharmacokinetic and Cytotoxicity Analyses
3.4. Target Fishing Analysis
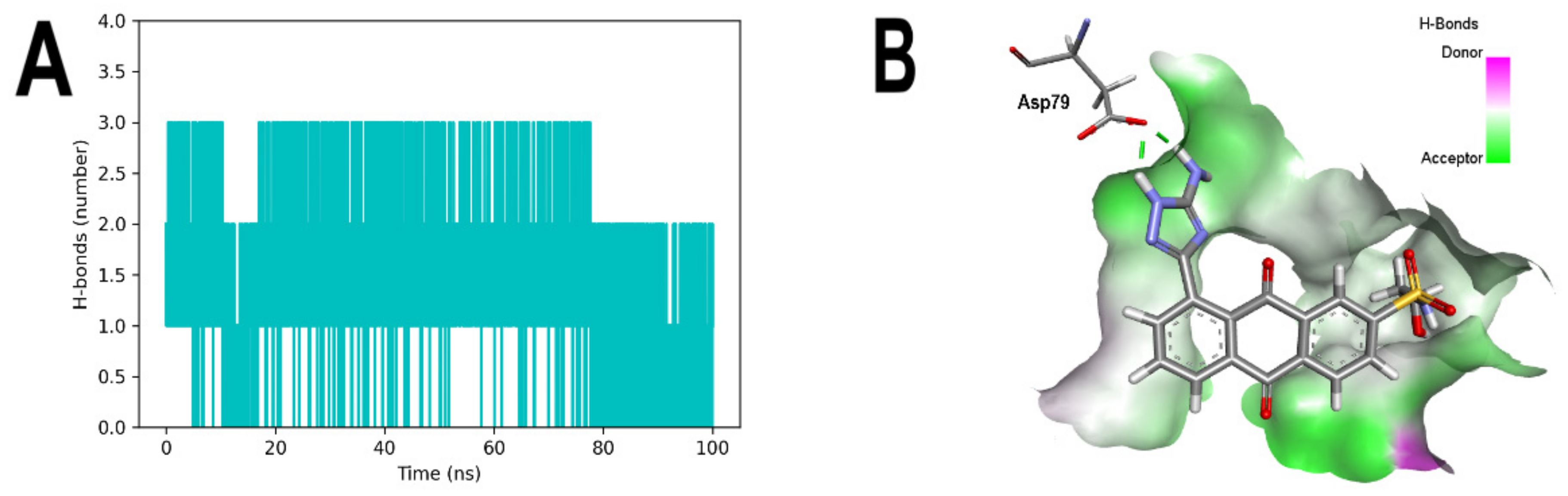
3.5. Molecular Dynamic Simulations Analyses
3.6. Binding Free Energy Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allué-Guardia, A.; García, J.I.; Torrelles, J.B. Evolution of Drug-Resistant Mycobacterium tuberculosis Strains and Their Adaptation to the Human Lung Environment. Front. Microbiol. 2021, 12, 612675. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2021; WHO: Geneva, Switzerland, 2021; ISBN 9789240037021. [Google Scholar]
- Zhai, W.; Wu, F.; Zhang, Y.; Fu, Y.; Liu, Z. The immune escape mechanisms of Mycobacterium Tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Bendre, A.D.; Peters, P.J.; Kumar, J. Tuberculosis: Past, present and future of the treatment and drug discovery research. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100037. [Google Scholar] [CrossRef] [PubMed]
- Emane, A.K.A.; Guo, X.; Takiff, H.E.; Liu, S. Drug resistance, fitness and compensatory mutations in Mycobacterium tuberculosis. Tuberculosis 2021, 129, 102091. [Google Scholar] [CrossRef]
- Nagaraja, V.; Godbole, A.A.; Henderson, S.R.; Maxwell, A. DNA topoisomerase I and DNA gyrase as targets for TB therapy. Drug Discov. Today 2017, 22, 510–518. [Google Scholar] [CrossRef]
- McKie, S.J.; Neuman, K.C.; Maxwell, A. DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. BioEssays 2021, 43, 2000286. [Google Scholar] [CrossRef]
- Vann, K.R.; Oviatt, A.A.; Osheroff, N. Topoisomerase II Poisons: Converting Essential Enzymes into Molecular Scissors. Biochemistry 2021, 60, 1630–1641. [Google Scholar] [CrossRef]
- Manjunatha, U.H.; Dalal, M.; Chatterji, M.; Radha, D.R.; Visweswariah, S.S.; Nagaraja, V. Functional characterization of mycobacterial DNA gyrase: An efficient decatenase. Nucleic Acids Res. 2002, 30, 2144–2153. [Google Scholar] [CrossRef]
- Ruiz, J. Mechanisms of resistance to quinolones: Target alterations, decreased accumulation and DNA gyrase protection. J. Antimicrob. Chemother. 2003, 51, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Heide, L. New aminocoumarin antibiotics as gyrase inhibitors. Int. J. Med. Microbiol. 2014, 304, 31–36. [Google Scholar] [CrossRef]
- Barančoková, M.; Kikelj, D.; Ilaš, J. Recent progress in the discovery and development of DNA gyrase B inhibitors. Future Med. Chem. 2018, 10, 1207–1227. [Google Scholar] [CrossRef] [PubMed]
- Shirude, P.S.; Madhavapeddi, P.; Tucker, J.A.; Murugan, K.; Patil, V.; Basavarajappa, H.; Raichurkar, A.V.; Humnabadkar, V.; Hussein, S.; Sharma, S.; et al. Aminopyrazinamides: Novel and specific GyrB inhibitors that kill replicating and nonreplicating Mycobacterium tuberculosis. ACS Chem. Biol. 2013, 8, 519–523. [Google Scholar] [CrossRef]
- Brvar, M.; Perdih, A.; Renko, M.; Anderluh, G.; Turk, D.; Solmajer, T. Structure-Based discovery of substituted 4,5′-bithiazoles as novel DNA gyrase inhibitors. J. Med. Chem. 2012, 55, 6413–6426. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Q.; Romero, J.A.C.; Cross, J.; Wang, B.; Poutsiaka, K.M.; Epie, F.; Bevan, D.; Wu, Y.; Moy, T.; et al. Discovery of Indazole Derivatives as a Novel Class of Bacterial Gyrase B Inhibitors. ACS Med. Chem. Lett. 2015, 6, 1080–1085. [Google Scholar] [CrossRef][Green Version]
- Sherer, B.A.; Hull, K.; Green, O.; Basarab, G.; Hauck, S.; Hill, P.; Loch, J.T.; Mullen, G.; Bist, S.; Bryant, J.; et al. Pyrrolamide DNA gyrase inhibitors: Optimization of antibacterial activity and efficacy. Bioorg. Med. Chem. Lett. 2011, 21, 7416–7420. [Google Scholar] [CrossRef]
- Ronkin, S.M.; Badia, M.; Bellon, S.; Grillot, A.L.; Gross, C.H.; Grossman, T.H.; Mani, N.; Parsons, J.D.; Stamos, D.; Trudeau, M.; et al. Discovery of pyrazolthiazoles as novel and potent inhibitors of bacterial gyrase. Bioorg. Med. Chem. Lett. 2010, 20, 2828–2831. [Google Scholar] [CrossRef]
- Durcik, M.; Tomašič, T.; Zidar, N.; Zega, A.; Kikelj, D.; Mašič, L.P.; Ilaš, J. ATP-competitive DNA gyrase and topoisomerase IV inhibitors as antibacterial agents. Expert Opin. Ther. Pat. 2019, 29, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Adriazola, I.O.; Amaral, A.E.D.; Amorim, J.C.; Correia, B.L.; Petkowicz, C.L.O.; Mercê, A.L.R.; Noleto, G.R. Macrophage activation and leishmanicidal activity by galactomannan and its oxovanadium (IV/V) complex in vitro. J. Inorg. Biochem. 2014, 132, 45–51. [Google Scholar] [CrossRef]
- Feuser, P.E.; Jacques, A.V.; Arévalo, J.M.C.; Rocha, M.E.M.; dos Santos-Silva, M.C.; Sayer, C.; de Araújo, P.H.H. Superparamagnetic poly(methyl methacrylate) nanoparticles surface modified with folic acid presenting cell uptake mediated by endocytosis. J. Nanopart. Res. 2016, 18, 104. [Google Scholar] [CrossRef]
- Amorim, J.C.; Vriesmann, L.C.; Petkowicz, C.L.O.; Martinez, G.R.; Noleto, G.R. Modified pectin from Theobroma cacao induces potent pro-inflammatory activity in murine peritoneal macrophage. Int. J. Biol. Macromol. 2016, 92, 1040–1048. [Google Scholar] [CrossRef]
- Carpio Arévalo, J.M.; Feuser, P.E.; Rossi, G.R.; Trindade, E.S.; da Silva Córneo, E.; Machado-de-Ávila, R.A.; Sayer, C.; Cadena, S.M.S.C.; Noleto, G.R.; Martinez, G.R.; et al. Preparation and characterization of 4-nitrochalcone-folic acid-poly(methyl methacrylate) nanocapsules and cytotoxic activity on HeLa and NIH3T3 cells. J. Drug Deliv. Sci. Technol. 2019, 54, 101300. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Saeed, B.Q.; Elseginy, S.A.; Al-Marzooq, F.; Ahmady, I.M.; El-Keblawy, A.A.; Hamdy, R. Critical discovery and synthesis of novel antibacterial and resistance-modifying agents inspired by plant phytochemical defense mechanisms. Chem. Biol. Interact. 2021, 333, 109318. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, G.; Mori, M.; Chiarelli, L.R.; Gelain, A.; Meneghetti, F.; Villa, S. Natural products against key Mycobacterium tuberculosis enzymatic targets: Emerging opportunities for drug discovery. Eur. J. Med. Chem. 2021, 224, 113732. [Google Scholar] [CrossRef] [PubMed]
- Baqi, Y. Anthraquinones as a privileged scaffold in drug discovery targeting nucleotide-binding proteins. Drug Discov. Today 2016, 21, 1571–1577. [Google Scholar] [CrossRef]
- Enas, M.M.; Christa, E.M. Anthraquinones As Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufosse, L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Xiang, W.; Song, Q.S.; Zhang, H.J.; Guo, S.P. Antimicrobial anthraquinones from Morinda angustifolia. Fitoterapia 2008, 79, 501–504. [Google Scholar] [CrossRef]
- Kemegne, G.A.; Mkounga, P.; Essia Ngang, J.J.; Sado Kamdem, S.L.; Nkengfack, A.E. Antimicrobial structure activity relationship of five anthraquinones of emodine type isolated from Vismia laurentii. BMC Microbiol. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Xin, G.; Niu, H.; Huang, W. Chlorinated emodin as a natural antibacterial agent against drug-resistant bacteria through dual influence on bacterial cell membranes and DNA. Sci. Rep. 2017, 7, 12721. [Google Scholar] [CrossRef]
- Pollo, L.A.E.; Martin, E.F.; Machado, V.R.; Cantillon, D.; Wildner, L.M.; Bazzo, M.L.; Waddell, S.J.; Biavatti, M.W.; Sandjo, L.P. Search for Antimicrobial Activity Among Fifty-Two Natural and Synthetic Compounds Identifies Anthraquinone and Polyacetylene Classes That Inhibit Mycobacterium tuberculosis. Front. Microbiol. 2021, 11, 622629. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Roué, M.; Spitzfaden, C.; Petrella, S.; Aubry, A.; Hann, M.; Bax, B.; Mayer, C. Mycobacterium tuberculosis DNA gyrase ATPase domain structures suggest a dissociative mechanism that explains how ATP hydrolysis is coupled to domain motion. Biochem. J. 2013, 456, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, J.M.C.; Amorim, J.C. Virtual screening, optimization and molecular dynamics analyses highlighting a pyrrolo[1,2-a]quinazoline derivative as a potential inhibitor of DNA gyrase B of Mycobacterium tuberculosis. Sci. Rep. 2022, 12, 4742. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A. Merck Molecular Force Field. I. Basis, Form, Scope, Parameterization, and Performance of MMFF94. J. Comput. Chem. 2000, 17, 520–552. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Korb, O.; Stützle, T.; Exner, T.E. PLANTS: Application of ant colony optimization to structure-based drug design. Lect. Notes Comput. Sci. 2006, 4150, 247–258. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Kochev, N.; Avramova, S.; Angelov, P.; Jeliazkova, N. Computational Prediction of Synthetic Accessibility of Organic Molecules with Ambit-Synthetic Accessibility Tool. Org. Chem. An Indian J. 2018, 14, 123. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W3664. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.M.; Mittal, J.; Feig, M.; MacKerell, A.D. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ1 and χ2 Dihedral Angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef]
- Ewald, P.P. Die Berechnung optischer und elektrostatischer Gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. Gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Stierand, K.; Maaß, P.C.; Rarey, M. Molecular complexes at a glance: Automated generation of two-dimensional complex diagrams. Bioinformatics 2006, 22, 1710–1716. [Google Scholar] [CrossRef]
- Tari, L.W.; Li, X.; Trzoss, M.; Bensen, D.C.; Chen, Z.; Lam, T.; Zhang, J.; Lee, S.J.; Hough, G.; Phillipson, D.; et al. Tricyclic GyrB/ParE (TriBE) inhibitors: A new class of broad-spectrum dual-targeting antibacterial agents. PLoS ONE 2013, 8, e84409. [Google Scholar] [CrossRef]
- Tari, L.W.; Trzoss, M.; Bensen, D.C.; Li, X.; Chen, Z.; Lam, T.; Zhang, J.; Creighton, C.J.; Cunningham, M.L.; Kwan, B.; et al. Pyrrolopyrimidine inhibitors of DNA gyrase B (GyrB) and topoisomerase IV (ParE). Part I: Structure guided discovery and optimization of dual targeting agents with potent, broad-spectrum enzymatic activity. Bioorg. Med. Chem. Lett. 2013, 23, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Allaka, B.S.; Basavoju, S.; Rekha, E.M.; Sriram, D.; Krishna, G.R. Design and synthesis of novel quinazolinyl-bisspirooxindoles as potent anti-tubercular agents: An ultrasound-promoted methodology. Mol. Divers. 2022. [Google Scholar] [CrossRef]
- Carpio Arévalo, J.M.; Amorim, J.C. An in-silico analysis reveals 7,7′-bializarin as a promising DNA gyrase B inhibitor on Gram-positive and Gram-negative bacteria. Comput. Biol. Med. 2021, 135, 104626. [Google Scholar] [CrossRef]
- Elseginy, S.A.; Anwar, M.M. Pharmacophore-Based Virtual Screening and Molecular Dynamics Simulation for Identification of a Novel DNA Gyrase B Inhibitor with Benzoxazine Acetamide Scaffold. ACS Omega 2022, 7, 1150–1164. [Google Scholar] [CrossRef]
- Saxena, S.; Renuka, J.; Yogeeswari, P.; Sriram, D. Discovery of novel mycobacterial DNA gyrase B inhibitors: In silico and in vitro biological evaluation. Mol. Inform. 2014, 33, 597–609. [Google Scholar] [CrossRef]
- Collin, F.; Karkare, S.; Maxwell, A. Exploiting bacterial DNA gyrase as a drug target: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 92, 479–497. [Google Scholar] [CrossRef]
- Gross, C.H.; Parsons, J.D.; Grossman, T.H.; Charifson, P.S.; Bellon, S.; Jernee, J.; Dwyer, M.; Chambers, S.P.; Markland, W.; Botfield, M.; et al. Active-site residues of Escherichia coli DNA gyrase required in coupling ATP hydrolysis to DNA supercoiling and amino acid substitutions leading to novobiocin resistance. Antimicrob. Agents Chemother. 2003, 47, 1037–1046. [Google Scholar] [CrossRef]
- Kharb, R.; Sharma, P.C.; Yar, M.S. Pharmacological significance of triazole scaffold. J. Enzyme Inhib. Med. Chem. 2011, 26, 1–21. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: A Promising Antitubercular Agent. Chem. Biol. Drug Des. 2015, 86, 410–423. [Google Scholar] [CrossRef]
- Kasinathan, N.; Jagani, H.V.; Alex, A.T.; Volety, S.M.; Venkata Rao, J. Strategies for drug delivery to the central nervous system by systemic route. Drug Deliv. 2015, 22, 243–257. [Google Scholar] [CrossRef]
- Liu, Y.; Mapa, M.S.T.; Sprando, R.L. Anthraquinones inhibit cytochromes P450 enzyme activity in silico and in vitro. J. Appl. Toxicol. 2021, 41, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and Induction of CYP Enzymes in Humans: An Update; Springer: Berlin/Heidelberg, Germany, 2020; Volume 94, ISBN 0123456789. [Google Scholar]
- Scheiber, J.; Chen, B.; Milik, M.; Sukuru, S.C.K.; Bender, A.; Mikhailov, D.; Whitebread, S.; Hamon, J.; Azzaoui, K.; Urban, L.; et al. Gaining insight into off-target mediated effects of drug candidates with a comprehensive systems chemical biology analysis. J. Chem. Inf. Model. 2009, 49, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.S.; Alsantali, R.I.; Jassas, R.S.; Alsimaree, A.A.; Syed, R.; Alsharif, M.A.; Kalpana, K.; Morad, M.; Althagafi, I.I.; Ahmed, S.A. Journey of anthraquinones as anticancer agents-a systematic review of recent literature. RSC Adv. 2021, 11, 35806–35827. [Google Scholar] [CrossRef]
- Cho, Y.C.; Park, J.E.; Park, B.C.; Kim, J.H.; Jeong, D.G.; Park, S.G.; Cho, S. Cell cycle-dependent Cdc25C phosphatase determines cell survival by regulating apoptosis signal-regulating kinase 1. Cell Death Differ. 2015, 22, 1605–1617. [Google Scholar] [CrossRef]
- Ostrov, D.A.; Hernández Prada, J.A.; Corsino, P.E.; Finton, K.A.; Le, N.; Rowe, T.C. Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob. Agents Chemother. 2007, 51, 3688–3698. [Google Scholar] [CrossRef]



| Score | Structure | Binding Affinity |
|---|---|---|
| BS-1 | 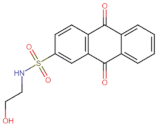 | −105.4 |
| BS-2 |  | −103.6 |
| BS-3 | 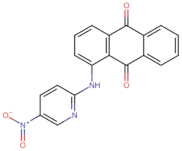 | −103.2 |
| BS-4 |  | −102.4 |
| BS-5 |  | −102.4 |
| BS-6 |  | −102.4 |
| BS-7 |  | −102.4 |
| BS-8 | 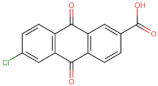 | −101.4 |
| BS-9 | 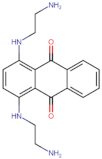 | −101.4 |
| BS-10 | 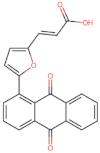 | −101.0 |
| ANP |  | −112.5 |
| Pharmacokinetic Analyses | |||
|---|---|---|---|
| AC | BS-1 | ATD | |
| GI absorption | High | High | Low |
| BBB permeant | Yes | No | No |
| P-gp substrate | No | No | Yes |
| CYP1A2 inhibitor | Yes | No | No |
| CYP2C19 inhibitor | Yes | No | No |
| CYP2C9 inhibitor | No | No | No |
| CYP2D6 inhibitor | No | No | No |
| CYP3A4 inhibitor | No | No | No |
| Cytotoxicity Prediction | Probability |
|---|---|
| AC | Inactive (0.85) |
| BS-1 | Inactive (0.72) |
| ATD | Inactive (0.66) |
| Target Fishing Analyses | Probability |
|---|---|
| Targets of AC | |
| Dual specificity phosphatase Cdc25B | 0.89 |
| Acyl coenzyme A: cholesterol Acyltransferase 1 | 0.06 |
| Apoptosis regulator Bcl-2 | 0.06 |
| Casein kinase I alpha | 0.06 |
| Caspase-3 | 0.06 |
| Targets of BS-1 | |
| Thymidine kinase cytoplasmatic | 0.10 |
| Thymidine kinase mitochondrial | 0.10 |
| Alkaline phosphatase | 0.10 |
| P-glycoprotein 1 | 0.10 |
| Tyrosine-protein kinase ABL | 0.10 |
| Targets of ATD | |
| 3-phosphoinositide-dependent protein kinase-1 | 0.00 |
| 78 kDa glucose-regulated protein | 0.00 |
| Adenosine A1 receptor | 0.00 |
| Adenosine A2a receptor | 0.00 |
| Adenosine A3 receptor | 0.00 |
| Energy Decomposition (Kcal/mol) ± Standard Deviation | ||
|---|---|---|
| BS-1 | ATD | |
| ΔE (Vdw) | −35.18 ± 1.95 | −45.50 ± 2.57 |
| ΔE (Ele) | 73.27 ± 6.41 | −30.83 ± 3.34 |
| ΔE (Polar, Solv) | −59.40 ± 4.50 | 46.02 ± 2.94 |
| ΔE (Non-Polar, Solv) | −4.03 ± 0.12 | −4.64 ± 0.11 |
| ΔG (Total) | −25.36 ± 3.11 | −34.96 ± 3.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, J.C.; Cabrera Bermeo, A.E.; Vásquez Urgilés, V.E.; Martínez León, M.R.; Carpio Arévalo, J.M. An In-Silico Evaluation of Anthraquinones as Potential Inhibitors of DNA Gyrase B of Mycobacterium tuberculosis. Microorganisms 2022, 10, 2434. https://doi.org/10.3390/microorganisms10122434
Amorim JC, Cabrera Bermeo AE, Vásquez Urgilés VE, Martínez León MR, Carpio Arévalo JM. An In-Silico Evaluation of Anthraquinones as Potential Inhibitors of DNA Gyrase B of Mycobacterium tuberculosis. Microorganisms. 2022; 10(12):2434. https://doi.org/10.3390/microorganisms10122434
Chicago/Turabian StyleAmorim, Juliana Carolina, Andrea E. Cabrera Bermeo, Viviana E. Vásquez Urgilés, Maritza R. Martínez León, and Juan M. Carpio Arévalo. 2022. "An In-Silico Evaluation of Anthraquinones as Potential Inhibitors of DNA Gyrase B of Mycobacterium tuberculosis" Microorganisms 10, no. 12: 2434. https://doi.org/10.3390/microorganisms10122434
APA StyleAmorim, J. C., Cabrera Bermeo, A. E., Vásquez Urgilés, V. E., Martínez León, M. R., & Carpio Arévalo, J. M. (2022). An In-Silico Evaluation of Anthraquinones as Potential Inhibitors of DNA Gyrase B of Mycobacterium tuberculosis. Microorganisms, 10(12), 2434. https://doi.org/10.3390/microorganisms10122434





