Evaluation of Control Strategies for Xylella fastidiosa in the Balearic Islands
Abstract
1. Introduction
2. Action Plans to Combat Xylella fastidiosa in the Balearic Islands
2.1. Elimination of Plants in Infected Areas
2.2. Measures against Vectors of the Specified Pest in Containment Zones
2.3. Annual Surveillance of Infected Areas
2.4. Authorization Regarding the Planting of Specified Plants in Infected Areas
2.5. Movement of Plant Material out of the Balearic Islands and Between Islands, Phytosanitary Passports, and Border Controls
2.6. Awareness Campaigns
3. Research on the Reconstruction of the Introduction and Spread of Xylella fastidiosa in the Balearic Islands
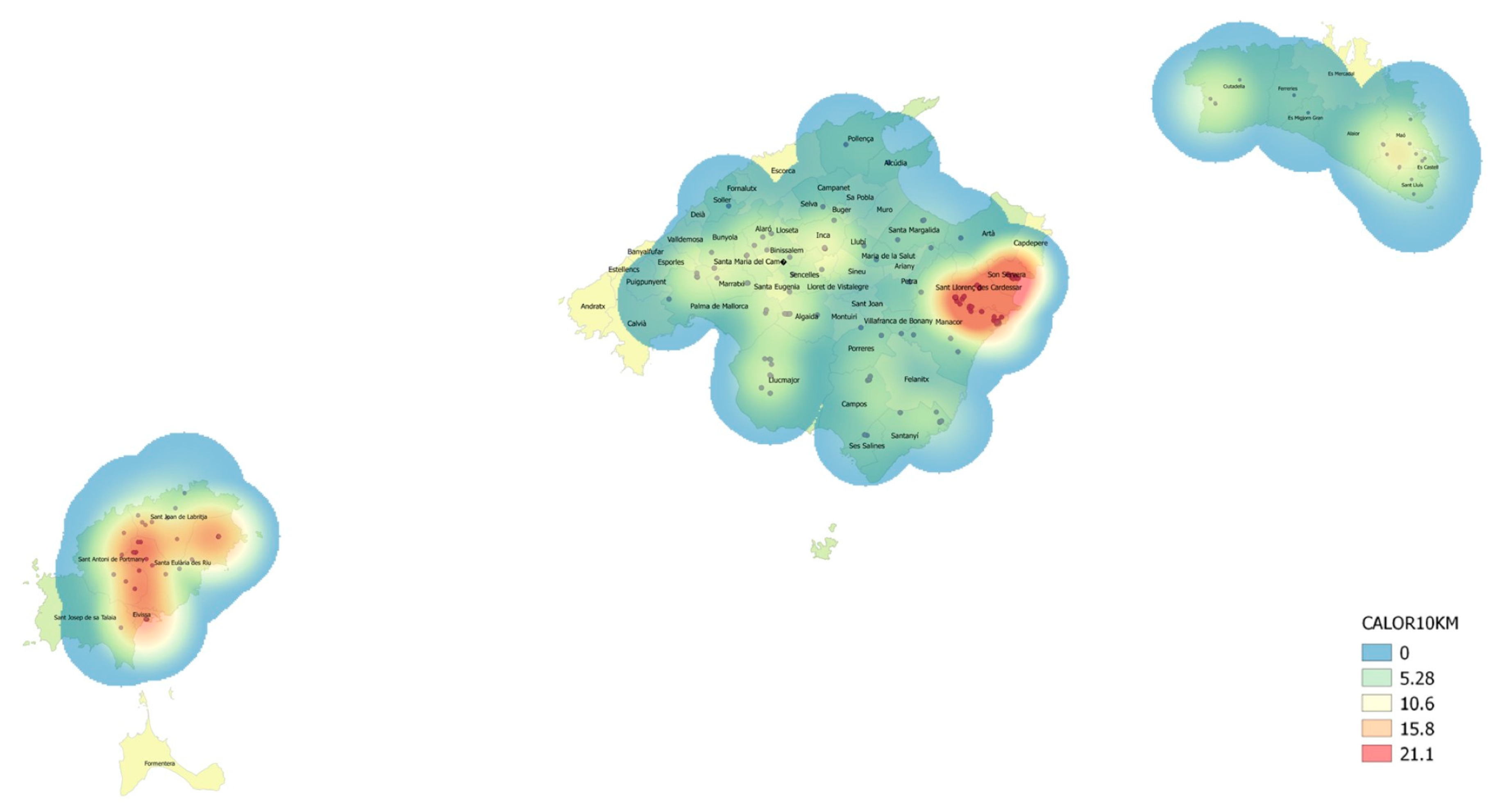
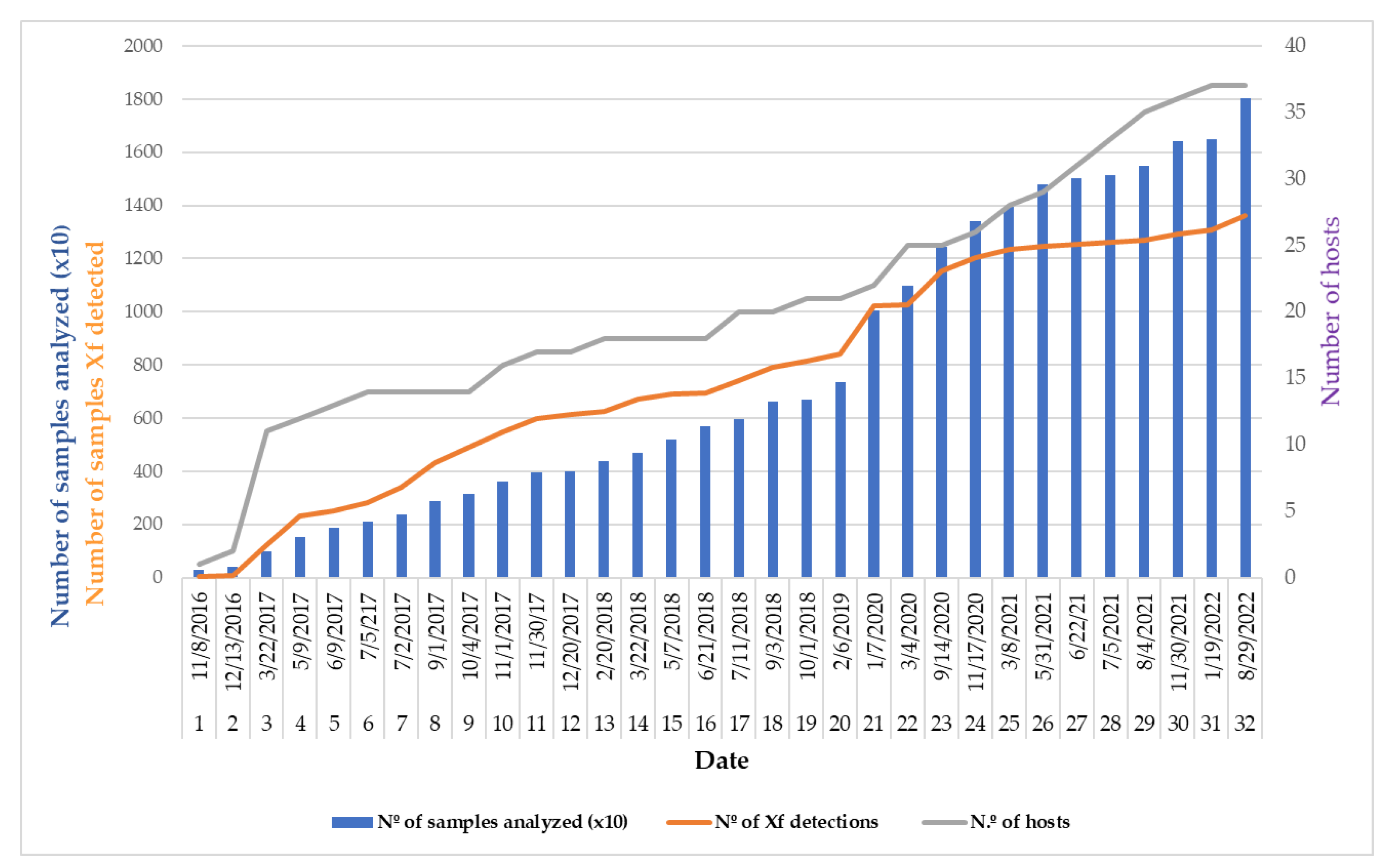
3.1. Disease Incidence
3.2. Phylogenetic Analysis
3.3. Vector Transmission
3.4. Climatic Conditions
3.5. Field Observations and Inoculation Experiments
4. Discussion
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Borkar, S.G. History of Plant Pathology; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1-351-37194-0. [Google Scholar]
- Brasier, C.M. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol. 2008, 57, 792–808. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.-N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Werres, S.; Marwitz, R.; In’t Veld, W.A.M.; De Cock, A.W.; Bonants, P.J.; De Weerdt, M.; Themann, K.; Ilieva, E.; Baayen, R.P. Phytophthora ramorum sp. nov., a new pathogen on rhododendron and viburnum. Mycol. Res. 2001, 105, 1155–1165. [Google Scholar] [CrossRef]
- McMullan, M.; Rafiqi, M.; Kaithakottil, G.; Clavijo, B.J.; Bilham, L.; Orton, E.; Percival-Alwyn, L.; Ward, B.J.; Edwards, A.; Saunders, D.G. The ash dieback invasion of Europe was founded by two genetically divergent individuals. Nat. Ecol. Evol. 2018, 2, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- Sicard, A.; Zeilinger, A.R.; Vanhove, M.; Schartel, T.E.; Beal, D.J.; Daugherty, M.P.; Almeida, R.P. Xylella fastidiosa: Insights into an emerging plant pathogen. Annu. Rev. Phytopathol. 2018, 56, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.; Blua, M.J.; Mizell Iii, R.F.; Andersen, P.C. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef] [PubMed]
- EFSA; Carrasco Cabrera, L.; Medina Pastor, P. The 2019 European union report on pesticide residues in food. EFSA J. 2021, 19, e06491. [Google Scholar]
- Hopkins, D.L. Effect of tetracycline antibiotics on Pierce’s disease of grapevine in Florida [caused probably by bacterium]. Proc. Annu. Meet. Fla. State Hortic. Soc. USA 1980, 92, 284–285. [Google Scholar]
- Kirkpatrick, B.; Anderson, P.; Civerolo, E.; Jones, D.; Purcell, A.; Smith, R.; Vargas, C.; Weber, E. Evaluation of bactericides and modes of delivery for managing Pierce’s disease. Pierce’s Dis. Res. Symp. 2003, 103, 101–103. [Google Scholar]
- EFSA; Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.-A.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S. Effectiveness of in planta control measures for Xylella fastidiosa. EFSA J. 2019, 17, e05666. [Google Scholar]
- Baccari, C.; Antonova, E.; Lindow, S. Biological control of Pierce’s disease of grape by an endophytic bacterium. Phytopathology 2019, 109, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, L.S.; Giorgiano, T.E.; Takita, M.A.; Forim, M.R.; Silva, L.F.; Coletta-Filho, H.D.; Machado, M.A.; de Souza, A.A. N-acetylcysteine in agriculture, a novel use for an old molecule: Focus on controlling the plant–pathogen Xylella fastidiosa. PLoS ONE 2013, 8, e72937. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M.; Chen, J.; De Caroli, M.; Dalessandro, G.; Pucci, N.; Modesti, V.; L’aurora, A.; Petriccione, M.; Zampella, L.; Mastrobuoni, F. A zinc, copper and citric acid biocomplex shows promise for control of Xylella fastidiosa subsp. pauca in olive trees in Apulia region (southern Italy). Phytopathol. Mediterr. 2018, 57, 48–72. [Google Scholar]
- Vanhove, M.; Retchless, A.C.; Sicard, A.; Rieux, A.; Coletta-Filho, H.D.; De La Fuente, L.; Stenger, D.C.; Almeida, R.P. Genomic diversity and recombination among Xylella fastidiosa subspecies. Appl. Environ. Microbiol. 2019, 85, e02972-18. [Google Scholar] [CrossRef] [PubMed]
- Landa, B.B.; Saponari, M.; Feitosa-Junior, O.R.; Giampetruzzi, A.; Vieira, F.J.; Mor, E.; Robatzek, S. Xylella fastidiosa’s relationships: The bacterium, the host plants, and the plant microbiome. New Phytol. 2022, 234, 1598–1605. [Google Scholar] [CrossRef]
- EFSA; Delbianco, A.; Gibin, D.; Pasinato, L.; Morelli, M. Update of the Xylella spp. host plant database–systematic literature search up to 30 June 2021. EFSA J. 2022, 20, e07039. [Google Scholar]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C. Open-access bacterial population genomics: BIGSdb software, the PubMLST. Org Website and Their Applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Nunney, L.; Azad, H.; Stouthamer, R. An experimental test of the host-plant range of nonrecombinant strains of north American Xylella fastidiosa subsp. multiplex. Phytopathology 2019, 109, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Berisha, B.; Chen, Y.D.; Zhang, G.Y.; Xu, B.Y.; Chen, T.A. Isolation of Peirce’s disease bacteria from grapevines in Europe. Eur. J. Plant Pathol. 1998, 104, 427–433. [Google Scholar] [CrossRef]
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in olive in Apulia: Where we stand. Phytopathology 2019, 109, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Denancé, N.; Legendre, B.; Briand, M.; Olivier, V.; De Boisseson, C.; Poliakoff, F.; Jacques, M.-A. Several subspecies and sequence types are associated with the emergence of Xylella fastidiosa in natural settings in France. Plant Pathol. 2017, 66, 1054–1064. [Google Scholar] [CrossRef]
- Olmo, D.; Nieto, A.; Adrover, F.; Urbano, A.; Beidas, O.; Juan, A.; Marco-Noales, E.; López, M.M.; Navarro, I.; Monterde, A. First detection of Xylella fastidiosa infecting cherry (Prunus avium) and Polygala myrtifolia plants, in Mallorca island, Spain. Plant Dis. 2017, 101, 1820. [Google Scholar] [CrossRef]
- Marco-Noales, E.; Barbé, S.; Monterde, A.; Navarro-Herrero, I.; Ferrer, A.; Dalmau, V.; Aure, C.M.; Domingo-Calap, M.L.; Landa, B.B.; Roselló, M. Evidence that Xylella fastidiosa is the causal agent of almond leaf scorch disease in Alicante, mainland Spain (Iberian peninsula). Plant Dis. 2021, 105, 3349–3352. [Google Scholar] [CrossRef] [PubMed]
- Zecharia, N.; Krasnov, H.; Vanunu, M.; Castillo, A.I.; Haberman, A.; Dror, O.; Vakel, L.; Almeida, R.; Blank, L.; Shtienberg, D. Xylella fastidiosa outbreak in Israel: Population genetics, host range and temporal and spatial distribution analysis. Phytopathology 2022. Online ahead of print . [Google Scholar] [CrossRef]
- Saponari, M.; D’Attoma, G.; Abou Kubaa, R.; Loconsole, G.; Altamura, G.; Zicca, S.; Rizzo, D.; Boscia, D. A new variant of Xylella fastidiosa subspecies multiplex detected in different host plants in the recently emerged outbreak in the region of Tuscany, Italy. Eur. J. Plant Pathol. 2019, 154, 1195–1200. [Google Scholar] [CrossRef]
- Carvalho-Luis, C.; Rodrigues, J.M.; Martins, L.M. Dispersion of the bacterium Xylella fastidiosa in Portugal. J. Agric. Sci. Technol. A 2022, 12, 35–41. [Google Scholar] [CrossRef]
- Landa, B.B.; Castillo, A.I.; Giampetruzzi, A.; Kahn, A.; Román-Écija, M.; Velasco-Amo, M.P.; Navas-Cortés, J.A.; Marco-Noales, E.; Barbé, S.; Moralejo, E. Emergence of a plant pathogen in Europe associated with multiple intercontinental introductions. Appl. Environ. Microbiol. 2020, 86, e01521-19. [Google Scholar] [CrossRef]
- Morelli, M.; García-Madero, J.M.; Jos, Á.; Saldarelli, P.; Dongiovanni, C.; Kovacova, M.; Saponari, M.; Baños Arjona, A.; Hackl, E.; Webb, S. Xylella fastidiosa in olive: A review of control attempts and current management. Microorganisms 2021, 9, 1771. [Google Scholar] [CrossRef]
- Wells, J.M.; Raju, B.C.; Hung, H.-Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa Gen. Nov., Sp. Nov: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Evol. Microbiol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- Reisman, E. Plants, pathogens, and the politics of care: Xylella fastidiosa and the intra-active breakdown of Mallorca’s almond ecology. Cult. Anthropol. 2021, 36, 400–427. [Google Scholar] [CrossRef]
- Landa, B.B.; Velasco-Amo, M.P.; Marco-Noales, E.; Olmo, D.; López, M.M.; Navarro, I.; Monterde, A.; Barbé, S.; Montes-Borrego, M.; Román-Écija, M. Draft genome sequence of Xylella fastidiosa subsp. fastidiosa strain IVIA5235, isolated fromPrunus avium in Mallorca island, Spain. Microbiol. Resour. Announc. 2018, 7, e01222-18. [Google Scholar] [PubMed]
- Gomila, M.; Moralejo, E.; Busquets, A.; Segui, G.; Olmo, D.; Nieto, A.; Juan, A.; Lalucat, J. Draft genome resources of two strains of Xylella fastidiosa XYL1732/17 and XYL2055/17 isolated from Mallorca vineyards. Phytopathology 2019, 109, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Olmo, D.; Nieto, A.; Borràs, D.; Montesinos, M.; Adrover, F.; Pascual, A.; Gost, P.A.; Quetglas, B.; Urbano, A.; García, J.d.D.; et al. Landscape epidemiology of Xylella fastidiosa in the Balearic Islands. Agronomy 2021, 11, 473. [Google Scholar] [CrossRef]
- Gramaje, D.; Agustí-Brisach, C.; Pérez-Sierra, A.; Moralejo, E.; Olmo, D.; Mostert, L.; Damm, U.; Armengol, J. Fungal trunk pathogens associated with wood decay of almond trees on Mallorca (Spain). Persoonia-Mol. Phylogeny Evol. Fungi 2012, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moralejo, E.; Gomila, M.; Montesinos, M.; Borràs, D.; Pascual, A.; Nieto, A.; Adrover, F.; Gost, P.A.; Seguí, G.; Busquets, A. Phylogenetic inference enables reconstruction of a long-overlooked outbreak of almond leaf scorch disease (Xylella fastidiosa) in Europe. Commun. Biol. 2020, 3, 560. [Google Scholar] [CrossRef]
- Moralejo, E.; Borràs, D.; Gomila, M.; Montesinos, M.; Adrover, F.; Juan, A.; Nieto, A.; Olmo, D.; Seguí, G.; Landa, B.B. Insights into the epidemiology of Pierce’s disease in vineyards of Mallorca, Spain. Plant Pathol. 2019, 68, 1458–1471. [Google Scholar] [CrossRef]
- López-Mercadal, J.; DelgaDo, S.; MeRCaDal, P.; Seguí, G.; Lalucat, J.; BusQuets, A.; Gomila, M.; LesteR, K.; KeNyoN, D.M.; Ruiz-Pérez, M. Collection of data and information in Balearic Islands on biology of vectors and potential vectors of Xylella fastidiosa (GP/EFSA/ALPHA/017/01). EFSA Support. Publ. 2021, 18, 6925E. [Google Scholar] [CrossRef]
- Cornara, D.; Saponari, M.; Zeilinger, A.R.; de Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Martelli, G.P.; Almeida, R.P.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef]
- Borràs, D. Studies on the competence of potential Xylella fastidiosa vectors in the Balearic Islands (Spain). In Proceedings of the 3rd European Conference on Xylella fastidiosa and XF-ACTORS Final Meeting (xylella21), Online, 26–30 April 2021. [Google Scholar] [CrossRef]
- Gimenez-Romero, A.; Galvan, J.; Montesinos, M.; Bauza, J.; Godefroid, M.; Fereres, A.; Ramasco, J.J.; Matias, M.A.; Moralejo, E. Global predictions for the risk of establishment of Pierce’s disease of grapevines. bioRxiv 2022. [Google Scholar] [CrossRef]
- Brasier, C. Rapid Evolution of Introduced Tree Pathogens via Episodic Selection and Horizontal Gene Transfer. In Proceedings of the Fourth International Workshop on the Genetics of Host-Parasite Interactions in Forestry: Disease and Insect Resistance in Forest Trees; Gen. Tech. Rep. PSW-GTR-240; Sniezko, R.A., Yanchuk, A.D., Kliejunas, J.T., Palmieri, K.M., Alexander, J.M., Frankel, S.J., Eds.; Pacific Southwest Research Station, Forest Service, US Department of Agriculture: Albany, CA, USA, 2012; Volume 240, pp. 133–142. [Google Scholar]
- Cruaud, A.; Gonzalez, A.-A.; Godefroid, M.; Nidelet, S.; Streito, J.-C.; Thuillier, J.-M.; Rossi, J.-P.; Santoni, S.; Rasplus, J.-Y. Using insects to detect, monitor and predict the distribution of Xylella fastidiosa: A case study in Corsica. Sci. Rep. 2018, 8, 15628. [Google Scholar] [CrossRef] [PubMed]
- Sabella, E.; Luvisi, A.; Aprile, A.; Negro, C.; Vergine, M.; Nicolì, F.; Miceli, A.; De Bellis, L. Xylella fastidiosa induces differential expression of lignification related-genes and lignin accumulation in tolerant olive trees cv. Leccino. J. Plant Physiol. 2018, 220, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Godefroid, M.; Cruaud, A.; Streito, J.-C.; Rasplus, J.-Y.; Rossi, J.-P. Xylella fastidiosa: Climate suitability of European continent. Sci. Rep. 2019, 9, 8844. [Google Scholar] [CrossRef] [PubMed]

| Host | Family | Islands 1 | Strain 2 |
|---|---|---|---|
| Acacia saligna | Leguminosae | Ma|Ib | ST81|ST80 |
| Calicotome spinosa Cistus albidus Cistus monspeliensis Clematis cirrhosa | Leguminosae Cistaceae Cistaceae Ranunculaceae | Ma Ma|Me|Ib Ma Me | ST1 ST81|ST81|ST80 ST1 ST81 |
| Elaeagnus angustifolia | Elaeagnaceae | Ib | Not determined |
| Ficus carica | Moraceae | Ma|Me | ST81|ST81 |
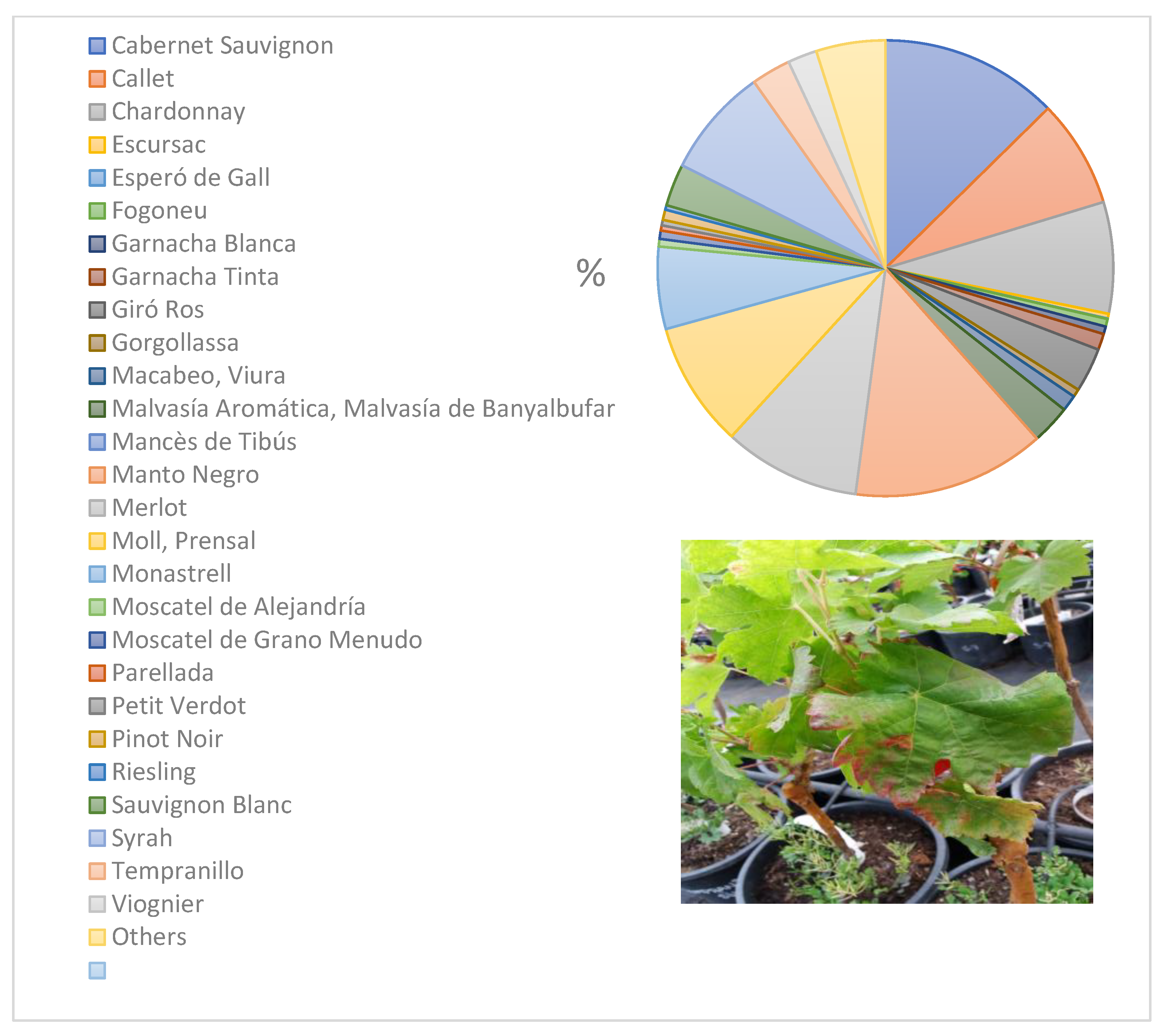
| Fraxinus angustifolia Genista hirsuta Genista lucida Genista valdes-bermejoi Helichrysum stoechas Juglans regia Lavandula angustifolia Lavandula dentata Nerium oleander Olea europaea var. europaea Olea europaea var. sylvestris Phagnalon saxatile Phillyrea angustifolia Phlomis italica Polygala myrtifolia Prunus avium Prunus domestica Prunus dulcis Rhamnus alaternus Rosmarinus officinalis Ruta chalepensis Santolina chamaecyparissus Santolina magonica Salvia officinalis Spartium junceum Teucrium capitatum Thymus vulgaris Ulex parviflorus Vitex agnus-castus Vitis vinifera | Oleaceae Leguminosae Leguminosae Leguminosae Compositae Juglandaceae Labiatae Labiatae Apocynaceae Oleaceae Oleaceae Compositae Oleaceae Labiatae Polygalaceae Rosaceae Rosaceae Rosaceae Rhamnaceae Labiatae Rutaceae Compositae Compositae Labiatae Leguminosae Labiatae Labiatae Leguminosae Verbenaceae Vitaceae | Ma Ib Ma Ma Ma|Me Ma Ma|Ib Ma|Ib Ma|Ib Ma|Me|Ib Ma|Me|Ib Ma Ma Me Ma|Ib Ma Ma Ma|Me|Ib Ma|Me Ma|Me|Ib Ma Ma|Me Me Ma Ma Ma Ib Ib Me Ma | ST81 Not determined ST1 ST81 Not determined|ST81 ST1 ST81|ST80 ST81|ST80 ST81|Not determined ST81|ST81|ST80 ST81|ST81|ST80 Not determined ST81 Not determined ST1, ST7, ST81|ST80 ST1 ST81 ST1, ST7, ST81|ST81|ST80 ST1, ST81|ST81 ST81|ST81|ST80 ST1 Not determined|ST81 ST81 ST81 ST81 ST1 Not determined ST80 Not determined ST1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quetglas, B.; Olmo, D.; Nieto, A.; Borràs, D.; Adrover, F.; Pedrosa, A.; Montesinos, M.; de Dios García, J.; López, M.; Juan, A.; et al. Evaluation of Control Strategies for Xylella fastidiosa in the Balearic Islands. Microorganisms 2022, 10, 2393. https://doi.org/10.3390/microorganisms10122393
Quetglas B, Olmo D, Nieto A, Borràs D, Adrover F, Pedrosa A, Montesinos M, de Dios García J, López M, Juan A, et al. Evaluation of Control Strategies for Xylella fastidiosa in the Balearic Islands. Microorganisms. 2022; 10(12):2393. https://doi.org/10.3390/microorganisms10122393
Chicago/Turabian StyleQuetglas, Bàrbara, Diego Olmo, Alicia Nieto, David Borràs, Francesc Adrover, Ana Pedrosa, Marina Montesinos, Juan de Dios García, Marta López, Andreu Juan, and et al. 2022. "Evaluation of Control Strategies for Xylella fastidiosa in the Balearic Islands" Microorganisms 10, no. 12: 2393. https://doi.org/10.3390/microorganisms10122393
APA StyleQuetglas, B., Olmo, D., Nieto, A., Borràs, D., Adrover, F., Pedrosa, A., Montesinos, M., de Dios García, J., López, M., Juan, A., & Moralejo, E. (2022). Evaluation of Control Strategies for Xylella fastidiosa in the Balearic Islands. Microorganisms, 10(12), 2393. https://doi.org/10.3390/microorganisms10122393





