Eosinopenia as Predictor of Poor Outcome in Hospitalized COVID-19 Adult Patients from Waves 1 and 2 of 2020 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Outcomes
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqi, H.K.; Mehra, M.R. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J. Heart Lung Transpl. 2020, 39, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, A.W.; Schwartz, J.T.; Rothenberg, M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dong, Y.; Wang, L.; Xie, H.; Li, B.; Chang, C.; Wang, F.-S. Characteristics and prognostic factors of disease severity in patients with COVID-19: The Beijing experience. J. Autoimmun. 2020, 112, 102473. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Fumagalli, L.A.M.; D’angelo, L.; Cerino, M.; Bonfanti, G.; Fumagalli, R.M.; Schiavo, G.; Lorini, C.; Lainu, E.; Terragni, S.; et al. Eosinopenia is a reliable marker of severe disease and unfavourable outcome in patients with COVID-19 pneumonia. Int. J. Clin. Pract. 2021, 75, e14047. [Google Scholar] [CrossRef] [PubMed]
- Soni, M. Original Article: Evaluation of eosinopenia as a diagnostic and prognostic indicator in COVID-19 infection. Int. J. Lab. Hematol. 2021, 43, e303. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Yang, J.; Xie, Y.; Tang, X. Relationship between blood eosinophil levels and COVID-19 mortality. World Allergy Organ J. 2021, 14, 100521. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.-S.; Kim, T.Y.; Lee, D.-G.; Kim, D.-W. Lymphopenia as a Biological Predictor of Outcomes in COVID-19 Patients: A Nationwide Cohort Study. Cancers 2021, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tu, L.; Zhu, P.; Mu, M.; Wang, R.; Yang, P.; Wang, X.; Hu, C.; Ping, R.; Hu, P.; et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study. Am. J. Respir. Crit. Care Med. 2020, 201, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Cheng, A.; Yuan, X.; Zhong, X.; Wang, H.; Zhou, W.; Xu, X.; Li, Y. Characteristics of peripheral white blood cells in COVID-19 patients revealed by a retrospective cohort study. BMC Infect. Dis. 2021, 21, 1236. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Chen, C.-H.; Chao, A.; Chen, E.-S.; Wei, M.-L.; Chen, L.-K.; Yang, K.D.; Lin, M.-C.; Wang, Y.-H.; Liu, J.-W.; et al. Molecular signature of clinical severity in recovering patients with severe acute respiratory syndrome coronavirus (SARS-CoV). BMC Genom. 2005, 6, 132. [Google Scholar] [CrossRef][Green Version]
- Garnacho-Montero, J.; Huici-Moreno, M.J.; Gutiérrez-Pizarraya, A.; López, I.; Márquez-Vácaro, J.A.; Macher, H.; Guerrero, J.M.; Puppo-Moreno, A. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit. Care 2014, 18, R116. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.I.; Osadnik, C.R.; Bulfin, L.; Hamza, K.; Leong, P.; Wong, A.; King, P.T.; Bardin, P.G. Low and High Blood Eosinophil Counts as Biomarkers in Hospitalized Acute Exacerbations of COPD. Chest 2019, 156, 92–100. [Google Scholar] [CrossRef]
- Chen, H.-T.; Xu, J.-F.; Huang, X.-X.; Zhou, N.-Y.; Wang, Y.-K.; Mao, Y. Blood eosinophils and mortality in patients with acute respiratory distress syndrome: A propensity score matching analysis. World J. Emerg. Med. 2021, 12, 131–136. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Wang, F.; Li, G.; Li, Y.; Xing, L.; et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020, 146, 119–127.e4. [Google Scholar] [CrossRef]
- Backes, Y.; van der Sluijs, K.F.; Mackie, D.P.; Tacke, F.; Koch, A.; Tenhunen, J.J.; Schultz, M.J. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: A systematic review. Intensive Care Med. 2012, 38, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S.; Mukhtar, N.; Aljomaiah, A.; Aljamei, H.; Bakhsh, A.; Alsudani, N.; Elsayed, T.; Alrashidi, N.; Fadel, R.; Alqahtani, E.; et al. The Impact of COVID-19 Viral Infection on the Hypothalamic-Pituitary-Adrenal Axis. Endocr. Pract. 2021, 27, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bro-Rasmussen, F. Effect of cortisol on the eosinophils in the rat spleen. Autoradiographic studies. Scand. J. Haematol. 1973, 11, 59–70. [Google Scholar] [CrossRef]
- Hoang, V.-T.; Colson, P.; Levasseur, A.; Delerce, J.; Lagier, J.-C.; Parola, P.; Million, M.; Fournier, P.-E.; Raoult, D.; Gautret, P. Clinical outcomes in patients infected with different SARS-CoV-2 variants at one hospital during three phases of the COVID-19 epidemic in Marseille, France. Infect. Genet. Evol. 2021, 95, 105092. [Google Scholar] [CrossRef]
- Dao, T.L.; Hoang, V.T.; Nguyen, N.N.; Delerce, J.; Chaudet, H.; Levasseur, A.; Lagier, J.C.; Raoult, D.; Colson, P.; Gautret, P. Clinical outcomes in COVID-19 patients infected with different SARS-CoV-2 variants in Marseille, France. Clin. Microbiol. Infect. 2021, 27, 1516.e1–1516.e6. [Google Scholar] [CrossRef]
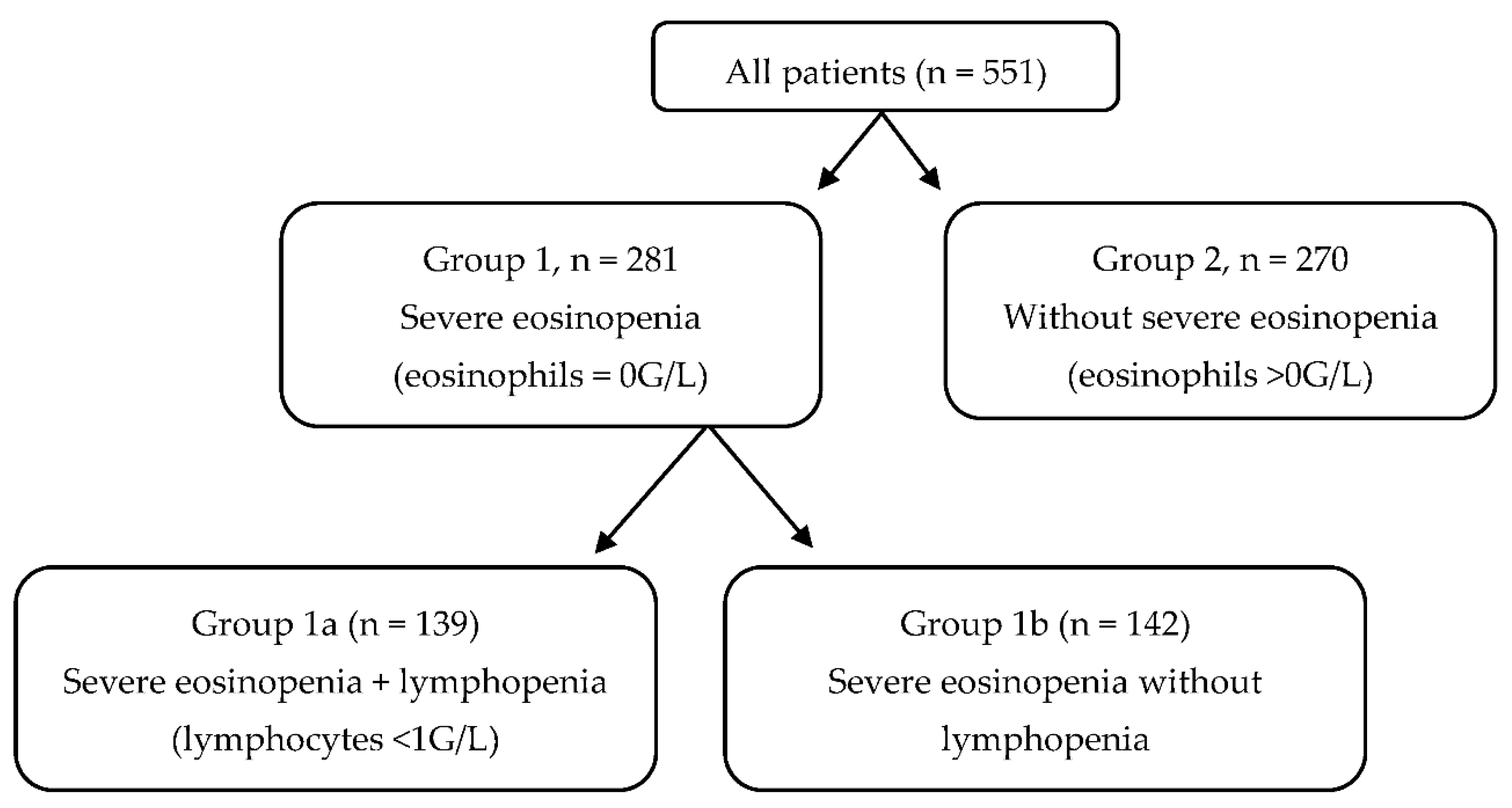
| Severe Eosinopenia (Group 1) | Without Severe Eosinopenia (Group 2) | p | |
|---|---|---|---|
| (n = 281) | (n = 270) | ||
| Demographics: mean (standard deviation) or absolute number (percentage) | |||
| Age, years | 65.13 (15.02) | 64.33 (16.32) | 0.552 |
| Male/female | 131 (46.6%)/150 (53.4%) | 139 (51.5%)/131 (48.5%) | 0.253 |
| Comorbidities: Absolute number (%) | |||
| Diabetes | 80 (28.47%) | 78 (28.89%) | 0.913 |
| High blood pressure | 121 (43.06%) | 140 (51.85%) | 0.042 |
| Body mass index > 30 kg/m2 | 72 (25.62%) | 53 (19.63%) | 0.064 |
| Cardiovascular disease * | 37 (13.17%) | 38 (14.07%) | 0.756 |
| Immunosuppression ** | 31 (11.03%) | 39 (14.44%) | 0.229 |
| Chronic respiratory disease *** | 43 (15.30%) | 52 (19.26%) | 0.219 |
| Tobacco smoking | 49 (17.44%) | 71 (26.30%) | 0.011 |
| Initial laboratory results: mean (SD) | |||
| Leucocytes, G/L | 7.66 (5.83) | 7.71 (4.65) | 0.908 |
| Neutrophils, G/L | 6.05 (4.44) | 5.38 (3.70) | 0.05 |
| Lymphocytes, G/L | 1.02 (0.81) | 1.53 (1.55) | <0.001 |
| CD3 + (/mm3) | 811 (522) | 1075 (620) | 0.021 |
| CD3 + CD4 + (mm3) | 485.8 (291.8) | 682.2 (419.5) | 0.001 |
| CD3 + CD8 + (/mm3) | 286.6 (237.6) | 364.8 (256.5) | 0.054 |
| CD19 + (/mm3) | 182.2 (193) | 181.8 (159) | 0.981 |
| CD16 + CD56 + CD3- (/mm3) | 196.1 (143.2) | 211.9 (183.1) | 0.543 |
| Neutrophil to lymphocyte ratio | 7.66 (13.95) | 6.07 (6.38) | <0.001 |
| Monocytes, G/L | 0.51 (0.64) | 0.96 (5.37) | 0.169 |
| Hemoglobin, g/dL | 13.23 (1.94) | 14.14 (10.95) | 0.179 |
| Platelets, G/L | 198 (82) | 240 (100) | <0.001 |
| C-reactive protein, mg/L | 112.3 (86.2) | 82.9 (82.2) | <0.001 |
| Ferritin, µg/L | 1367 (1146) | 890 (917) | <0.001 |
| Fibrinogen, g/L | 6.29 (1.57) | 5.89 (1.70) | 0.018 |
| Albumin, g/L | 36.74 (4.87) | 36.70 (6.10) | 0.945 |
| Creatinin, µmol/L | 92.86 (54.05) | 94.17 (63.19) | 0.794 |
| ASAT, IU/L | 61.51 (46.77) | 45.21 (28.75) | 0.003 |
| LDH, IU/L | 373.2 (129.5) | 322.4 (130.4) | <0.001 |
| Troponin, ng/L | 42.34 (146.80) | 30.69 (39.43) | 0.4 |
| CT scan: Absolute number (%) | |||
| Severe or critical CT scan | 170 (60.5%) | 137 (50.7%) | 0.033 |
| Severe Eosinopenia (Group 1) | Without Severe Eosinopenia (Group 2) | p | |
|---|---|---|---|
| Mean (SD) | |||
| suPAR | 10.210 (5.4) | 6.643 (6.6) | 0.0334 |
| IP-10 | 142.8 (122.9) | 84.04 (70.2) | 0.0301 |
| Interleukin-6 (pg/mL) | 47.37 (53.7) | 51.35 (55.4) | 0.7935 |
| Blood cortisol (nmol/L) | 458 (318) | 483 (174) | 0.7345 |
| Severe Eosinopenia (Group 1) | Without Severe Eosinopenia (Group 2) | p | |
|---|---|---|---|
| (n = 281) | (n = 270) | ||
| Absolute number (%) | |||
| Composite poor outcome | 98 (34.87%) | 57 (21.11%) | <0.001 |
| Death | 43 (15.30%) | 19 (7.03%) | <0.001 |
| Severe Eosinopenia and Lymphopenia (Group 1a) | Other Patients | p | |
|---|---|---|---|
| (n = 139) | (n = 412) | ||
| Absolute number (%) | |||
| Composite poor outcome | 75 (53.95%) | 81 (19.66%) | <0.001 |
| Death | 24 (17.26%) | 38 (9.22%) | <0.001 |
| OR (IC 95%) | p | |
|---|---|---|
| Eosinophils = 0 G/L | 2.58 (1.77–3.75) | <0.0001 |
| Male gender | 1.48 (1.02–2.15) | 0.0371 |
| BMI ≥ 30 kg/m2 | 2.34 (1.50–3.66) | 0.0002 |
| Age ≥ 65 | 1.35 (0.90–2.02) | 0.1447 |
| High blood pressure | 1.36 (0.90–2.05) | 0.1492 |
| Type 2 diabetes | 0.99 (0.64–1.51) | 0.9465 |
| Etiology of ARDS Not Related to SARS-CoV2 (n = 118), Absolute Number (%) | |
|---|---|
| Bacterial pneumonia | 67 (56.78%) |
| Ventilator-associated pneumonia | 8 (6.78%) |
| Viral pneumonia | 11 (9.32%) |
| Pulmonary embolism | 3 (2.54%) |
| Pulmonary fibrosis | 5 (4.24%) |
| Hemoptysis | 4 (3.39%) |
| Atelectasis | 6 (5.08%) |
| Drowning | 1 (0.85%) |
| Traumatic | 5 (4.24%) |
| Others | 8 (6.78%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cauchois, R.; Pietri, L.; Dalmas, J.-B.; Koubi, M.; Capron, T.; Cassir, N.; Potere, N.; Polidoro, I.; Jean, R.; Jarrot, P.-A.; et al. Eosinopenia as Predictor of Poor Outcome in Hospitalized COVID-19 Adult Patients from Waves 1 and 2 of 2020 Pandemic. Microorganisms 2022, 10, 2423. https://doi.org/10.3390/microorganisms10122423
Cauchois R, Pietri L, Dalmas J-B, Koubi M, Capron T, Cassir N, Potere N, Polidoro I, Jean R, Jarrot P-A, et al. Eosinopenia as Predictor of Poor Outcome in Hospitalized COVID-19 Adult Patients from Waves 1 and 2 of 2020 Pandemic. Microorganisms. 2022; 10(12):2423. https://doi.org/10.3390/microorganisms10122423
Chicago/Turabian StyleCauchois, Raphael, Lea Pietri, Jean-Baptiste Dalmas, Marie Koubi, Thibaut Capron, Nadim Cassir, Nicola Potere, Ildo Polidoro, Rodolphe Jean, Pierre-André Jarrot, and et al. 2022. "Eosinopenia as Predictor of Poor Outcome in Hospitalized COVID-19 Adult Patients from Waves 1 and 2 of 2020 Pandemic" Microorganisms 10, no. 12: 2423. https://doi.org/10.3390/microorganisms10122423
APA StyleCauchois, R., Pietri, L., Dalmas, J.-B., Koubi, M., Capron, T., Cassir, N., Potere, N., Polidoro, I., Jean, R., Jarrot, P.-A., Andre, B., Veit, V., Carvelli, J., Pauly, V., Chanez, P., Papazian, L., & Kaplanski, G. (2022). Eosinopenia as Predictor of Poor Outcome in Hospitalized COVID-19 Adult Patients from Waves 1 and 2 of 2020 Pandemic. Microorganisms, 10(12), 2423. https://doi.org/10.3390/microorganisms10122423






