Seroepidemiology of Borrelia burgdorferi s.l. among German National Cohort (NAKO) Participants, Hanover
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Blood Sample Analyses
2.3. Defining B. burgdorferi s.l. Seropositivity
- A positive or equivocal screening test (ELISA) with a subsequent positive confirmatory test (line blot), which corresponds to the current standard MiQ12 [33];
2.4. Force of Infection
2.5. Regression Analysis
3. Results
4. Discussion
4.1. Hanoverian Seropositivity in Context
4.2. Age-Specific Seropositivity
4.3. Risk Factors for Seropositivity
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Cunze, S.; Glock, G.; Kochmann, J.; Klimpel, S. Ticks on the move-climate change-induced range shifts of three tick species in Europe: Current and future habitat suitability for Ixodes ricinus in comparison with Dermacentor reticulatus and Dermacentor marginatus. Parasitol. Res. 2022, 121, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut. RKI-Ratgeber Lyme-Borreliose; Robert Koch-Institut: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Müllegger, R. Infektionen: Lyme-Borreliose, Leptospirose und Rückfallfieber. In Braun-Falco’s Dermatologie, Venerologie und Allergologie; Plewig, G., Ruzicka, T., Kaufmann, R., Hertl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–17. ISBN 978-3-662-49546-9. [Google Scholar]
- van den Wijngaard, C.C.; Hofhuis, A.; Simões, M.; Rood, E.; van Pelt, W.; Zeller, H.; van Bortel, W. Surveillance perspective on Lyme borreliosis across the European Union and European Economic Area. Euro Surveill. 2017, 22, 30569. [Google Scholar] [CrossRef]
- Enkelmann, J.; Böhmer, M.; Fingerle, V.; Siffczyk, C.; Werber, D.; Littmann, M.; Merbecks, S.-S.; Helmeke, C.; Schroeder, S.; Hell, S.; et al. Incidence of notified Lyme borreliosis in Germany, 2013–2017. Sci. Rep. 2018, 8, 14976. [Google Scholar] [CrossRef]
- Akmatov, M.K.; Holstiege, J.; Dammertz, L.; Heuer, J.; Kohring, C.; Lotto-Batista, M.; Boeing, F.; Ghozzi, S.; Castell, S.; Bätzing, J. Epidemiology of Lyme borreliosis based on outpatient claims data of all people with statutory health insurance, Germany, 2019. Euro Surveill. 2022, 27, 2101193. [Google Scholar] [CrossRef] [PubMed]
- Knoll, S.; Springer, A.; Hauck, D.; Schunack, B.; Pachnicke, S.; Fingerle, V.; Strube, C. Distribution of Borrelia burgdorferi s.l. and Borrelia miyamotoi in Ixodes tick populations in Northern Germany, co-infections with Rickettsiales and assessment of potential influencing factors. Med. Vet. Entomol. 2021, 35, 595–606. [Google Scholar] [CrossRef]
- Boehnke, D.; Brugger, K.; Pfäffle, M.; Sebastian, P.; Norra, S.; Petney, T.; Oehme, R.; Littwin, N.; Lebl, K.; Raith, J.; et al. Estimating Ixodes ricinus densities on the landscape scale. Int. J. Health Geogr. 2015, 14, 23. [Google Scholar] [CrossRef]
- Brugger, K.; Boehnke, D.; Petney, T.; Dobler, G.; Pfeffer, M.; Silaghi, C.; Schaub, G.A.; Pinior, B.; Dautel, H.; Kahl, O.; et al. A Density Map of the Tick-Borne Encephalitis and Lyme Borreliosis Vector Ixodes ricinus (Acari: Ixodidae) for Germany. J. Med. Entomol. 2016, 53, 1292–1302. [Google Scholar] [CrossRef]
- Hansford, K.M.; Wheeler, B.W.; Tschirren, B.; Medlock, J.M. Questing Ixodes ricinus ticks and Borrelia spp. in urban green space across Europe: A review. Zoonoses Public Health 2022, 69, 153–166. [Google Scholar] [CrossRef]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-Wide Meta-Analysis of Borrelia burgdorferi Sensu Lato Prevalence in Questing Ixodes ricinus Ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef]
- Tappe, J.; Jordan, D.; Janecek, E.; Fingerle, V.; Strube, C. Revisited: Borrelia burgdorferi sensu lato infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany). Parasit. Vectors 2014, 7, 441. [Google Scholar] [CrossRef] [PubMed]
- Blazejak, K.; Raulf, M.-K.; Janecek, E.; Jordan, D.; Fingerle, V.; Strube, C. Shifts in Borrelia burgdorferi (s.l.) geno-species infections in Ixodes ricinus over a 10-year surveillance period in the city of Hanover (Germany) and Borrelia miyamotoi-specific Reverse Line Blot detection. Parasit. Vectors 2018, 11, 304. [Google Scholar] [CrossRef]
- Hauck, D.; Springer, A.; Chitimia-Dobler, L.; Strube, C. Two-year monitoring of tick abundance and influencing factors in an urban area (city of Hanover, Germany). Ticks Tick Borne Dis. 2020, 11, 101464. [Google Scholar] [CrossRef]
- Böhmer, M.M.; Ens, K.; Böhm, S.; Heinzinger, S.; Fingerle, V. Epidemiological Surveillance of Lyme Borreliosis in Bavaria, Germany, 2013–2020. Microorganisms 2021, 9, 1872. [Google Scholar] [CrossRef] [PubMed]
- Deutsches Klima-Konsortium (DKK); Deutsche Meteorologische Gesellschaft (DMG); Deutscher Wetterdienst (DWD); Extremwetterkongress Hamburg; Helmholtz-Klima-Initiative; klimafakten.de. Was wir Heute Übers Klima Wissen: Basisfakten zum Klimawandel, die in der Wissenschaft Unumstritten Sind. Available online: https://www.klimafakten.de/meldung/was-wir-heute-uebers-klima-wissen-basisfakten-zum-klimawandel-die-der-wissenschaft (accessed on 24 July 2022).
- Perret, J.L.; Guigoz, E.; Rais, O.; Gern, L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 2000, 86, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Borsche, M.; Friedrich, A.; Friedrich, K.; Fröhlich, K.; Früh, B.; Kreienkamp, F.; Pietzsch, S.; Rauthe, M.; Riecke, W.; Walter, A.; et al. Klimareport Niedersachsen: Fakten bis zur Gegenwart—Erwartungen für die Zukunft, 1. Auflage; Deutscher Wetterdienst Abt. Klima- und Umweltberatung Regionales Klimabüro Hamburg; Druckerei des BMVI: Hamburg, Bonn, Germany, 2018; ISBN 978-3-88148-509-8. [Google Scholar]
- Tucker, P.; Gilliland, J. The effect of season and weather on physical activity: A systematic review. Public Health 2007, 121, 909–922. [Google Scholar] [CrossRef]
- Dehnert, M.; Fingerle, V.; Klier, C.; Talaska, T.; Schlaud, M.; Krause, G.; Wilking, H.; Poggensee, G. Seropositivity of Lyme borreliosis and associated risk factors: A population-based study in Children and Adolescents in Germany (KiGGS). PLoS ONE 2012, 7, e41321. [Google Scholar] [CrossRef]
- Wilking, H.; Fingerle, V.; Klier, C.; Thamm, M.; Stark, K. Antibodies against Borrelia burgdorferi sensu lato among Adults, Germany, 2008–2011. Emerg. Infect. Dis. 2015, 21, 107–110. [Google Scholar] [CrossRef]
- Woudenberg, T.; Böhm, S.; Böhmer, M.; Katz, K.; Willrich, N.; Stark, K.; Kuhnert, R.; Fingerle, V.; Wilking, H. Dynamics of Borrelia burgdorferi-Specific Antibodies: Seroconversion and Seroreversion between Two Population-Based, Cross-Sectional Surveys among Adults in Germany. Microorganisms 2020, 8, 1859. [Google Scholar] [CrossRef]
- Coors, A.; Hassenstein, M.J.; Krause, G.; Kerrinnes, T.; Harries, M.; Breteler, M.M.B.; Castell, S. Regional seropositivity for Borrelia burgdorferi and associated risk factors: Findings from the Rhineland Study, Germany. Parasit. Vectors 2022, 15, 241. [Google Scholar] [CrossRef]
- Wilking, H.; Stark, K. Trends in surveillance data of human Lyme borreliosis from six federal states in eastern Germany, 2009–2012. Ticks Tick Borne Dis. 2014, 5, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, J.S.P.; Semper, A.E.; Brooks, T.J.G.; Russell, K.; Halsby, K.D.; Christley, R.M.; Radford, A.D.; Vivancos, R.; Warner, J.C. The demographics and geographic distribution of laboratory-confirmed Lyme disease cases in England and Wales (2013–2016): An ecological study. BMJ Open 2019, 9, e028064. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, J.S.P.; Christley, R.M.; Radford, A.D.; Warner, J.C.; Beadsworth, M.B.J.; Beeching, N.J.; Vivancos, R. A descriptive epidemiological study of the incidence of newly diagnosed Lyme disease cases in a UK primary care cohort, 1998–2016. BMC Infect. Dis. 2020, 20, 285. [Google Scholar] [CrossRef] [PubMed]
- Hjetland, R.; Nilsen, R.M.; Grude, N.; Ulvestad, E. Seroprevalence of antibodies to Borrelia burgdorferi sensu lato in healthy adults from western Norway: Risk factors and methodological aspects. APMIS 2014, 122, 1114–1124. [Google Scholar] [CrossRef]
- Linard, C.; Lamarque, P.; Heyman, P.; Ducoffre, G.; Luyasu, V.; Tersago, K.; Vanwambeke, S.O.; Lambin, E.F. Determinants of the geographic distribution of Puumala virus and Lyme borreliosis infections in Belgium. Int. J. Health Geogr. 2007, 6, 15. [Google Scholar] [CrossRef]
- Sočan, M.; Blaško-Markič, M.; Erčulj, V.; Lajovic, J. Socio-Economic Characteristics in Notified Erythema Migrans Patients. Zdr. Varst. 2015, 54, 267–273. [Google Scholar] [CrossRef]
- Hens, N.; Shkedy, Z.; Aerts, M.; Faes, C.; van Damme, P.; Beutels, P. Modeling Infectious Disease Parameters Based on Serological and Social Contact Data; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-4071-0. [Google Scholar]
- Peters, A.; Greiser, K.H.; Göttlicher, S.; Ahrens, W.; Albrecht, M.; Bamberg, F.; Bärnighausen, T.; Becher, H.; Berger, K.; Beule, A.; et al. Framework and baseline examination of the German National Cohort (NAKO). Eur. J. Epidemiol. 2022, 37, 1107–1124. [Google Scholar] [CrossRef]
- Lyme-Borreliose; Mauch, H.; Fingerle, V.; Eiffert, H.; Gessner, A.; Göbel, U.; Hofmann, H.; Hunfeld, K.-P.; Krause, A. (Eds.) 2. Auflage; Urban & Fischer in Elsevier: München, Germany, 2017; ISBN 978-3-437-22605-2. [Google Scholar]
- Woessner, R.; Gaertner, B.C.; Grauer, M.T.; Weber, K.; Mueller-Lantzsch, N.; Hunfeld, K.P.; Treib, J. Incidence and prevalence of infection with human granulocytic ehrlichiosis agent in Germany. A prospective study in young healthy subjects. Infection 2001, 29, 271–273. [Google Scholar] [CrossRef]
- Thortveit, E.T.; Aase, A.; Petersen, L.B.; Lorentzen, Å.R.; Mygland, Å.; Ljøstad, U. Human seroprevalence of antibodies to tick-borne microbes in southern Norway. Ticks Tick Borne Dis. 2020, 11, 101410. [Google Scholar] [CrossRef]
- German Federal Statistical Office. GENESIS-Online Database. Available online: www.destatis.de (accessed on 19 July 2022).
- Lumley, T. Package ‘Survey’; CRAN: Vienna, Austria, 2021. [Google Scholar]
- Lomax, N.; Norman, P. Estimating Population Attribute Values in a Table: “Get Me Started in” Iterative Proportional Fitting. Prof. Geogr. 2016, 68, 451–461. [Google Scholar] [CrossRef]
- Kalish, R.A.; McHugh, G.; Granquist, J.; Shea, B.; Ruthazer, R.; Steere, A.C. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin. Infect. Dis. 2001, 33, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Muench, H. Derivation of Rates from Summation Data by the Catalytic Curve. J. Am. Stat. Assoc. 1934, 29, 25. [Google Scholar] [CrossRef]
- Muench, H. Catalytic Models in Epidemiology; Harvard University Press: Cambridge, MA, USA, 1959. ISBN 978067442 8928.
- Griffiths, D.A. A Catalytic Model of Infection for Measles. Appl. Stat. 1974, 23, 330. [Google Scholar] [CrossRef]
- Grenfell, B.T.; Anderson, R.M. The estimation of age-related rates of infection from case notifications and serological data. J. Hyg. 1985, 95, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Schenk, L.; Bau, A.-M.; Borde, T.; Butler, J.; Lampert, T.; Neuhauser, H.; Razum, O.; Weilandt, C. Mindestindikatorensatz zur Erfassung des Migrationsstatus. Empfehlungen für die epidemiologische Praxis. Bundesgesundheitsblatt Gesundh. Gesundh. 2006, 49, 853–860. [Google Scholar] [CrossRef]
- International Standard Classification of Education ISCED 1997. English Edition—Re-Edition; UNESCO-UIS: Montreal, QC, Canada, 2006; ISBN 92-9189-035-9. [Google Scholar]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef]
- Lohse, T.; Rohrmann, S.; Bopp, M.; Faeh, D. Heavy Smoking Is More Strongly Associated with General Unhealthy Lifestyle than Obesity and Underweight. PLoS ONE 2016, 11, e0148563. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Soft. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K.; Vink, G.; Schouten, R. Package ‘Mice’; CRAN: Vienna, Austria, 2021. [Google Scholar]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef] [PubMed]
- van Buuren, S. Flexible Imputation of Missing Data, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2021; ISBN 9781032178639. [Google Scholar]
- Royston, P.; Altman, D.G. Regression Using Fractional Polynomials of Continuous Covariates: Parsimonious Parametric Modelling. Appl. Stat. 1994, 43, 429. [Google Scholar] [CrossRef]
- Heinze, G.; Ambler, G.; Benner, A. Package ‘mfp’; CRAN: Vienna, Austria, 2022. [Google Scholar]
- Fox, J. Linear Statistical Models and Related Methods: With Applications to Social Research; Wiley: New York, NY, USA, 1984; ISBN 9780471099130. [Google Scholar]
- Akinwande, M.O.; Dikko, H.G.; Samson, A. Variance Inflation Factor: As a Condition for the Inclusion of Suppressor Variable(s) in Regression Analysis. OJS 2015, 05, 754–767. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kalmár, Z.; Briciu, V.; Coroian, M.; Flonta, M.; Rădulescu, A.-L.; Topan, A.; Mihalca, A.D.; Lupșe, M. Seroprevalence of antibodies against Borrelia burgdorferi sensu lato in healthy blood donors in Romania: An update. Parasit. Vectors 2021, 14, 596. [Google Scholar] [CrossRef]
- Schwarz, A.; Hönig, V.; Vavrušková, Z.; Grubhoffer, L.; Balczun, C.; Albring, A.; Schaub, G.A. Abundance of Ixodes ricinus and prevalence of Borrelia burgdorferi s.l. in the nature reserve Siebengebirge, Germany, in comparison to three former studies from 1978 onwards. Parasit. Vectors 2012, 5, 268. [Google Scholar] [CrossRef]
- Kampen, H.; Rötzel, D.C.; Kurtenbach, K.; Maier, W.A.; Seitz, H.M. Substantial rise in the prevalence of Lyme borreliosis spirochetes in a region of western Germany over a 10-year period. Appl. Environ. Microbiol. 2004, 70, 1576–1582. [Google Scholar] [CrossRef]
- Kempner, T.; Hein, S.; Neht, A.; Prenger-Berninghoff, K.; Welfers, L.; Witte, A.; Bamminger, C.; Grothues, E.; Gründeberg, C.; Kauke, N.; et al. Klimabericht NRW 2021: Klimawandel und seine Folgen—Ergebnisse aus dem Klimafolgen- und Anpassungsmonitoring; LANUV Fachbericht 120: Recklinghausen, Germany, 2021. [Google Scholar]
- Zeman, P.; Benes, C. Peri-urbanisation, counter-urbanisation, and an extension of residential exposure to ticks: A clue to the trends in Lyme borreliosis incidence in the Czech Republic? Ticks Tick Borne Dis. 2014, 5, 907–916. [Google Scholar] [CrossRef]
- Kamtsiuris, P.; Lange, M.; Hoffmann, R.; Schaffrath Rosario, A.; Dahm, S.; Kuhnert, R.; Kurth, B.M. Die erste Welle der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1): Stichprobendesign, Response, Gewichtung und Repräsentativität. Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 620–630. [Google Scholar] [CrossRef]
- Thefeld, W.; Stolzenberg, H.; Bellach, B.-M. Bundes-Gesundheitssurvey: Response, Zusammensetzung der Teilnehmer und Non-Responder-Analyse. Gesundh. Sonderh. 2 1999, 57–61. [Google Scholar]
- Cunze, S.; Glock, G.; Klimpel, S. Spatial and temporal distribution patterns of tick-borne diseases (Tick-borne Encephalitis and Lyme Borreliosis) in Germany. PeerJ 2021, 9, e12422. [Google Scholar] [CrossRef]
- Lohr, B.; Müller, I.; Mai, M.; Norris, D.E.; Schöffski, O.; Hunfeld, K.-P. Epidemiology and cost of hospital care for Lyme borreliosis in Germany: Lessons from a health care utilization database analysis. Ticks Tick Borne Dis. 2015, 6, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut. Epidemiologisches Bulletin: Aktuelle Daten und Informationen zu Infektionskrankheiten und Public Health No. 5, Berlin. 2009. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2009/Ausgaben/05_09.pdf?__blob=publicationFile (accessed on 10 October 2022).
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Wen, C.; Albert, C.; von Haaren, C. Equality in access to urban green spaces: A case study in Hannover, Germany, with a focus on the elderly population. Urban For. Urban Green. 2020, 55, 126820. [Google Scholar] [CrossRef]
- Lampert, T.; Kroll, L.; Müters, S.; Stolzenberg, H. Messung des sozioökonomischen Status in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Juhász-Horváth, L.; Trájer, A.; Pintér, L.; Rounsevell, M.D.A.; Harrison, P.A. Lifestyle, habitat and farmers’ risk of exposure to tick bites in an endemic area of tick-borne diseases in Hungary. Zoonoses Public Health 2018, 65, e248–e253. [Google Scholar] [CrossRef] [PubMed]
- Schielein, L.; Tizek, L.; Biedermann, T.; Zink, A. Tick bites in different professions and regions: Pooled cross-sectional study in the focus area Bavaria, Germany. BMC Public Health 2022, 22, 234. [Google Scholar] [CrossRef]
- Schüle, S.A.; Gabriel, K.M.A.; Bolte, G. Relationship between neighbourhood socioeconomic position and neighbourhood public green space availability: An environmental inequality analysis in a large German city applying generalized linear models. Int. J. Hyg. Environ. Health 2017, 220, 711–718. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Powell, K.E.; Campbell, W.W.; DiPietro, L.; Pate, R.R.; Pescatello, L.S.; Collins, K.A.; Bloodgood, B.; Piercy, K.L. Physical Activity and the Prevention of Weight Gain in Adults: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1262–1269. [Google Scholar] [CrossRef]
- Beyer, K.M.M.; Szabo, A.; Hoormann, K.; Stolley, M. Time spent outdoors, activity levels, and chronic disease among American adults. J. Behav. Med. 2018, 41, 494–503. [Google Scholar] [CrossRef]
- Hanson, S.; Jones, A. Is there evidence that walking groups have health benefits? A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 710–715. [Google Scholar] [CrossRef]
- Laiou, P.; Kaliukhovich, D.A.; Folarin, A.A.; Ranjan, Y.; Rashid, Z.; Conde, P.; Stewart, C.; Sun, S.; Zhang, Y.; Matcham, F.; et al. The Association Between Home Stay and Symptom Severity in Major Depressive Disorder: Preliminary Findings From a Multicenter Observational Study Using Geolocation Data From Smartphones. JMIR Mhealth Uhealth 2022, 10, e28095. [Google Scholar] [CrossRef] [PubMed]
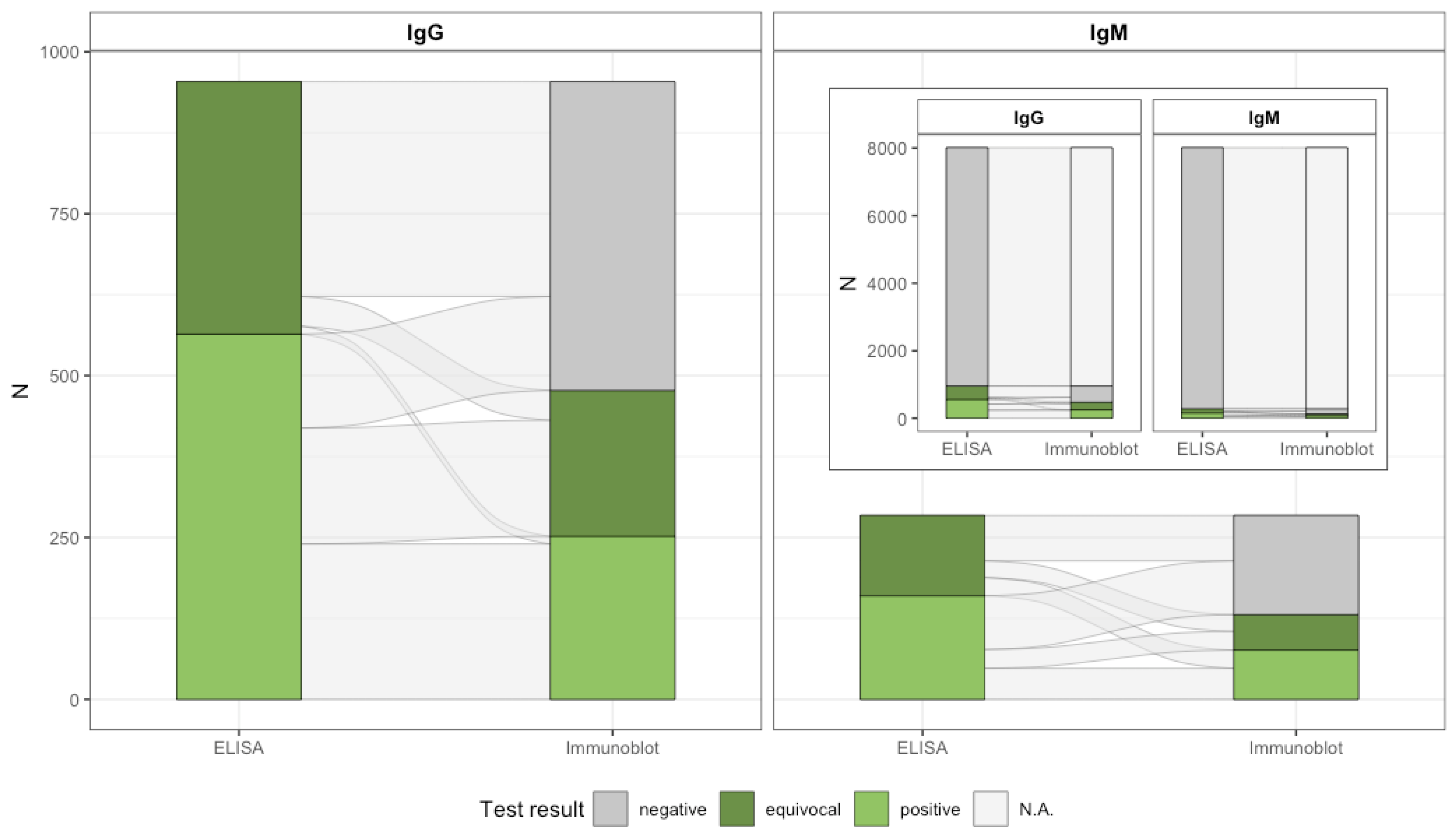
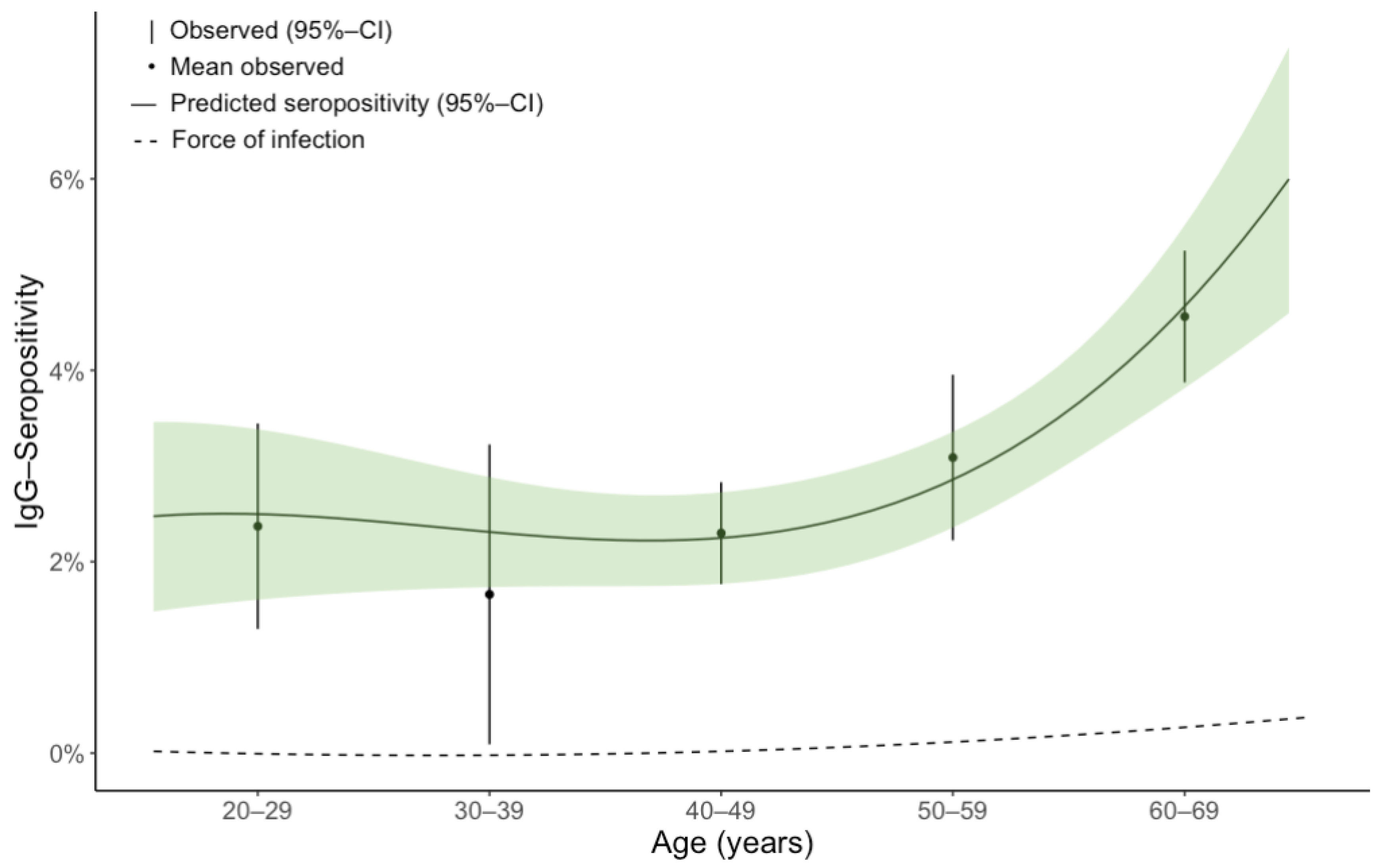
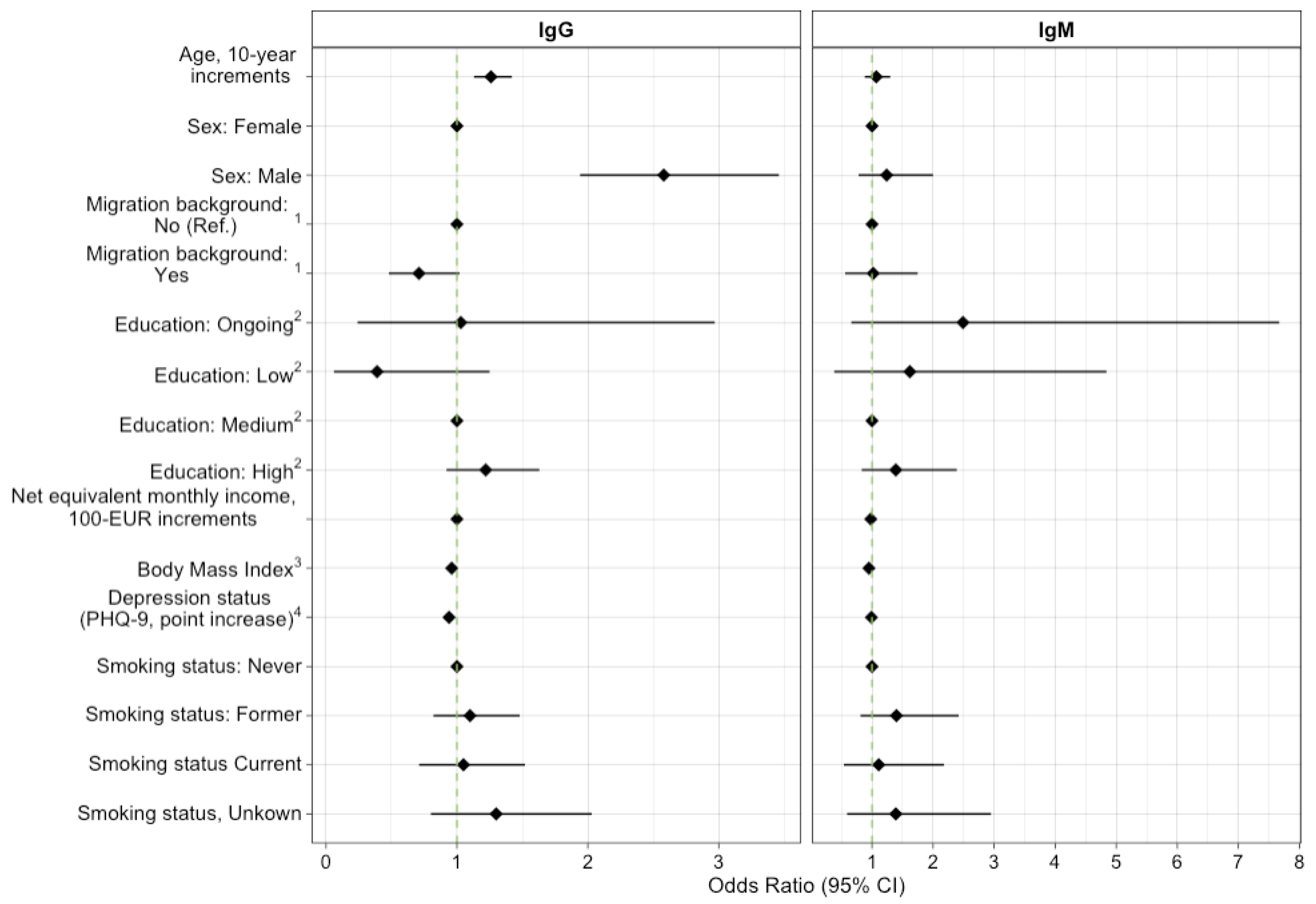
| Characteristics | Total (N = 8009) | IgG Seropositive (n = 252), Proportion (%, 95% CI) | IgM Seropositive (n = 76), Proportion (%, 95% CI) |
|---|---|---|---|
| Age | |||
| 20–29 years | 831 (10.4%) | 23/831 (2.8; 1.8–3.7) | 11/831 (1.3; 0.7–2.0) |
| 30–39 years | 767 (9.6%) | 14/767 (1.8; 1.0–2.6) | 5/767 (0.7; 0.2–1.1) |
| 40–49 years | 2102 (26.2%) | 47/2102 (2.2; 1.7–2.8) | 22/2102 (1.0; 0.7–1.4) |
| 50–59 years | 2117 (26.4%) | 67/2117 (3.2; 2.5–3.8) | 17/2117 (0.8; 0.5–1.1) |
| 60–69 years | 1999 (25.0%) | 91/1999 (4.6; 3.8–5.3) | 19/1999 (1.0; 0.6–1.3) |
| 70 years and older | 193 (2.4%) | 10/193 (5.2; 2.6–7.8) | 2/193 (1.0; 0.0–2.2) |
| Sex | |||
| Male | 3991 (49.8%) | 181/3991 (4.5; 4.0–5.1) | 41/3991 (1.0; 0.8–1.3) |
| Female | 4018 (50.2%) | 71/4018 (1.8; 1.4–2.1) | 35/4018 (0.9; 0.6–1.1) |
| Migration Background 1 | |||
| No | 6389 (79.8%) | 217/6389 (3.4; 3.0–3.8) | 58/6389 (0.9; 0.7–1.1) |
| Yes | 1616 (20.2%) | 35/1616 (2.2; 1.6–2.8) | 17/1616 (1.1; 0.6–1.5) |
| Missing | 4 (0.1%) | 0/4 (0.0; 0.0–0.0) | 1/4 (25.9; 0.0–60.6) |
| Education 2 | |||
| Ongoing | 172 (2.1%) | 2/172 (1.2; 0.0–2.5) | 3/172 (1.5; 0.1–3.4) |
| Low | 203 (2.6%) | 2/203 (1.0; 0.0–2.1) | 3/203 (1.5; 0.1–2.9) |
| Medium | 2680 (33.5%) | 73/2680 (2.7; 2.2–3.2) | 21/2680 (0.8; 0.5–1.1) |
| High | 4480 (55.9%) | 166/4480 (3.7; 3.2–4.2) | 45/4480 (1.00; 0.8–1.2) |
| Missing | 472 (6.9%) | 9/472 (1.9; 0.9–2.9) | 4/463 (0.9; 0.2–1.6) |
| Net equivalent monthly income (Euro) | |||
| Median income (IQR) | 2150 (1520–2917) | 2150 (1633–3167) | 1900 (1471–2533) |
| Quartile 1 | 1852 (23.1%) | 49/1852 (2.6; 2.0–3.3) | 18/1852 (1.0; 0.6–1.3) |
| Quartile 2 | 1972 (24.6%) | 74/1972 (3.8; 3.0–4.5) | 23/1972 (1.2; 0.8–1.6) |
| Quartile 3 | 1745 (21.8%) | 46/1745 (2.6; 2.0–3.3) | 18/1745 (1.0; 0.6–1.4) |
| Quartile 4 | 1813 (22.6%) | 60/1813 (3.3; 2.6–4.0) | 11/1813 (0.6;0.3–0.9) |
| Missing | 621 (7.8%) | 23/621 (3.7; 2.5–5.0) | 6/621 (1.0; 0.3–1.6) |
| Body Mass Index 3 | |||
| Underweight | 81 (1.0%) | 1/81 (1.2; 0.0–3.3) | 1/81 (1.2; 0.0–3.3) |
| Normal | 3581 (44.7%) | 128/3581 (3.6; 3.1–4.1) | 37/3581 (1.0; 0.8–1.3) |
| Pre-obesity | 2812 (35.1%) | 78/2812 (2.8; 2.3–3.3) | 30/2812 (1.1; 0.7–1.4) |
| Obesity class I | 974 (12.2%) | 29/974 (3.0; 2.1–3.9) | 4/974 (0.4; 0.1–0.7) |
| Obesity class II | 268 (3.3%) | 9/268 (3.4; 1.5–5.2) | 1/268 (0.4; 0.0–1.0) |
| Obesity class III | 125 (1.6%) | 2/125 (1.6; 0.0–3.4) | 2/123 (1.6; 0.0–3.5) |
| Missing | 168 (2.1%) | 5/168 (3.0; 0.8–5.1) | 1/168 (0.6; 0.0–1.6) |
| Depression symptoms 4 | |||
| None/minimal | 4917 (61.4%) | 183/4917 (3.7; 3.3–4.2) | 49/4917 (1.0; 0.8–1.2) |
| Mild | 1780 (22.2%) | 32/1780 (1.8; 1.3–2.3) | 12/1780 (0.7; 0.4–1.0) |
| Moderate | 370 (4.6%) | 6/370 (1.6; 0.5–2.7) | 2/370 (0.5; 0.0–1.2) |
| Moderately severe | 134 (1.7%) | 2/134 (1.5; 0.0–3.2) | 2/134 (1.5; 0.0–3.2) |
| Severe | 36 (0.4%) | 0/36 (0.0; 0.0–0.0) | 0/36 (0.0; 0.0–0.0) |
| Missing | 772 (9.6%) | 29/772 (3.8; 2.6–4.9) | 11/772 (1.4; 0.7–2.1) |
| Smoking status | |||
| Never | 1695 (21.2%) | 48/1695 (2.8; 2.2–3.5) | 17/1695 (1.0; 0.6–1.4) |
| Former | 1396 (17.4%) | 49/1396 (3.5; 2.7–4.3) | 11/1396 (0.8; 0.4–1.2) |
| Current | 694 (8.7%) | 23/694 (3.3; 2.2–4.4) | 8/694 (1.2; 0.5–1.8) |
| Unknown | 393 (4.9%) | 15/393 (3.8; 2.2–5.4) | 7/393 (1.8; 0.7–2.9) |
| Missing | 3831 (47.8%) | 117/3831 (3.1; 2.6–3.5) | 33/3831 (0.9; 0.6–1.1) |
| Antibody Type | Seropositivity Definition | Crude Numbers | Crude % (95% CI) | Weighted Estimate % (95% CI) 1 |
|---|---|---|---|---|
| IgG | ELISA: positive or equivocal and line blot: positive (MiQ12) 2 | 252/8009 | 3.1 (2.8–3.5) | 3.0 (2.7–3.4) |
| ELISA: positive and line blot: positive or equivocal or ELISA: equivocal and line blot: positive | 431/8009 | 5.4 (4.9–5.9) | 5.2 (4.7–5.7) | |
| ELISA: positive 3 | 564/8009 | 7.0 (6.5–7.6) | 6.8 (6.3–7.4) | |
| IgM | ELISA: positive or equivocal and line blot: positive (MiQ12) 2 | 76/8009 | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) |
| ELISA: positive and line blot: positive or equivocal or ELISA: equivocal and line blot: positive | 105/8009 | 1.3 (1.1–1.6) | 1.4 (1.2–1.7) | |
| ELISA: positive 3 | 160/8009 | 2.0 (1.7–2.3) | 2.1 (1.8–2.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassenstein, M.J.; Janzen, I.; Krause, G.; Harries, M.; Melhorn, V.; Kerrinnes, T.; Kemmling, Y.; Castell, S. Seroepidemiology of Borrelia burgdorferi s.l. among German National Cohort (NAKO) Participants, Hanover. Microorganisms 2022, 10, 2286. https://doi.org/10.3390/microorganisms10112286
Hassenstein MJ, Janzen I, Krause G, Harries M, Melhorn V, Kerrinnes T, Kemmling Y, Castell S. Seroepidemiology of Borrelia burgdorferi s.l. among German National Cohort (NAKO) Participants, Hanover. Microorganisms. 2022; 10(11):2286. https://doi.org/10.3390/microorganisms10112286
Chicago/Turabian StyleHassenstein, Max J., Irina Janzen, Gérard Krause, Manuela Harries, Vanessa Melhorn, Tobias Kerrinnes, Yvonne Kemmling, and Stefanie Castell. 2022. "Seroepidemiology of Borrelia burgdorferi s.l. among German National Cohort (NAKO) Participants, Hanover" Microorganisms 10, no. 11: 2286. https://doi.org/10.3390/microorganisms10112286
APA StyleHassenstein, M. J., Janzen, I., Krause, G., Harries, M., Melhorn, V., Kerrinnes, T., Kemmling, Y., & Castell, S. (2022). Seroepidemiology of Borrelia burgdorferi s.l. among German National Cohort (NAKO) Participants, Hanover. Microorganisms, 10(11), 2286. https://doi.org/10.3390/microorganisms10112286





